Abstract
Offensive odorant emissions from a food waste (FW) treatment plant that uses hydrothermal hydrolysis and aerobic fermentation technology were studied for 1 month through in situ monitoring and laboratory testing. Results showed that the emission flux (7000 μg kg−1FW h−1) of total volatile organic compounds and concentrations of most volatile organic compounds (VOCs) were highest at the discharge outlet of the hydrothermal hydrolysis reactor. Furthermore, VOC composition analysis showed that the concentrations of most hydrocarbons detected during the sorting/crushing process were higher than those in the aerobic fermentation process, but more oxygenated organic compounds and pinenes were released in the aerobic treatment process. The analysis of VOC temporal characteristics via t test indicated that even with various FW loads during the day and night, most VOC concentrations sampled in the storing room were not significantly different. However, great variances among most VOC concentrations were observed during the sorting/crushing process and at the hydrothermal hydrolysis reactor. The annoyance degrees of offensive gases were also determined via analysis of odor indices. The results suggested that sulfocompounds mainly dominated in terms of high odor activate values during the sorting/crushing process, and the fractional content of oxygenated organic compounds increased in the aerobic treatment processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution caused by food waste (FW) is a serious problem around the world, and the proportion of FW in municipal solid waste is expanding with rapid industrialization, urbanization and economic development [1]. Many studies innovated or improved some efficient or economical ways to process FW. Among the various technologies for FW treatment, the combination of hydrothermal hydrolysis and aerobic fermentation is preferred by FW treatment plants in China.
Most previous works focused on the FW processing technology, but not on the negative effects of FW processing technology on the surrounding environment and workers’ health. In the collection and transportation of FW, a large amount of offensive odorants and leachates could be generated easily because of the high content of volatile organic solids and water. Several researchers have studied the offensive odorants, including ammonia, hydrogen sulfide and some volatile organic compounds (VOCs), emitted from municipal solid waste processing [2, 3]. These substances are present not only in decaying FW [4, 5], but also in FW aerobic landfill [6]. In fact, the unpleasant smell is the main reason for the complaints and the social impact of any waste treatment plant, and such smell is not limited to the final solid waste disposal [7]. Because of the lower sensory threshold among the different VOCs, sulfur compounds significantly contribute to the nuisance odor even if they are present in trace concentrations [8, 9].
A typical full-scale FW treatment plant using hydrothermal hydrolysis and aerobic fermentation technology was chosen for this study. This treatment technology would aggravate air pollution and accelerate the diffusion of odorous gases under open and unsealed work conditions. By analyzing the composition and concentration of each compound in air samples collected at five sampling points in the FW treatment process, the present research focused on the emission characteristics of odorous VOCs, as well as the concentrations of H2S and NH3 in the plant. The spatial and temporal changes of odorant concentrations at different treatment stages were determined. Furthermore, the odorous degree at each sampling point and the contributing odorant groups were also discussed extensively in this paper. Significantly, some suggestions on the sound management of FW treatment factories had been proposed to alleviate the negative effects of odorous gases.
Monitoring plan and methods
Site description
A full-scale FW treatment plant was selected for the study. The plant is a 300 t day−1 treatment capacity plant located in Suzhou, China. The plant started its operation in 2004 and it is one of the representative FW treatment plants in China because of its relatively advanced technology, good management and large-scale, long-term normal operation.
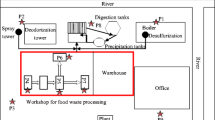
The plant operates for 9 h per workday, from 9:00 am to 6:00 pm. The highest FW loads come in at around 11:00 am to 1:00 pm. The FW treatment process is as follows: after receiving and keeping the FW in the storeroom, the FW is sorted mechanically and manually to remove inorganic materials. Then, FW is crushed and transferred for hydrothermal hydrolysis treatment. The hydrolysis process would take 3 h at 100 °C. At this temperature, macromolecules are not only converted to simple organic matter, but the raw materials are also sterilized and disinfected. The hydrolysis products are separated into solid and liquid. The liquid phase is utilized for biogas production in anaerobic reactors, whereas the solid phase is utilized for aerobic fermentation of final products, such as animal feeds. All treatment processes are run in the same workshop. Air extractors are used for organizing odorous gas emissions from hydrothermal hydrolysis reactors. Twelve ventilators are installed around the workshop to supply fresh air, but the discharged gases do not undergo further treatment. The entire treatment flowchart is shown in Fig. 1.
In situ monitoring description
According to Escalas et al. [10], the concentration of the majority of the analytes tested in weekdays and weekends were not significantly different because of the similarities of the compounds in the plant influent, but the time effect on the variance of analyte concentrations was observed. Monitoring was conducted at noon (12:00 am) and night (8:00 pm) during July 2013, when the temperature was highest (more than 30 °C at noon and 20 °C at night) and barometric pressure was lowest. The two monitoring times also coincided with the phases when facilities are operated and stopped. Sampling was conducted three times per week, and the entire sampling period lasted for 1 month. Data with large errors (because of factory accidents) were excluded. The average values of the measurements during different times were calculated using Microsoft Excel 2013.
Five monitoring points were selected according to the key processes of the treatment plant, and these points were clustered in a large, closed workshop, as shown in Fig. 2. These monitoring points included two pre-treatment processes (storing room and sorting/crushing process), two treatment processes (hydrothermal hydrolysis and aerobic fermentation) and the boundary of the plant.
Offensive odorants from the pre-treatment processes were mainly the result of the anaerobic environment formed during FW stacking and transportation. However, hydrothermal hydrolysis and aerobic fermentation were high-temperature and high-moisture processes. Thus, the composition of odorous gas emitted from the two phases would vary.
Sampling
The odorant gases were tested first by portable detectors in situ, near the facilities. Moreover, the air at each sampling point was pumped into FEP Teflon sample bags (8 L) and its compounds were analyzed in an authorized laboratory.
Analytical methods
The concentrations of H2S, NH3 and TVOC were monitored in situ using portable detectors. MX6iBird detector (Industrial Scientific Corporation, USA) was used for H2S test. OEM detector (Beijing Hongchangxin, China) was used for NH3 testing, and TVOC was tested by TVOC analyzer with PID detector (RAE system, USA, PGM-7340), using a PID with a gas-discharge lamp (9.8, 10.6 or 11.7 eV).
The compositions of TVOC were analyzed by the State Environmental Protection Key Laboratory of Odor Control of China in Tianjin, with the USEPA method TO-14/TO-15 as reference. The instrument type and the test program have been reported previously [11].
Quality assurance and control
The following measures were adopted to ensure the accuracy and reliability of data obtained from monitoring points. First, clean air was tested as a blank sample to determine possible contamination resulting from the sampling, transportation, or storage. The blank sample should have a background value of less than 0.1 μg/m3 [6]; second, at each sampling point, parallel samples were collected and long-term monitoring was performed. Finally, bromochloromethane, 1,4-difluorobenzene, chlorobenzene-d5 and 4-bromofluorobenzene were used as internal standards, and a 65-component determined by the USEPA was used as external standard to qualitatively identify each type of VOC according to retention time. By comparing the mass spectra of samples with the VOC standard spectrum, the concentration of each VOC type was determined.
Results and discussion
Emission characteristics of NH3, H2S and TVOC
The concentrations of NH3, H2S and TVOC in the waste treatment plant have been discussed in other reports [12, 13]. In this study, the concentrations of NH3 at each sampling point are shown in Table 1. NH3 concentration at the sampling point of aerobic fermentation was higher than that at the other points in the working process. The NH3 concentrations were nearly equal to the value obtained in the plant boundary (about 0.3 mg m−3). This finding indicted that in the aerobic vessel, NH3 was much easier to generate because the macromolecular proteins of FW transformed into molecular proteins, and the resultant amino acid would be further degraded under the condition of high air flow rate [14]. The low NH3 concentration at most sampling points showed that NH3 was not a serious pollutant in this FW treatment facility.
The variance of H2S concentration was strongly correlated with the change in monitoring point. The hydrothermal hydrolysis reactor and sorting/crushing process were the main sources of H2S emission, thereby indicating that H2S was generated easily under the conditions of high temperature, high moisture, and low oxygen concentration. Furthermore, the H2S concentration detected during hydrothermal hydrolysis was higher compared with that detected during the sorting/crushing process.
The hydrothermal hydrolysis reactor and aerobic fermentation tank were also the main sources of TVOC release in the plant. The sorting/crushing process is also a dominant pollution source, because some substances could be generated in an anaerobic environment.
The temporal distribution of NH3 and TVOC concentration has been reported in our previous studies [15]. In the previous study, NH3 concentration was relatively stable, and little difference was observed between concentrations during the day and at night. However, TVOC concentration during the day was much higher compared with concentration at night, because more FWs were received during the day and high temperature favored the release of odorous gases.
Flux evaluation of TVOC emission
VOCs are the most important contaminants generated from the FW treatment process, and the amount of VOCs emitted was much higher than those of H2S and NH3. To measure the emission fluxes and quantities in the different treatment processes, Eq. (1) was used to calculate their values [16, 17], as follows:
where Q is the airflow rate of odorous gases from the treatment reactor discharge outlet (L h−1), C 0 (t) is the concentration monitored in the discharge outlet (µg L−1), M W is the mass of the disposed waste in an hour (kg) and t is the work hour (assuming that each workday had 9 h). The dimensions of the VOC discharge outlets where each process was conducted were as follows: storing room, 3 m × 3 m; sorting/crushing, 0.6 m × 0.6 m; hydrothermal hydrolysis reactor, 10 circle holes with a radius of 0.3 m; and aerobic fermentation tank, 0.5 m × 0.5 m. The data are shown in Fig. 3.
The descending order of TVOC emission fluxes had little difference compared with the order of TVOC concentrations. The TVOC emission flux in the storing room was higher than the TVOC emission flux measured during the sorting/crushing phase and in the aerobic fermentation tanks because of the larger outlet area in the storing room. The emission flux of the hydrothermal hydrolysis reactor was higher than 7000 μg kg−1 FW h−1. The present study indicated that a closed environment was helpful in preventing the spread of contaminants.
Spatial VOC characterization
In the laboratory tests, 50 VOC species were detected using GC–MS and these compounds could be classified into four major groups: oxygenated organic compounds (9 species), aromatic hydrocarbons (12 species), hydrocarbons (24 species) and sulfocompounds (5 species). Table 2 shows the mean concentrations of the dominant substances (C ≥ 0.01 mg m−3) in the VOC composition analysis at five monitoring points.
In the treatment unit of hydrothermal hydrolysis, the concentrations of most VOCs were higher compared with those in other units, because high temperature and high moisture promoted the emission of offensive odorants, with some odorants generated from the front treatment units. The treatment processes of sorting/crushing and aerobic fermentation were the two other main sources of odorous VOC release. However, the VOC types at the two above-mentioned sampling points differed.
The concentrations of some hydrocarbons, including 1,2-dichloroethane, heptane, propane, isobutene and butane, were much higher during the sorting/crushing process than during other processes. This indicated that nutrients of raw materials can be converted to this VOC group under anaerobic conditions during FW transportation and stacking. Furthermore, these compounds were hardly further degraded under conditions of lower oxygen concentration, even if the mechanism underlying the degradation activity has not been reported.
On the contrary, the emission amount of oxygenated organic compounds from the process of aerobic fermentation was higher than that generated during the sorting/crushing process. In addition, concentrations of the terpenes, (α-pinene, β-pinene and limonene) were also maintained at a high level, even if these terpenes were not oxygenated organic compounds and this finding has been confirmed in previous FW treatment studies [18]. The reason for the high terpene concentration may be related to the presence of citrus fruits or citrus oils in FW [16].
The concentrations of aromatic hydrocarbons (including benzene, toluene, ethylbenzene and paraxylene) during the sorting/crushing process and in aerobic fermentation were approximate values, because aromatic hydrocarbons were difficult to generate or degrade whether in an anaerobic environment or in an aerobic environment. These compounds were monitored from the FW itself without treatment processes.
Ethanol was another important VOC in this study, and its concentration remained at a higher level (higher than 10 mg m−3) during the entire process. In the hydrothermal hydrolysis reactor, the ethanol value was about 59.5 mg m−3. This was an interesting and exciting phenomenon because as a new and clean energy resource, ethanol production from waste has been reported widely [19, 20]. The ethanol production potential of FW has been proven by Ma et al. [21] and Hatfield [22]. The compositions of FW, such as sugars, starches and cellulose, were the preferred substrates for producing ethanol and other bioenergies. In the hydrolysis process, these complex carbohydrates are converted to simple monomers in an acidic environment.
To evaluate the ambient air pollution of the surroundings resulting from these offensive odorants, concentrations were detected at 100 m away from the plant boundary. The result showed that the detector failed to sense the 15 VOC species. Moreover, the concentrations of certain tested substances were lower than the pollutants’ emission standards in China (Emission Standards for Odor Pollutants, China), as shown in Table 3. Among the eight pollutants, trimethylamine and styrene were not monitored at any sampling point in the plant. Thus, the FW treatment plant only slightly influenced the surroundings.
Temporal VOC characterization
For the different inlet organic loads during the day and night, some VOC emission concentrations in most waste treatment plants were greatly diverse [10]. In this study, two sampling times were selected. During the day, many FWs are transported from restaurants, and all the processes are run. At night, most of the facilities in the plant do not function.
The effect of time on the differences between the mean concentrations of each compound monitored at noon and night was computed via a statistical method. A t test was run to determine the temporal effect of each analyte, at a significance level of 0.05, and the results are shown in Table 4. The symbol ‘+’ indicates that the concentrations of analytes showed significant differences between the two times. On the contrary, the symbol ‘−’ indicates that no significant difference was found. The results indicated that in the storing room, only a few compounds were significantly different in terms of detected concentration. Meanwhile, in the aerobic fermentation monitoring site, the number of compounds was low. The temporal effects were positive only for 3 out of 39 and 11 out of 39 VOCs analyzed in the storing room and in the aerobic fermentation process, respectively, at the 0.05 significance level. This means that for most VOCs, the emission concentrations were not significantly higher during the day than at night. By contrast, the t test indicated that the temporal effect was significant for 27 out of 39 and 29 out of 39 target compounds at the sorting/crushing point and in the hydrothermal hydrolysis tank, respectively, at the 0.05 significance level.
Few analytes differed in terms of concentrations in the storing room, because only oxidized gases were emitted from the surface of the FWs. Thus, the spontaneous course was nearly the same for the two monitoring times. However, the VOCs generated during the sorting/crushing phase were mainly from the anaerobic part of FW inner, and they spread rapidly and easily during mechanical stirring when the facility was operating. In the process of hydrothermal hydrolysis, except for the continuous stirring, the high temperature could speed up the release of VOC to the environment during the day. Furthermore, most of the compounds that showed negative difference results for concentration values were hydrocarbons and aromatic hydrocarbons. These results may have resulted from the low correlation between the formation of these compounds and the operation conditions.
Characterization of the odor properties from the treatment process
Analysis of odor indices
For the different odorants, no absolute equation exists between concentration and their annoyance degree because of the various odor thresholds (OTs). Thus, people were sensitive to some offensive odorants because of their low odor threshold, even if the concentrations of such compounds are at trace level; these odorants would cause annoyance and public health problems of nearby residences. To assess the annoyance degree of any substance i, odor activity value (OAV) of i is calculated by Eq. (2) [23, 24]. The OT and OAV of H2S, NH3 and main VOCs are summarized in Table 5:
People would have negative perception of the odor when OAV exceeds 1. Most OAVs of the monitored VOCs in the treatment processes were below one. However, previous studies suggest that malodorants have a cumulative effect on olfaction annoyance [24]. Thus, the sum of odor activity value (SOAV) was calculated to evaluate annoyance degree for any malodorant group, as described by Eq. (3):
where k represents the amount of offensive odorant in any group (four groups were present) as previously mentioned. Except for VOC, the odorous smell caused by H2S and NH3 could not be ignored. Therefore, a total odor activity value (TOAV) was adopted to show the olfactory annoyance degree of each treatment process by adding the SOAVs of four VOC groups and the OAVs of H2S and NH3, as Eq. (4) shows:
Furthermore, odor contribution (OC) was studied to assess the fractional content of H2S, NH3 and TVOC in TOAV and the odor quotient of each VOC group in SOAV.
Because air pollution caused by offensive odorants was more serious under high FW loads than under low FW loads, only the annoyance degrees of offensive odorants monitored during the day were discussed here.
Odor indices variance in different treatment processes
Although the odorous gases had little influence on surroundings according to previous discussions, their olfactory nuisances and occupational health and safety to the workers in the factory cannot be ignored. From the discussion of the VOC spatial emission characterization, the types and concentrations of VOC showed great differences at various sampling points. Consequently, the annoyance degrees of odorous gases generated from different processes varied, as shown in Fig. 4. Both TOAV and SOAV had the same change tendencies, and the orders of the two values from the highest to lowest were as follows: hydrothermal hydrolysis, aerobic fermentation, sorting/crushing and sorting room. The high-temperature and moisture conditions in hydrothermal hydrolysis process had positive effects on odorant emissions, and the aeration in the aerobic fermentation increased the speed of release of odorous gases and VOCs. Additionally, the two values at the boundary were approximately 1, which was smaller by two to three orders compared with those in the work processes.
Figure 4a shows the fractional contents of H2S, NH3 and TVOC, with NH3 showing little effect on the TOAV except at the plant boundary. H2S monitored at the sorting/crushing unit comprised a considerable fraction of the TOAV (about 20 %). Furthermore, at sorting/crushing warehouse and in the hydrothermal hydrolysis process, H2S was about 5–10 % of the calculated TOAV. VOCs, as the most important pollutants generated from FW treatment, generally contributed more than 80 % of the offensive odors to the ambient air of the plant. To present more detailed information on the VOC odors, Fig. 4b was plotted to indicate the nuisance odors caused by the four VOC groups and the changes in their values at different monitoring points. From the fractional content of each group, we concluded that the groups of oxygenated organic compounds and sulfocompounds are worth noticing. The sulfocompounds were dominant in the odors caused by VOCs, because this offensive odor group is mainly generated in the process of microbial anaerobic metabolism [27, 28]. However, the fraction of monitored sulfocompounds did not sharply decrease at the hydrothermal hydrolysis reactor (about 50 %) and the value reached up to 20 % in the aerobic fermentation process, thereby suggesting that they could not be oxidized easily via aerobic fermentation. By contrast, the fractional ratio of oxygenated organic compounds gradually increased in the following order: sorting/crushing phase, hydrothermal hydrolysis tank and aerobic fermentation reactor. Thus, several suggestions have been proposed here to improve the work conditions and to alleviate the damages caused by odorous gases. First, the ventilated condition should be further strengthened and more ventilators are needed, especially near the sampling points of hydrothermal hydrolysis and aerobic fermentation, in which high values of TVOA and SOAV analyzed. Moreover, some further treatment facilities could be equipped for reducing the environmental pollution caused by collected odorous gases.
Conclusion
In this work, a typical FW treatment plant that uses hydrothermal hydrolysis and aerobic fermentation technology was selected to study the emission of offensive odorants in the different treatment processes during the day and night.
TVOC generated in the plant was mainly from the hydrothermal hydrolysis reactor and the aerobic fermentation process. The emission flux of TVOC was highest (up to 7000 μg kg−1 FW h−1) at the discharge outlet of the hydrolysis monitoring reactor.
The spatial variability analysis of VOC composition indicated that the concentrations of hydrocarbons during the sorting/crushing process were higher compared with the concentrations of hydrocarbons emitted in the aerobic fermentation process. On the contrary, oxygenated organic compounds, which are also typical odorous VOCs, had higher concentrations in the aerobic fermentation process. Additionally, aromatic hydrocarbon concentrations were nearly the same under aerobic and anaerobic conditions.
The t test was used to analyze the temporal characteristics of VOC emissions, and the results indicated that the concentrations of compounds monitored at storing room and the aerobic fermentation tank were not significantly correlated with the FW load changes from day to night. However, significant differences were found in the VOC concentrations obtained at noon and at night at the sorting/crushing sampling point and at the hydrothermal hydrolysis reactor, because the conditions of continuous stirring and high temperature favored the release of odorous gases in the daytime.
Finally, the annoyance odor analysis showed that sulfocompounds were dominant contributors to the nuisance smell at each sampling point because of their low threshold values. Moreover, the SOAV analysis showed that sulfocompounds were easily generated under anoxic conditions. In the aerobic treatment processes, the fractional content of the oxygenated organic compounds greatly increased.
References
Schneider F (2013) The evolution of food donation with respect to waste prevention. Waste Manag 33(3):755–763
Pierucci P, Porazzi E, Martinez MP, Adani F, Carati C, Rubino FM, Colombi A, Calcaterra E, Benfenati E (2005) Volatile organic compounds produced during the aerobic biological processing of municipal solid waste in a pilot plant. Chemosphere 59(3):423–430
Sundberg C, Yu D, Franke-Whittle I, Kauppi S, Smårs S, Insam H, Romantschuk M, Jönsson H (2013) Effects of pH and microbial composition on odour in food waste composting. Waste Manag 33(1):204–211
Büyüksönmez F (2012) Full-scale VOC emissions from green and food waste windrow composting. Compost Sci Util 20(1):57–62
Kim KH, Pal R, Ahn JW, Kim YH (2009) Food decay and offensive odorants: a comparative analysis among three types of food. Waste Manag 29(4):1265–1273
Zou SC, Lee SC, Chan CY, Ho KF, Wang XM, Chan LY, Zhang ZX (2003) Characterization of ambient volatile organic compounds at a landfill site in Guangzhou, South China. Chemosphere 51(9):1015–1022
Font X, Artola A, Sanchez A (2011) Detection, composition and treatment of volatile organic compounds from waste treatment plant. Sensors 11(4):4043–4059
Kim KH, Choi YJ, Jeon EC, Sunwoo Y (2005) Characterization of malodorous sulfur compounds in landfill gas. Atmos Environ 39(6):1103–1112
Kim KH (2006) Emissions of reduced sulfur compounds (RSC) as a landfill gas (LFG): a comparative study of young and old landfill facilities. Atmos Environ 40(34):6567–6578
Escalas A, Guadayol JM, Cortina M, Rivera J, Caixach J (2003) Time and space patterns of volatile organic compounds in a sewage treatment plant. Water Res 37(16):3913–3920
Yue DB, Han B, Sun Y, Yang T (2014) Sulfide emissions from different areas of a municipal solid waste landfill in China. Waste Manage 34(6):1041–1044
Fang JJ, Yang N, Cen DY, Shao LM, He PJ (2012) Odor compounds from different sources of landfill: characterization and source identification. Waste Manag 32(7):1401–1410
Li JJ, Wu YD, Zhang YL, Zeng PY, Tu X, Xu MY, Sun GP (2015) Emission of odorous volatile organic compounds from a municipal manure treatment plant and their removal using a biotrickling filter. Envrion Technol 36(8):1050–1056
Zhang L, Lee YW, Jahng D (2011) Anaerobic co-digestion of food waste and piggery wastewater: focusing on the role of trace elements. Bioresour Technol 102(8):5048–5059
Liu JG, Wang XW, Nie XQ, Li RD, Song MY (2013) In-situ emission characteristics of odorous gases from two food waste processing plants. J Mater Cycles Waste Manage 15(4):510–515
Wang XM, Wu T (2008) Release of isoprene and monoterpenes during the aerobic decomposition of orange wastes from laboratory incubation experiments. Environ Sci Technol 42(9):3265–3270
Wu T, Wang XM (2012) Release of volatile organic compounds from food waste during the aerobic decomposition. Acta Sci Circumst 32(10):2575–2583
Mao IF, Tsai CJ, Shen SH, Lin TF, Chen WK, Chen ML (2006) Critical components of odors in evaluating the performance of food waste composting plants. Sci Total Environ 370(2–3):323–329
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37(1):19–27
Prasad S, Singh A, Joshi HC (2007) Ethanol as an alternative fuel from agriculture, industrial and urban residue. Resour Conserv Recycl 50:1–39
Ma HZ, Wang QH, Qian DY, Gong LJ, Zhang WY (2009) The utilization of acid-tolerant bacteria on ethanol production from kitchen garbage. Renew Energy 34(6):1466–1470
Hatfield JL, Smith DD (2013) Food and agricultural waste: sources of carbon for ethanol production. Carbon Manag 4(2):203–213
Feilberg A, Liu D, Adamsen APS, Hansen MJ, Jonassen KEN (2010) Odorant emissions from intensive pig production measured by online proton-transfer-reaction mass spectrometry. Environ Sci Technol 44(15):5894–5900
Lee HD, Jeon SB, Choi WJ, Lee SS, Lee MH, Oh KJ (2013) A novel assessment of odor sources using instrumental analysis combined with resident monitoring records for an industrial area in Korea. Atmos Environ 74:277–290
Nagata Y (2003) Odor intensity and odor threshold value. Bulletin of Japan, Environmental Sanitation Center, Kawasaki, pp 17–25
Rosenfeld PE, Clark JJJ, Hensley AR, Suffet IH (2007) The use of an odour wheel classification for the evaluation of human health risk criteria for compost facilities. Water Sci Technol 55:345–357
He PJ, Zeng Y, Tang JF, Shao LM (2010) Emission of volatile organic compounds during initial decomposition phase of municipal solid waste. J Tongji Univ (Nat Sci) 38(6):854–869
Wu T, Wang XM, Li DJ, Yi ZG (2010) Emission of volatile sulfur compounds (VOSCs) during aerobic decomposition of food waste. Atmos Environ 44(39):5065–5071
Acknowledgments
The project is supported by the Environmental Protection Public Welfare Project (grant number 201109035).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kong, X., Liu, J., Song, M. et al. Spatial and temporal variability of odorous VOC in a food waste treatment plant using hydrothermal hydrolysis and aerobic fermentation technology. J Mater Cycles Waste Manag 17, 626–636 (2015). https://doi.org/10.1007/s10163-015-0407-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-015-0407-6