Abstract
A diagnostic study was conducted to examine the effectiveness of malodor removal from a large-scale municipal waste treatment plant in an urban area. To this end, the odor pollution status was investigated from a total of 16 spots in the treatment facility to cover the dual treatment lines consisting of regenerative thermal oxidation (first stage) and a wet chemical scrubber (second stage). As a simple means to learn more about the odorant removal efficiency of different treatment units, samples collected from ambient spots as well as before and after each treatment unit were analyzed for 22 key offensive odorants (i.e., reduced sulfur compounds, carbonyl compounds, nitrogenous compounds, volatile organic compounds, and fatty acids) along with dilution-to-threshold ratios based on the air dilution sensory test. The removal patterns differed greatly between different odorant groups across different processing units. The effectiveness of this dual treatment system was optimized for such odorants as hydrogen sulfide and ammonia, while it was not the case for others (e.g., some aldehydes and organic acids). The results thus suggest the need for the validation of the efficiency in many types of odor processing units and for establishing new control techniques to cover a list of odorants un-subordinate to preexisting methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The nuisance stemming from inadequate handling of food waste has become a prominent environmental issue not only in highly modernized urban areas but also in rural areas. Odor-related nuisances can be a common cause of legal conflicts. Hence, construction of a treatment plant is often considered as an inevitable option for odor emanation control in many urban areas. As such, densely populated areas treatment plant operation requires a highly deliberate management scheme, especially in densely populated urban areas (Dincer et al. 2006; Dincer and Muezzinoglu 2008). This is because the processing of waste, its sludge and compost is prone to generate all types of odorants that are often offensive to the surrounding residents (Bell et al. 1993; Cetin et al. 2003). Those odorants generated in each processing stage in turn became key evidence for the corresponding anaerobic and/or septic conditions.
As a means to handle massive quantities of municipal wastes, a large waste treatment plant named “Environment Resource Center (ERC)” has been in operation in the Dong Dae Mun (DDM) district near the central part of metropolitan Seoul, Korea since November 2010. ERC has adopted a dual operation line consisting of regenerative thermal oxidation (RTO) and a 3-stage wet chemical scrubbing (WCS) system with a full deodorizing capacity of 3,600 m3 min−1. Built as a modernized underground facility, this ERC represents the largest ecofriendly waste treatment plant of all the major cities in Korea.
In light of complexities and concerns associated with this plant’s operation in a densely populated locale, a field study was undertaken to obtain diagnostic data sets to assess the status of odor propagation along the main process lines and the pattern of their reduction through the treatment system. To comply with the study purpose, it was focused on a total of 22 offensive odorants regulated by the malodor prevention law in Korea (KMOE 2008) as the primary target of this investigation. These 22 odorants can be divided into five chemical groups: (1) reduced sulfur compounds (RSCs: H2S, CH3SH, DMS, and DMDS); (2) carbonyls [propionaldehyde (PA), butyraldehyde (BA), valeraldehyde (VA), iso-valeraldehyde (IA), and acetaldehyde (AA)]; (3) nitrogenous compounds [ammonia and trimethylamine (TMA)]; (4) VOCs [toluene (T), styrene (S), para-xylene (p-X), methyl ethyl ketone (MEK), methyl isobutyl ketone (MIBK), butyl acetate (BuAc), and isobutyl alcohol (i-BuAl)]; and (5) volatile fatty acids (VFA) [propionic acid (PPA), butyric acid (BTA), isovaleric acid (IVA), and valeric acid (VLA)]. This study reports the initial measurements of 22 odorants using samples collected from 16 spots at the ERC facility and provide some insights into the odor treatment along its main process lines under routine operating conditions. Field measurements were carried out on 27 December 2010. The collection of field samples for odorant analysis was made from 1 to 5 PM of the day as a collaborative team work made by three sampling groups each of which were deployed to cover the treatment units of the similar types or in the same underground floor.
Materials and methods
Table 1 summarizes the basic physicochemical properties (e.g., chemical formula, structural formula, molecular weight, CAS number, etc.) of all target compounds along with their properties as odorants (e.g., threshold values). Our field study was intended to measure these 22 offensive odorants along with some reference components [including benzene, formaldehyde, and total hydrocarbon (THC)] as supplementary variables due to their abundance. General information of measurement method for the major target compounds is described in Table 2. In addition to the analysis of target compounds, the strength of odor pollution was also assessed by the direct (olfactometry) method in terms of the dilution-to-threshold (D/T) ratios on the basis of the air dilution sensory (ADS) test (Kim and Park 2008).
Site characteristics and sampling locations
The DDM district, where the model municipal waste treatment facility is located, has long served as the hub of eastern Seoul while functioning as a second downtown with Cheong Nyang Ni Station and Gyeong Dong Market in its district. It also serves as a hub of transportation, as it is accessible to major highways such as Cheon Ho Ro, Wang San Ro, and Go San Ja Ro, as well as Subway lines No. 1 and 2. DDM covers 4.22 km2 (2.35 %) of Seoul city with a district population of 385,825 (and 156,777 households) as of 2007.
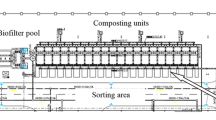
As shown in the facility map (Fig. 1), ERC is a five story building (3 above and 2 under ground) with an area coverage of 15,041 m2. The main treatment facility of ERC is built underground beneath Yong Doo Park, which is in the area intersecting Yong Doo station (subway line no. 2) and Go San Ja bridge. The construction of ERC was initiated in November. 2006 and completed in May 2010 at a cost of approximately 60 million USD on a build-transfer-operate (BTO) basis. This facility has a dual fan, the maximum deodorizing capacity of which is 1,800 m3 min−1 per single unit.
As described above, ERC was the first ecofriendly and modernized treatment facility in an urban area. However, that does not necessarily guarantee that its operation is free from complaints as a source of malodors. ERC pursues a comprehensive treatment of diverse waste types: food waste, household waste, large-scale waste, and recyclable materials, with the treatment capacity of 98, 270, 408, and 20 tons per day, respectively. The procedures of waste treatment consist of sorting out non-food materials from food wastes and fermenting the remainder over 30 days via a hybrid anaerobic digester. The biogas produced from this fermenting process is used to generate electricity. In the meantime, waste heat is also reused inside the system by making steam via a boiler. The unfermented portions of organic wastes are first dehydrated and separated into sludge and waste water. The latter is sent to a sewage treatment plant, while the former, fermented over 15 days, is made into compost. The non-recyclable portion of the wastes is sorted separately, pressurized, and buried in landfill facilities. As a means to properly evaluate the reliability of this deodorizing treatment, a preliminary field study was carried out to measure odorant species under normal operation conditions both in and outside this facility.
To precisely describe the odor reduction patterns of odorants in the model municipal waste treatment facility, its reduction efficiency for target compounds needs to be evaluated with respect to each treatment unit. To this end, the distribution of the target offensive odorants was measured from a total of 16 spots selected to represent the transfer/processing loop of the facility (Fig. 2). All of the measurements at 11 spots can cover the major transport route of odorants along this facility: (a) transport and duct lines for odor transfer between the units (5 spots) and (b) double control units of RTO and WCS (6 spots). In addition, concentrations of odorant levels inside the facility in ambient air were also measured from four spots in the bordering area and one in the open area of this facility. The odorant measurements which were made at a total of 11 transfer processing units are presented along with those made at a total of five reference (ambient) spots.
Sample collection and analysis
ADS test
The locations of individual sampling sites within the facility are briefly depicted in Fig. 2. Sampling in most treatment units was made from the designated sampling holes (or spots) for routine monitoring purposes. The collection of samples for the ADS test and many odorants (e.g., RSC) was made through a lung sampler (ACEN, Korea) for a duration of 5–10 min interval to fill up 10-L Tedlar bags (SKC Corp., PA, USA). These samples were brought to the laboratory and analyzed within 12 h. Because of the development in analytical techniques, major odorants contained in the ambient air samples can be measured and quantified using standardized methods. However, if a number of major odorants co-exist in the air, it is very difficult to accurately evaluate their contribution as a mixture in terms of sensory data. This is why one needs to rely on the sensory test method to quantitatively describe their odor strengths (Kim and Park 2008). The human olfactory system is capable of detecting odors at very low concentrations and over very short time intervals (Hasin-Brumshtein et al. 2009). Among such direct approaches, the ADS test has been improved and adopted by many researchers (Kim and Park 2008). In this study, the odor strength of each air sample has been measured initially by the standard protocol of the ADS test established by KMOE (2008).
To conduct this test, a panel (e.g., five panelists) was determined based on the olfactory sensitivity test using four standard odors (Kim and Park 2008). In this application, each sample was tested for the derivation of the “dilution-to-threshold (D/T)” ratio through a combination of the ‘yes/no’ opinions from all five panel members. The static dilution of odorant samples for the ADS test was made in a stepwise manner by mixing original samples with odorless air using a 3 L odor bag made of polyethylene terephthalate film. Odorless air was prepared by passing normal air through activated charcoals. This test was conducted until the last panel member reached the minimum detection (threshold values) of a given odor sample. The level of dilution for the ADS test by each panel can be finally expressed as X values as follows:
where Z is a multiplying factor (either 1 or 3) and n corresponds to an integer value of 0, 1, 2, 3,…, n. As a result, X values determined by each individual panel are computed as 1, 3, 10, 30, 100, 300, and so forth. The final D/T ratio of a given sample is then determined as a square root of the product of three X values (after excluding the maximum and minimum values taken from all 5 panel members). The odor index or odor intensity (OI) value for each odorant in a given sample is then determined by the conversion formula developed in a stipulated method (KMOE 2008), as shown in Table 3.
RSCs analysis
The analysis of RSCs was done by gas chromatography (GC) equipped with a pulsed flame photometric detector (PFPD) interfaced with a multi-function thermal desorber (TD) system with an air server (AS) unit. Details of the operating conditions for the RSC analysis have been listed in Table 2. The analytical procedures for RSCs in ambient air samples have been described in a number of previous publications by the authors (Kim 2005; Kim et al. 2006). The detection limits (DL) of the system fell in the range of 0.5 [or 0.12 ppb (DMDS)] to 0.7 pg [or 0.52 ppb (H2S)] (in a sampling volume of 120 mL). If the precision of this method is evaluated in terms of relative standard error (RSE), it generally ranges from 1.43 (H2S) to 4.57 % (DMDS).
VOC analysis
For VOC analysis, the combination of gas chromatography (GC) with mass spectrometry (MS) coupled with a multi-function thermal desorber (TD) was used. The samples in the Tedlar bag were transported to the TD system for analysis based on the thermal desorption. Chromatographic separation was achieved by the Vocol column (60 m × 0.32 mm i.d. and 1.8-μm film thickness: Supelco) at a column flow rate 1.2 mL min−1 (99.9 % pure He as carrier gas). Detailed analytical conditions of this system are listed in Table 2. The DL values fell in the range of 1.27 [0.33 ppb (MIBK)] to 1.81 ng [0.39 ppb (BuAc)]. If the precision of this method is evaluated in terms of RSE, it generally varied from 1.98 % (BuAc) to 2.59 % (MEK).
VFA analysis
A TD system interfaced with a GC-flame ionization detector (FID) was used for the analysis of VFA (refer to Table 2). The collection of all the acid components was initially made by a Carbopack X tube (60/80 mesh, Supelco, PA, USA) by transferring samples at a flow rate of 200 mL min−1 for 5 min with the help of a minipump (SIBATA, Japan). The analysis of VFA was made in a manner analogous to that of RSCs by interfacing the GC system with TD. The DL values of the acid compounds were 0.82 ng [0.39 ppb (PA)], 0.60 ng [0.20 ppb (BA)], 0.50 ng [0.14 ppb (IA)], and 0.60 ng [0.21 ppb (VA)]. The precision of the TD-based analysis, if expressed in terms of RSE, fell in the range of 4.4–7.3 %.
Carbonyl analysis
The analysis of carbonyl compounds was carried out by high performance liquid chromatography (HPLC) equipped with a UV detector and dsCHROM software (for peak integration). Analytical conditions of the HPLC system are shown in Table 2. To initiate the analysis of carbonyls, air samples were passed through Lp DNPH cartridges (Supelco, USA) at a normal set-up value of 10 min (at a fixed sampling flow rate, 0.8 L min−1) via a Sep-Pak ozone scrubber (Waters, USA). After that, the cartridges were eluted slowly with 5 mL methanol and filtered through 0.45 μm, 13 mm, GHP Acrodisc filters (PALL, NY, USA) into a 25 mL capacity borosilicate glass volumetric flask. The eluate was manually injected into the HPLC system equipped with a 20 μL sample loop. The DL values, expressed in terms of mixing ratio (assuming a total sampling volume of 15 L), were 14.1 [or 0.77 ppb (FA)], 19.1 [or 0.71 ppb (AA)], and 13.9 ng [or 0.39 ppb (AC)]. The precision of analysis, assessed in terms of RSE, tended to vary in the range of 0.61 % (FA) to 1.16 % (AC).
Ammonia analysis
Samples for ammonia analysis were collected by passing the ambient air on top of the grates through 50 mL boric acid solution as an absorbent. A total of 30 L air was passed through the boric acid solution at a flow rate of 2.5 mL min−1 for 12 min through a minipump (SIBATA, Japan). These solution samples were covered after each reagent addition to avoid any possible interference or NH3 loss into the atmosphere. A UV/Vis detector was used to determine the NH3 concentration (refer to Table 2). The boric acid solution, used as absorbent, was then transferred and kept in Teflon bottles. Five milliliter of both phenol and sodium hypochlorite (NaOHCl) solutions was added to 10 mL of the boric acid solution. Each of these mixtures was then left to stand for 1 h to allow color development. Quantification of NH3 was carried out against a five-point calibration with an outstanding linearity (R 2 = 0.997) to yield a DL value of 0.81 ng (or 117 ppb) and RSE of around 1.43 %.
Results and discussion
General pattern of odorant emissions from waste treatment facility
The results of odor measurements from each sampling point in and around this treatment facility are described in Table 4. Although it was intended to measure the total of 22 offensive odorants shown in Table 1, six compounds consisting of styrene, MIBK, valeraldehyde, iso-valeraldehyde, isobutyl alcohol, and isovaleric acid were measured as below detection limit (BDL) from all sampling locations. Hence, detailed analysis of the measured data was made after excluding these six compounds. The results of the analysis indicate that the measured concentrations at the bordering areas (B-N, B-E, B-S, and B-W) are fairly low to show BDL values in many instances. The level of odor pollution is thus difficult to assess for certain odorants (e.g., TMA) with their quantification frequently limited by the instrumental detectability. As such, many odorants are found well below the regulation guidelines set by the malodor prevention law of KMOE in Table 1 (e.g., toluene, TMA, etc.). Nonetheless, a number of odorants including H2S, aldehydes, NH3, and VFA are detected at considerably high concentration levels, especially in the duct and control unit.
As the threshold of odorants differs greatly, the actual intensity of individual odorants cannot be assessed simply by the magnitude of odorant concentration (Kim and Park 2008). Hence, the strengths of each odorant were compared after conversion into odor intensity, as shown in Table 3. If the results are compared in this respect, odorant emissions appear to be dominated by such components as RSCs, NH3, acetaldehyde, propionaldehyde, butyraldehyde, and propionic acid. In contrast, the relative contribution of VOCs is insignificant in terms of OI (Table 3).
The results of odorant measurements, compared between different treatment units, indicate that the pattern of odor pollution along the transfer/process line of this facility should be distinguished in several respects. The highest concentrations of ammonia (13.37 ppm) and H2S (64.7 ppb) were measured from the duct line (DU-1). It is, however, surprising to find that the maximum values of some odorants are detected even after treatment stages. For instance, the maximum values of acetaldehyde (180 ppb) and THC (485 ppm) are seen at R-1 unit. This observation thus implies that some pollutants are not easy to remove by the combined processing of the dual treatment. Likewise, the maximum concentration of Form-A (429 ppb) seen at S-1 unit is also striking, as S-1 is the last stage of the treatment. As a simple guideline to assess the degree of odor pollution inside this treatment facility, the results can be simply compared against permissible emission guidelines of KMOE in Table 1. According to this evaluation, the level of exceedance from such guideline values was observed most intensely from VFAs such as propionic acid (maximum value of 221 ppb) and butyric acid (18.9 ppb) in reference to their respective guideline values of 70 and 2 ppb. In addition to these VFA species, such exceedance pattern is also observed from NH3, H2S, and acetaldehyde.
The emission patterns of odorants measured in this study were also investigated by examining the relationship between direct and indirect measurement methods. If one attempts to assess the actual odor strengths of each sample, the use of OI concept alone is very limited to account for the complexity arising from the mixing effects. It is clear that the effect of mixing can be reflected into several categories such as masking, averaging, etc. (Kim 2010, 2011). Despite such complexity, one may use a simple approach like the sum of individual odor intensity (SOI) as a reference value for the mixed odor (Kim and Park 2008). As the SOI concept is dominated by the major contributing component, it can be an efficient tool to assess the masking effect in some senses. Hence, it can be used as a reference information to understand the D/T ratio values taken directly based on the ADS test (Fig. 3). Comparison of these two concepts indicates a relatively weak tie between the two methods. In fact, the results between different treatment units tend to be distinguished more effectively by D/T. It should be recalled that the SOI patterns are not that sensitive enough to discriminate subtle differences between minor odorant species, as its magnitude is governed mainly by the predominant components.
Effectiveness of odor control between different treatment units
To learn more about the nature of odor pollution, several studies have been undertaken to assess the factors and processes for odorant abatement at various manmade sources (Mahin 2001; Van Harreveld 2001; Sucker et al. 2001; Kim et al. 2006; Santos et al. 2009; Zhang et al. 2009). As the model waste treatment plant was constructed and is currently operated in central urban areas, the feasibility of its operation needs to be assessed with respect to the effectiveness of odor control for its proper management. To this end, the regulation guidelines of KMOE were used as primary criteria. Nonetheless, there is also a proprietary quality criterion for the management of this facility in the form of an internal audit set by the city executive team, managing company, and residents.
As described in Fig. 2, the ERC adopted a two-stage hybrid treatment system to control odor produced by complicated waste and intermittent emissions in the ERC. As a basic means to evaluate the extent of odor reduction, the odorant levels determined prior to (duct line) and after treatment (RTO and WSC) are compared in Table 5. The analysis of these data sets indicates that the patterns are distinguished both between odorants and between different processing steps. It appears that odorant pollution inside ERC facility should be dominated by NH3 and organic acids, as they tend to exceed their respective guidelines most frequently.
The first stage of the control system consists of one can-type rotary wing RTO. In the ERC, two RTOs built with the same capacity are employed to eliminate or suppress odor emissions transported from two different transport lines (Fig. 2). The RTO unit 1 was placed to draw air directly from the main processing lines, while the RTO unit 2 was to do so at all the miscellaneous spots. In addition, the second stage of control unit is made up of a three-step wet chemical scrubber system in which target gases are drawn into the three different containers, each of which is filled with 50 % H2SO4, 33 % NaOH, and NaCl electrolyte solutions. This three stage scrubber is devised to remove or suppress odorants of alkaline, acidic, and neutral nature, respectively. As such, all the gases initially treated by the dual RTO units are re-processed by the second stage treatment for further purification.
The overall patterns of odorant treatment can be assessed by the results depicted in Fig. 4. Here, the odorant concentrations measured from the same treatment units of the different systems are put together to derive the representative values for each transfer or treatment unit. The results are then compared for each odorant group between different units. Error bars are calculated as the standard error (SE) value for each transfer or treatment unit. According to this analysis, it may be possible to derive four different types of patterns between the different treatment approaches and odorants investigated in this work: (1) odorants treated fairly effectively by thermal treatment, the reduced sulfur species (e.g., H2S); (2) odorants treated fairly effectively by wet scrubber treatment (e.g., NH3); (3) odorants that are recalcitrant against both treatments (acetaldehyde, propionic acid, etc.); and (4) odorants of which removal efficiency cannot be judged by this study due to their minimal presence in processing units (valeraldehyde, iso-valeraldehyde, etc.). The suitability of thermal treatment like incineration has already been demonstrated for reduced sulfur species based on a series of comparative analyses between different abatement techniques (Smet and Van Langenhove 1998). It is also interesting to find that the concentration level of NH3 is raised after the RTO treatment. However, due to the high solubility, ammonia appears to be easily removed after the second processing stage of the ERC, WCS system (Susaya and Kim 2010).
Comparison of odorant concentration levels across transfer and process line: On X-axis, + symbol for 2 units and ~ symbol for multiple sites (Refer to Table 1 for short names). a RSC and NH3. b VOC. c Aldehyde. d Acid
As certain odorants such as aldehydes and acids were not effectively removed from the existing treatment facility (as discussed above in category 3), surveying plausible techniques for the effective removal of these odorants would seem worthwhile. Although not falling in the concentration range found in the present study (ppb range), certain new or modified techniques had been proposed for direct application to these types of odorants. For instance, the removal of unpleasant odor gases was exercised by an Ag–Mn catalyst (Watanabe et al. 1996). These authors treated acetaldehyde and trimethylamine as the model malodorants and measured their removal from a concentration of 50 ppm. A corona-discharge reactor (a deposition-type reactor) was applied to remove acetaldehyde and skatole from nitrogen and an oxygen–nitrogen mixture (Sano et al. 1997). They were able to remove their mixture (20 ppm acetaldehyde + 2.3 ppm skatole) almost completely by the corona-discharge method, which involves the negative ionization of odorants and the subsequent depositing at the anode surface. In another report, Ibrahim et al. (2001) tested the removal efficiency of acetaldehyde and propionaldehyde from waste gas by the packed column containing the immobilized activated sludge gel beads together with the hollow plastic ball. To test the reliability of this approach, they supplied both aldehydes at the identical concentration (10 ppm) at the inlet for the prolonged operation time (30 days). Through an application of this approach, they observed their removal at 92 and 95 %, respectively. In continuation of this effort, Asada et al. (2002) utilized Bamboo charcoal for the removal of formaldehyde, acetaldehyde, and benzene from waste gases. They confirmed the differential removal capacity of these odorants at varying temperature ranges. Likewise, Spigno et al. (2003) applied the biofilter method (prepared by inoculation of Aspergillus niger) to remove hexane from contaminated air streams. Based on this biofilter technique, they were able to achieve a removal efficiency of 80 % for hexane in the 2–7 g m−3 range. Hence, to find a better solution for the odorants released in the ERC facility, one may look for new and improved techniques along with those introduced previously for the synergetic removal of odorants from waste gases.
Conclusion
In order to explore the reliability of odor control systems in a gigantic municipal waste treatment plant, distribution of 22 key offensive odorants was investigated throughout its processing unit and the outdoor air in the surrounding areas. By acquiring the odorant concentration data before and after each of the dual processing units, the effectiveness of the treatment units against most of the key offensive odorants released from this facility was investigated.
As the field conditions do not necessarily represent the maximum occurrence of each odorant, the acquired odorant data may not be ideal to judge the overall effectiveness of this treatment facility. However, the observed patterns indicate that the system is removing certain odorant groups (RSCs, NH3, etc.) more effectively, while it is not for other volatile organic species, especially several aromatics, carbonyls, and fatty acids. It should be recalled that the dual combination of treatment systems investigated investigated in this study was selected as a practical option for the physical treatment of odor. However, as the efficiency of this treatment facility is unlikely to be sufficient for some volatile organics, it needs to be further developed to find more reliable tools, or certain selective techniques (e.g., biological or chemical properties) should be used additionally to reduce such recalcitrant odorants.
References
Asada T, Ishihara S, Yamane T, Toba A, Yamada A, Oikawa A (2002) Science of bamboo charcoal: study on carbonizing temperature of bamboo charcoal and removal capability of harmful gases. J Health Sci 48:473–479
Bell J, Melcer H, Monteith H, Osinga I, Steel P (1993) Striping of volatile organic compounds at full-scale municipal wastewater treatment plants. Water Environ Res 65(6):708–716
Cetin E, Odabasi M, Seyfioglu R (2003) Ambient volatile organic compound (VOC) concentrations around a petrochemical complex and a petroleum refinery. Sci Total Environ 312:103–112
Dincer F, Muezzinoglu A (2008) Odor-causing volatile organic compounds in wastewater treatment plant units and sludge management areas. J Environ Sci Health 43(13):1569–1574
Dincer F, Odabasi M, Muezzinoglu A (2006) Chemical characterization of odorous gases at a landfill site by gas chromatography–mass spectrometry. J Chromatogr A 1122:222–229
Hasin-Brumshtein Y, Lancet D, Olender T (2009) Human olfaction: from genomic variation to phenotypic diversity. Trends Genet 25(4):178–184
Ibrahim MA, Mizuno H, Yasuda Y, Fukunaga K, Nakao K (2001) Removal of mixtures of acetaldehyde and propionaldehyde from waste gas in packed column with immobilized activated sludge gel beads. Biochem Eng J 8:9–18
Kim K-H (2005) Some insights into the gas chromatographic determination of reduced sulfur compunds (RSC) in air. Environ Sci Technol 39(17):6765–6769
Kim K-H (2010) Experimental demonstration of masking phenomenon between competing odorants via an air dilution sensory test. Sensors 10(8):7287–7302
Kim K-H (2011) The averaging effect of odorant mixing via air dilution sensory test: a case study on reduced sulfur compounds. Sensors 11:1405–1417
Kim K-H, Park S-Y (2008) A comparative analysis of malodor samples between direct (olfactometry) and indirect (instrumental) methods. Atmos Environ 42:5061–5070
Kim K-H, Jeon EC, Choi YJ, Koo YS (2006) The emission characteristics and related malodor intensities of gaseous reduced sulfur compounds (RSC) in a large industrial complex. Atmos Environ 40:4478–4490
KMOE (2008) Annual report of ambient air quality in Korea. Korean Ministry of Environment (KMOE)
Mahin TD (2001) Comparison of different approaches used to regulate odours around the world. Water Sci Technol 44:87–102
Nagata Y (2003a) Measurement of odor threshold by triangle odor bag method. Odor Measurement Review. Ministry of Environment (MOE), Japan, pp 118–127
Nagata Y (2003b) Odor Intensity and Odor Threshold Value. Environ Sanitation Cent, Japan, pp 17–25
Sano N, Nagamoto T, Tamon H, Suzuki T, Okazaki M (1997) Removal of acetaldehyde and skatole in gas by a corona-discharge reactor. Ind Eng Chem Res 36:3783–3791
Santos JM, Lopes ES, Reis NC, Melo de Sá L, Hora NJ (2009) Mathematical modelling of hydrogen sulphide emission and removal in aerobic biofilters comprising chemical oxidation. Water Res 43(14):3355–3364
Smet E, Van Langenhove H (1998) Abatement of volatile organic sulfur compounds in odorous emissions from the bio-industry. Biodegradation 9:273–284
Spigno G, Pagella C, Daria Fumi M, Molteni R, Marco De Faveri D (2003) VOCs removal from waste gases: gas-phase bioreactor for the abatement of hexane by Aspergillus niger. Chem Eng Sci 58:739–746
Sucker K, Both R, Winneke G (2001) Adverse effects of environmental odours: reviewing studies on annoyance responses and symptom reporting. Water Sci Technol 44(9):43–51
Susaya J, Kim K-H (2010) Removal of gaseous NH3 by water as a sorptive media: the role of water volume and absorption time. Fresenius Environ Bull 19(4a):745–750
Van Harreveld AP (2001) From odorant formation to odour nuisance: new definitions for discussing a complex process. Water Sci Technol 44(9):9–15
Watanabe N, Yamashita H, Miyadera H, Tominaga S (1996) Removal of unpleasant odor gases using an Ag-Mn catalyst. Appl Catal B 8:405–415
Zhang W, Lau AK, Wen ZS (2009) Preventive control of odor emissions through manipulation of operational parameters during the active phase of composting. J Environ Sci Health 44(5):496–505
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology (MEST) (No. 2009-0093848). The fourth author acknowledges partial support made by Small & medium business association (SMBA) in Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, KH., Jo, SH., Song, HC. et al. Diagnostic analysis of offensive odorants in a large municipal waste treatment plant in an urban area. Int. J. Environ. Sci. Technol. 10, 261–274 (2013). https://doi.org/10.1007/s13762-012-0134-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-012-0134-7