Abstract
In the present investigation, bulk and chemical partitioning of elements (Cu, Mn, Ni, Zn, Fe, Ca) together with organic matter as a loss in ignition in the Qarechay River bed sediments have been studied. The concentration of metals in Qarechay River bed sediments is governed by the geological units of the study area. The study of anthropogenic portion shows that a small proportion of elemental concentration belongs to this phase. However, Mn has a large portion of anthropogenic sources (43 %). Also, Mn has a share of 13.6 % in sulfide fractions. This result indicates that Mn is a highly mobile element and can easily enter the water column. The presence of Mn in sulfide fraction might be indicative of initial stages of conversion of oxidation state into reduction in Qarechay River. Share of metals in anthropogenic portion is in the following order: Mn (43 %) > Cu (19 %) > Zn (10 %) > Ni (3 %) > Fe (0 %). Organic metallic bonds are not significantly present in the study area. Geochemical index (I geo), pollution index (I poll), enrichment factor (EF), and pollution load index (PLI) values are indicative of a clean environment throughout the river course. These values are in well agreement with results of chemical partitioning data. Eventually, based on the results of chemical partitioning, regional standard of elements for Qarechay River bed sediments has been established.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are among the most common environmental pollutants, and their occurrence in waters and biota indicates the presence of natural or anthropogenic sources. The existence of trace metals in aquatic environments has led to serious concerns about their influence on plant and animal life (Zvinowanda et al. 2009; Sheikh et al. 2007). Many researchers have used sediments to study the behavior of metals over time of sedimentation (Bellucci et al. 2003; Bertolotto et al. 2003; Al-Masri et al. 2002; Borretzen and Salbu 2002; Weis et al. 2001; Lee and Cundy 2001; Karbassi 1989). Forstner and Muller (1973) used a sediment pollution index to evaluate the heavy metal pollution of rivers of Germany.
Sediment quality values are a useful tool to screen the potential for contaminants within sediment to induce biological effects and to compare sediment contaminant concentration with corresponding quality guideline (Mohan 2000). These indexes evaluate the degree to which the sediment-associated chemical status might adversely affect aquatic organisms and are designed to assist sediment assessors and manager responsible for the interpretation of sediment quality (Borole et al. 1982). It is also to rank and prioritize the contaminated areas or the chemicals for further investigation (Adams et al. 1992).
Qarechay is one of two permanent and big rivers in Markazi Province in the central part of Iran. This river is almost 540 km long, and its catchment area is 1,800 km2. It originates from the heights of Shazand and finally flows into the Qom salt lake. In recent years, due to the reduced rainfall and construction of “Alghadir” dam for agricultural purposes, the mean annual discharge of this river has been decrease. Trace element pollution in rive, lake, estuary, and bay sediments has been reported by many researchers around the world (Al-Masri et al. 2002; Coker et al. 1995; Farmer 1991). However, study on the geochemistry of Qarechay River sediments in the present study area has not been undertaken by previous researchers so far.
Materials and methods
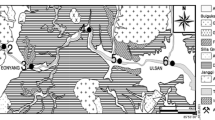
Riverine sediments were collected from six stations in Qarechay River in winter 2012 using a Peterson grab sampler. Location of samples is shown in Fig. 1. The riverbed sediments are brownish clayey silts deposited in the recent times. These samples were instantly placed in polyethylene bags, refrigerated, and carried to the laboratory. Sediments were dried at 70 °C for 24 h and passed through a mesh smaller than 63 μm. The sediment grains were then powdered using an agate mortal and pestle. Bulk digestion was carried out by HF–HNO3–HCl–HClO4. Chemical partition studies were carried out in four sequential steps: (1) 25 % v/v acetic acid, (2) 25 % v/v acetic acid–0.1 M hydroxylamine hydrochloride, (3) 30 % H2O2 “extraction with 1 M ammonium acetate,” and (4) 50 % hot HCl (Malo 1977; Gupta and Chen 1975; Gibbs 1973; Chester and Hughes 1967). Metal concentrations were measured by the Varian SpectrAA-200 at Science and Research Branch, I.A. University, Tehran, Iran, in spring 2013.
Procedural blanks and duplicates were run with the samples in a similar way for quality assurance of the laboratory analysis. The accuracy of analysis was about ±5 % for all elements. A standard sample (MESS-1) was analyzed in the same manner for analysis accuracy check. Results showed that the errors in the analysis were <5 %.
Organic matter was estimated by recording the loss on ignition (LOI) of samples heated for 4 h at 450 °C in a muffle furnace (Carver 1972). The weighted pair-group method (GWP) was used to identify clustering tendencies among the samples (Davis 1973).
Results and discussion
Bulk digestion
Concentrations of Cu, Ni, Zn, Mn, Fe, Ca, and other organic matters as the LOI in riverbed sediments of Qarechay are presented in Table 1.
Shale values and mean crust (Bowen 1979) are generally used as background values in sediment studies (Datta and Subramanian 1998; Jones and Turki 1997; López-Sánchez et al. 1993). The evaluation of the results reveals that Cu, Ni, Mn, Ca, and Fe values found in sediments from Qarechay River are lower than those for average shale. So, we can conclude that there is no pollution concerning these elements in the Qarechay River bed sediments.
A close look on the data shows that concentrations of Ni almost tally with the average concentrations of world sediments. However, concentrations of the other studied elements (except for Zn) are considerably lower than the average concentrations of mean crust, world sediments, and shale values (Table 1). Such discrepancies between concentrations of elements in the study area with those of shale, mean crust, and mean world sediments are indicative of considerable deviation in geological units. Most of the study area is covered with sandstone and limestone; thus, lower concentrations of element could be expected. The considerable difference between the concentration of Zn in samples obtained from sediments and shale is also explainable due to the fact that typical background concentrations of Zn found in soil may vary from 10 to 300 mg/kg (Alloway 1990).
Further, cluster analysis (Davis 1973) was used to know about the interrelation among studied elements. As shown in Fig. 2, dendrogram of cluster analysis depicts four distinct clusters, namely, “A,” “B,” “C,” and “D.” There is an agreement between results of cluster analysis and those of chemical partitioning studies. For instance, chemical partitioning studies do not show a considerable affinity among studied metals and organics (Table 2). In cluster B, Zn, Fe, and Ni are joined together with a high similarity coefficient that shows a lithogenous origin of Zn and Ni, but the correlation of this cluster with the other clusters is not meaningful. Therefore, further interpretations are depended on the results of chemical partitioning.
Chemical partitioning
The results of chemical partitioning studies show a share of metals in various sedimentary phases (Table 2). These results were grouped into lithogenous and anthropogenic ones (Fig. 3). Finally, pollution intensity was subjected to geochemical index (I geo), pollution index (I poll), enrichment factor (EF) (Table 3), and pollution load index (PLI) analysis (Table 4). The I geo and EF values are compared with the percentile of pollution obtained from chemical partitioning studies.
Percentile of elements in various sedimentary phases is in the following order:
-
Loose ions Mn (28.9 %) > Zn (13.5 %) > Cu (13.3 %) > Ni (12.2 %) > Fe (2.8 %)
-
Sulfide ions Mn (13.6 %) > Zn (0.3 %) > Fe (0.2 %) > Cu,Ni (0 %)
-
Organic ions Cu (13.4 %) > Mn (6 %) > Zn (5.5 %) > Fe (2.5 %) > Ni (0 %)
-
Resistant ions Zn (73.6 %) > Fe (62.9 %) > Ni (58.4 %) > Cu (47.8 %) > Mn (37.3 %)
-
Within lattice ions Fe (31.6 %) > Ni (29.4 %) > Cu (25.5 %) > Mn (14.2 %) > Zn (7.1 %).
In this study, Mn is the metal showing the highest mobility (upper than 28 %) in the loose ions fraction. It can be concluded that this element might be present in the form of carbonates in the area of study. Therefore, Mn shows more concentration in the loose ions fraction. The study of anthropogenic portion shows that a large portion of total concentration of Mn is related to this phase (43 %). Also, Mn has a share of 13.6 and 6 % in sulfide and organic fractions, respectively. These results indicate that Mn is a highly mobile element and can easily enter the water column. The presence of Mn in sulfide fraction might be indicative of initial stages of conversion of oxidation state into reduction (Karbassi 1996) in Qarechay River.
Further, the results of chemical partitioning were grouped into anthropogenic and lithogenous ones. As shown in Fig. 3, a small proportion of elemental concentration is in anthropogenic phase (except for Mn). Among all of the studied elements, Mn and Ni have the highest and the lowest share of anthropogenic sources, respectively, but Fe showing no anthropogenic portion remains constant in all sampling stations.
Although results of bulk digestion show a higher concentration of Zn than those of shale, mean crust, and mean world sediments, chemical partitioning proves a lithogenous source for this element (90 %), and share of anthropogenic portion is not significant.
Share of anthropogenic and lithogenous portions can be summarized as follows:
-
Lithogenous Fe (100 %) > Ni (97 %) > Zn (90 %) > Cu (81 %) > Mn (57 %)
-
Anthropogenic Mn (43 %) > Cu (19 %) > Zn (10 %) > Ni (3 %) > Fe (0 %).
Calculation of pollution intensity
The concentrations of various metals provide measures for determining the level of pollution in Qarechay sediments, based on Muller’s Index (I geo), EF, and I poll. One of the evaluation methods for the intensity of metal contamination in Qarechay sediments is the geochemical accumulation index
where I geo, C n, and B n are the geochemical accumulation index, the sediment metal concentration, and the metal concentration in the shale, respectively (Gonzalez et al. 2000; Forstner et al. 1991; Muller 1979). In this formula, the constant number 1.5 is applied as a factor for normalization to background metal concentrations.
The enrichment factor can be calculated for metals using
where (C n/C Fe)sample is the ratio of the element concentration (C n) to the concentration of Fe (C Fe).
In the sediment sample, (C n/C Fe)crust is the same ratio in an unpolluted reference sample (Pekey 2006; Ergin et al. 1991; Rule 1986; Buat-Menard and Chesselet 1979).
Based on the results of chemical partitioning, the anthropogenic portion of metals can be separated from their lithogenic portion. Therefore, Muller’s formula has been modified in order to correctly measure the pollution intensity of metal contamination in sediments (Nasrabadi et al. 2010; Karbassi et al. 2008)
In previous equation, I poll is the pollution intensity, B c is the sediment metal concentration, and L p is the lithogenous portion. The lithogenous portion is calculated by subtraction of the anthropogenic portion of elements from their bulk concentration. As metal concentrations in the shale play no role in this equation, the constant factor (1.5) is not required for normalization. EF and I geo formulae compare bulk concentrations of elements to their background levels in crust and shale, respectively. However, I poll shows the chemical partitioning results of the study area. Table 3 shows a comparison among various pollution indices in Qarechay River sediments.
The pollution load index (PLI) is obtained by calculating concentration factors (CFs). The PLI of the stations is calculated by obtaining the n-root from the n-CFs from all metals (Soares et al. 1999; Tomlinson et al. 1980).
where CF is the contamination factor, n is the number of metals, C metal is the metal concentration in contaminated sediments, and C background value is the background value of that metal (shale values). Table 4 shows the contamination factor and PLI in sampling stations for Qarechay River sediments.
Table 5 shows the categories of sediment pollution intensity based on the calculated indexes. According to geochemical accumulation index (I geo), this region is unpolluted in all sampling stations. Iwuoha et al. (2012) and Karbassi et al. (2006) obtained the same result in their investigations. Moreover, the sediments are unpolluted to moderately polluted with all of the measured elements in all sampling stations according to I poll, except for station 2 which is moderately polluted with Mn (I poll = 1.25) (Table 3). It should be pointed out that according to I poll, the highest pollution intensity in all sampling stations belongs to Mn.
The enrichment factor values for Cu and Mn indicate no or minor enrichment, and those for Ni and Zn show minor and moderate enrichment, respectively. This result is in agreement with the findings of Kumar and Patterson Edward (2009). They stated that the level of metal pollution in Manakudy estuary is low, and it is due to the absence of major factories in and around the study area. Karbassi et al. (2006) reported that the quantification of EF values is not logically possible, and therefore, I geo values can be used more effectively. In contrast, Iwuoha et al. (2012) reported that EF not only shows the class of pollution status of the riverbed sediments but also could be an exact indicator (i.e., showing the percentage contribution) from the sources (lithogenous or anthropogenic) of the contaminant and is very simple to use.
The PLI of the stations (Table 4) shows that the sediments of the river are perfect to baseline pollution condition (Table 5). The same result has been reported by Iwuoha et al. (2012) and Kumar and Patterson Edward (2009). Moreover, the value of CFs for all stations is lower than 1 which is indicative of low contamination. Therefore, all of calculated indexes are indicative of a clean environment throughout the river course. These values are in well agreement with results of chemical partitioning data.
Establishing metals regional standards
Based on the results of chemical partitioning, regional standard for the studied elements in Qarechay River bed sediments has been established. Concentrations of metals in lithogenous portion have been considered as a background concentration and based on the fact that 10 % of total concentration of metals could naturally be present in anthropogenic portion, and allowable variations have been calculated (Table 6). Therefore, in the future investigations, chemical partitioning studies are not necessary for the studied metals. By comparison of the results of bulk digestion with the amount of established regional standards, the amount of metal pollution could be determined. Comparison of the results of bulk digestion with the established regional standard shows that all of the studied elements are within the standard range except for Mn. This result is compatible with the results of chemical partitioning. As it was mentioned before, Mn is a mobile element, and its presence in sulfide fraction might be indicative of initial stages of conversion of oxidation state into reduction.
Conclusion
Geological units in the study area have totally governed the concentration of trace metals in bed sediments of Qarechay River. Manganese might be present in the form of carbonates in the study area. Therefore, Mn shows more concentration in the fraction of loose ions. A large amount of Mn is in anthropogenic portion, and as it is a highly mobile element, it can easily enter the water column. The presence of Mn in sulfide fraction might be indicative of initial stages of conversion of oxidation state into reduction. Based on the results of chemical partitioning, a small proportion of the other elements are in anthropogenic phase. According to I poll, the highest pollution intensity in all sampling stations belongs to Mn.
I geo, I poll, EF, and PLI values are indicative of a clean environment throughout the river course. These values are in well agreement with the results of chemical partitioning data. Finally, the comparison of results of bulk digestion with the established regional standard shows that all of the studied elements are within the standard range except for Mn.
References
Adams, W. J., Kimerle, R. A., & Barnet, J. W., Jr. (1992). Sediment quality and aquatic life assessment. Environmental Science and Technology, 26, 1865–1875.
Alloway, B. J. (1990). The origin of heavy metals in soils. New York: Springer. ISBN 0470215984k.
Al-Masri, M. S., Aba, A., Khalil, H., & Al-Hares, Z. (2002). Sedimentation rates and pollution history of a dried lake: Al-Oteibeh Lake. Science of the Total Environment, 293(1–3), 177–189.
Bellucci, L. G., El Moumni, B., Collavini, F., Frignani, M., & Albertazzi, S. (2003). Heavy metals in Morocco Lagoon and river sediments. Journal de Physique IV, 107(1), 139–142.
Bertolotto, R. M., Tortarolo, B., Frignani, M., Bellucci, L. G., Albanese, S., & Cuneo, C. (2003). Heavy metals in coastal sediments of the Ligurian sea off Vado Ligure. Journal de Physique IV, 107(1), 159–162.
Borole, D. V., Sari, M. M., & Somayajulu, B. L. K. (1982). Comparison of Normada and Tapti estuarine particulates and adjacent Arabian Sea sediments. Indian Journal of Marine Sciences, 11, 57–62.
Borretzen, P., & Salbu, B. (2002). Fixation of Cs to marine sediments estimated by a stochastic modeling approach. Journal of Environmental Radioactivity, 61(1), 1–20.
Bowen, H. J. M. (1979). Environmental chemistry of the elements (p. 333). London: Academic.
Buat-Menard, P., & Chesselet, R. (1979). Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth and Planetary Science Letters, 42, 398–411.
Carver, G. A. (1972). Glacial geology of the mountain lakes wilderness and adjacent parts of the cascade range, Oregon [PhD. Dissertation]: Seattle, University of Washington.
Chester, R., & Hughes, R. M. (1967). A chemical technique for the separation of ferromanganese minerals, carbonate minerals and adsorbed trace elements from pelagic sediments. Chemical Geology, 2, 249–262.
Coker, W. B., Kettles, I. M., & Shilts, W. W. (1995). Comparison of mercury concentrations in modern lake sediments and glacial drift in the Canadian Shield in the region of Ottawa/Kingston to Georgian Bay, Ontario, Canada. Water, Air, and Soil Pollution, 80, 1025–1029.
Datta, D., & Subramanian, V. (1998). Distribution and fractionation of heavy metals in the surface sediments of the Ganges-Brahmaputra-Meghna river system in the Bengal basin. Environmental Geology, 36(1–2), 93–101.
Davis, J. C. (1973). Statistics and data analysis in geology. New York: Wiley.
Ergin, M., Saydam, C., Basturk, O., Erdem, E., & Yoruk, R. (1991). Heavy metal concentrations in surface sediments from the two coastal inlets (Golden Horn Estuary and Izmit Bay) of the northeastern Sea of Marmara. Chemical Geology, 91, 269–285. doi:10.1016/0009-2541(91)90004-B.
Farmer, J. G. (1991). The perturbation of historical pollution records in aquatic systems. Environmental Geochemistry and Health, 13(2), 76–83.
Forstner, U., & Muller, G. (1973). Heavy metal accumulation in river sediments: a response to environmental pollution. Geoforum, 14(17), 53–61.
Forstner, U., Ahlf, W., Calmano, W., & Kersten, M. (1991). Sediment criteria development. in sediments and environmental geochemistry (pp. 311–338). Berlin: Springer.
Gibbs, R. J. (1973). Mechanisms of trace metal transport in rivers. Science, 180, 71–72.
Gonzalez, A., Rodriguez, M., Sanchez, J., Espinosa, A. J., & De La Rosa, F. J. (2000). Assessment of metals in sediments in a tributary of Guadalquiver River (Spain). Heavy metal partitioning and relation between the water and sediment system. Water, Air, and Soil Pollution, 121(1–4), 11–29.
Gupta, K. S., & Chen, K. Y. (1975). Partitioning of trace metals in selective chemical fractions of near shore sediments. Environmental Letters, 10, 129–159.
Iwuoha, G. N., Osuji, L. C., & Horsfall, M. J. (2012). Index model analysis approach to heavy metal pollution assessment in sediments of Nworie and Otamiri Rivers in Imo State of Nigeria. Research Journal of Chemical Sciences, 2(8), 1–8.
Jones, B., & Turki, A. (1997). Distribution and speciation of heavy metals in surficial sediments from the tees estuary, north-east England. Marine Pollution Bulletin, 34(10), 768–779.
Karbassi, A. R. (1989). Geochemical and magnetic studies of riverine, estuarine and marine sediments. Ph.D. thesis, Mangalore University, India, 196.
Karbassi, A. R. (1996). Geochemistry of Ni, Zn, Cu, Pb, Co, Cd, V, Mn, Fe, Al and Ca in sediments of North Western part of the Persian Gulf. International Journal of Environmental Studies, 54, 205–212.
Karbassi, A. R., Bayati, I., & Moattar, F. (2006). Origin and chemical partitioning of heavy metals in riverbed sediments. International Journal of Environmental Science and Technology, 3(1), 35–42.
Karbassi, A. R., Monavari, S., Nabi Bidhendi, G., Nouri, J., & Nematpour, K. (2008). Metal pollution assessment of sediment and water in the Shur River. Environmental Monitoring and Assessment, 147(1–3), 107–116.
Kumar, S. P., & Patterson Edward, J. K. (2009). Assessment of metal concentration in the sediment core of Manakudy estuary, south west coast of India. Indian Journal of Marine Sciences, 8(2), 235–248.
Lee, S. V., & Cundy, A. B. (2001). Heavy metal contamination and mixing processes in sediments from the Humber Estuary, Eastern England. Estuarine, Coastal and Shelf Science, 53(5), 619–636.
López-Sánchez, J. F., Rubio, R., & Rauret, G. (1993). Comparison of two sequential extraction procedures for trace metal partitioning in sediments. International Journal of Environmental Analytical Chemistry, 51(1–4), 113–121.
Malo, B. A. (1977). Partial extraction of metals from aquatic sediments. Environmental Science and Technology, 11, 277–282.
Mohan, P. M. (2000). Sediment transport mechanism in the Vellar estuary, east coast of India. Indian Journal of Marine Sciences, 29, 27–31.
Muller, G. (1979). Schwermetalle in den sediments des rheins-veranderungen seitt 1971. Umschan, 9, 778–783.
Nasrabadi T., Nabi Bidhendi G. R., Karbassi AR., & Mehrdadi N. (2010). Evaluating the efficiency of sediment metal pollution indices in interpreting the pollution of Haraz river sediments, Southern Caspian Sea basin. Environmental Monitoring and Assessment, 171, 395–410.
Pekey, H. (2006). Heavy metal pollution assessment in sediments of Izmit Bay, Turkey. Environmental Monitoring and Assessment, 123, 219–232.
Rule, J. (1986). Assessment of traces elements geochemistry of Hampton Roads Harbour and Lower Chesapeake Bay area sediments. Environmental Geology, 8, 209–219.
Sheikh, M. A., Noah, N. M., Tsuha, K., & Oomoti, T. (2007). Occurrence of tributyltin compounds and characteristics of heavy metals. International Journal of Environmental Science and Technology, 4(1), 49–60.
Soares, H. M., Boaventura, R. A. R., & Esteves da Silva, J. (1999). Sediments as monitors of heavy metal contamination in the Ave River basin (Portugal): multivariate analysis of data. Environmental Pollution, 105, 311–323.
Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffney, D. W. (1980). Problems in the assessment of heavy metal levels in estuaries and the formation of a pollution index. Helgoländer Wissenschaftliche Meeresuntersuchungen, 33, 566–572.
Weis, D. A., Callaway, A. B., & Gersberg, R. M. (2001). Vertical accretion rates and heavy metal chronologies in wetland sediments of the Tijuana Estuary. Estuaries, 24(6A), 840–850.
Zvinowanda, C. M., Okonkwo, J. O., Shabalala, P. N., & Agyei, N. M. (2009). A novel adsorbent for heavy metal remediation in aqueous environments. International Journal of Environmental Science and Technology, 6(3), 425–435.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biati, A., Karbassi, A.R. & Keyhani, Z. Origination and assessment of metal pollution in Qarechay River bed sediments. Environ Monit Assess 186, 4289–4297 (2014). https://doi.org/10.1007/s10661-014-3698-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3698-5