Abstract
In this study, feasibility of using seawater to neutralize alkaline red mud for its safe disposal has been studied using Taguchi’s design of experimental methodology. Parameters such as weight of red mud, volume of seawater, stirring time and temperature were tested at three levels to study their effect on response characteristic, i.e., pH of the neutralized slurry. The analysis of variance showed that volume of seawater added and quantity of red mud are the two significant parameters with 53.59 and 44.92 % contribution each, respectively. Under the optimized parameters, pH value of red mud slurry reaches to about 8.0 which is within disposable limits. When seawater or other Ca- and Mg-rich brines are added to caustic red mud, the pH of the mixture is reduced causing hydroxide, carbonate or hydroxy carbonate minerals to be precipitated. This mechanism of neutralization process has been explained with emphasis on chemical analysis, mineralogy and morphology of the neutralized red mud. The process improved the physical characteristics of red mud with entrained liquor becoming non-hazardous water with reduced alkalinity. The results would be extremely useful in the process of safe disposal of red mud.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Bayer process of extraction of alumina from bauxite remains the most economical process till date. In the Bayer process, the insoluble product generated after bauxite (containing 40–60 % Al2O3) digestion with sodium hydroxide at elevated temperature and pressure to produce alumina is known as ‘red mud’ or ‘bauxite residue’. The waste product gets its name as red mud due to the oxidized iron content present in it. Red mud is a mixture of compounds originally present in the parent mineral, bauxite and of compounds formed during the Bayer cycle. As the bauxite has been subjected to sodium hydroxide treatment, red mud is highly caustic with a pH of 10.5–12.5.
Red mud is disposed as dry or semi dry material in red mud pond or abandoned bauxite mines and as slurry having a high solid concentration of 30–60 % and with a high ionic strength. The environmental concerns relate to two aspects: very large quantity of the red mud generated (world wide generation is 70 million tons every year) and its causticity. Depending upon the type of bauxite processed, about 1–2.5 tons of red mud is generated per ton of alumina produced (Paramguru et al. 2005). Problems associated with the disposal of red mud waste include: its high pH, alkali seepage into underground water, safety in storage, alkaline air-borne dust impact on plant life and vast area of land required for disposal. Up to 2 tons of liquor with a significant alkalinity of 5–20 g/L caustic (as Na2CO3) accompany every ton of dry mud.
Safe treatment and storage of high volume industrial waste streams pose unique waste management challenges. Though red mud appears to be consolidated, it has a muddy consistency because of the fineness of the material involved and their colloidal nature due to the caustic soda present in it. Neutralization of red mud will help to reduce the environmental impact caused due to storage activities of the residue and also lessen significantly the ongoing management of the deposits after closure. It will also open opportunities for re-using the residue, which was prevented until recently because of the high pH. The cost of neutralization will, to some degree at least, be offset by a reduction in the need for long-term management of the residue deposits. Instead of accruing funds to deal with a future liability, the funds can be invested in process improvements, which reduce or remove the liability. By reducing the pH of residue leachate, the potential risk of long-term degradation to clay or synthetic liners will reduce. Also, any leachate which does escape from the impoundment will have a reduced impact on the receiving waters; hence, the overall risk of groundwater contamination would be reduced significantly.
Efforts to ameliorate red mud are being carried out by possibly incorporating a pH-reduction processing step. These are treatments of red mud with CO2, with acid/chemicals having acidic properties/acidic wastes, bioleaching, sintering and seawater neutralization being one of them. The paper describes feasibility of neutralization of red mud with seawater for its treatment using Taguchi’s methodology. Different parameters have been optimized and characterization of neutralized red mud and entrained liquor has been studied to show that both the red mud and entrained liquor after filtration become safe for disposal.
Seawater neutralization has been studied by researchers who have found that when seawater or other Ca- and Mg-rich brines (e.g., Salt lake brines) are added to caustic red mud, the pH of the mixture is reduced causing hydroxide, carbonate or hydroxycarbonate minerals to be precipitated (Palmer and Ray 2009). Average seawater contains 965 g of water and 35 g of salts (i.e., 3.5 % salinity). The concentration of various salt ions in seawater is 55 % chloride (Cl−), 30.6 % sodium (Na+), 7.7 % sulfate (SO4 2−), 3.65 % magnesium (Mg2+), 1.17 % calcium (Ca2+), 1.13 % potassium (K+) and 0.7 % others (Oceanplasma. Chemistry of Seawater. http://oceanplasma.org/documents/chemistry.html#concentrations). Seawater neutralization does not eliminate hydroxide from the system but converts the readily soluble, strongly caustic wastes into less soluble, weakly alkaline solids. The carbonate and bicarbonate alkalinity of the waste is removed primarily by reaction with calcium to form aragonite and calcite (McConchie et al. 2000). Seawater neutralization makes the red mud non-caustic, but does not reduce its acid-neutralizing capacity. This allows the red mud to be used without the need for further chemical treatment. It has been established that reductions in both pH and aluminum from the seawater neutralization process are due to the formation of ‘Bayer’ hydrotalcite Mg7Al2OH18CO3 2−, SO4 2−·xH2O. This is the primary mechanism involved in the removal of aluminum from solution.
In post-disposal reuse, researchers (Brunori et al. 2005) studied the possibility of reusing treated red mud (through the technology patented by Virotec), consisting of a seawater treatment for pH neutralization, in Eurallumina plants, located in Sardinia (Italy) for treating contaminated waters and soils. The effectiveness of using thermally activated seawater-neutralized red mud for the removal of arsenate, vanadate, and molybdate in individual and mixed solutions has been investigated (Palmer et al. 2010). The investigators found that thermally activated seawater-neutralized red mud removes at least twice the concentration of anionic species than thermally activated red mud alone, due to the formation of 40–60 % Bayer hydrotalcite during the neutralization process. Arsenic removal from water using five sorbents namely seawater-neutralized red mud (Bauxsol), acid-treated Bauxsol (ATB), activated Bauxsol (AB), Bauxsol-coated sand (BCS), and activated Bauxsol-coated sand (ABCS) was studied (Fuhrman et al. 2004). Sorptive capacity of all tested sorbents compares well with conventional sorbents such as activated alumina and ferric oxides. A detailed review of the neutralization processes and utilization of red mud is stated by Rai et al. (2012).
Taguchi’s design of experiments has been used extensively in product quality assessment and several other studies (Taguchi and Yu-in 1979; Taguchi 1986; Ross 1996; Barker 1990; Roy 1990; Srivastava et al. 2007). Taguchi method is a scientifically disciplined mechanism for evaluating and implementing improvements in products, processes, materials, equipment, and facilities. These improvements are aimed at improving the desired characteristics and simultaneously reducing the number of defects by studying the key variables controlling the process and optimizing the procedures or design to yield the best results. Taguchi method has been used to study the multicomponent sorption of pyridine and its derivatives from aqueous solution onto rice husk ash, granular-activated carbon and bagasse fly ash (Lataye et al. 2008, 2009). It has been used in other areas of engineering and medicine as well. In this study, the feasibility of using seawater to neutralize alkaline red mud in Indian context using Taguchi methodology has been studied. Taguchi’s fractional factorial design of experiments has been used to examine the effect of significant parameters such as weight of red mud, volume of seawater added, stirring/contact time and reaction temperature on pH of red mud slurry. A comprehensive study of these effects is of great significance as the ways in which these factors interact in the neutralization process are poorly understood. Multifactorial experimental design, the Taguchi method has been evaluated to approach this problem of evaluation of pH values. The average values and the signal-to-noise (S/N) ratio of the quality response characteristic (pH) for each parameter at three levels of their values have been calculated from the experimental data. The response curves have been graphically represented to reflect any change in the quality characteristic and S/N ratio with the variation in process parameters. Significant parameters have been identified using the analysis of variance (ANOVA) on the experimental data.
Materials and methods
Materials
Red mud
Red mud from an alumina refinery situated at the eastern coast of India (18°49′9″N,82°57′52″E) which uses highly gibbsitic bauxite was ground to 100 mesh size and used for the study. Red mud taken for the study as determined by wet chemical method has the chemical composition as Al2O3 (16–17.5 %), Fe2O3 (53.5–56 %), SiO2 (6–7 %), TiO2 (5–6 %), Na2O (4–5 %), and CaO (2–3 %). Red mud is a very fine material in terms of particle size distribution. Average particle size of red mud is less than 10 μm. A few particles of greater than 20 μm are also available. The specific surface area (BET) of red mud is between 10 and 30 m2/g, depending on the degree of grinding of bauxite.
Mineralogically, red muds have phases of undigested alumina, aluminosilicates, phases of iron and titania. These phases are hematite (Fe2O3), goethite Fe(1−x)Al x OOH(x = 0.33), gibbsite Al(OH)3, boehmite AlO(OH), calcite(CaCO3), calcium aluminum hydrate (x.CaO.yAl2O3 .zH2O), rutile (TiO2), anatase (TiO2), CaTiO3, Na2TiO3, kaolinite Al2O3·2SiO2·2H2O, sodalites, aluminum silicates, cancrinite (NaAlSiO4)6CaCO3 and hydrogarnet Ca3Al2(SiO4) n (OH)12−4n .
Sodium present in red mud (total soda) is in two forms: free soda and bound soda. Free soda is the entrained liquor in the red mud slurry which gets incorporated during digestion process and remains with red mud in spite of repeated washings. Free soda is in the form of NaOH, Na2CO3, NaAlO2, etc. The pH of the red mud is due to the presence of these alkaline solids in red mud. Inclusion of caustic soda in bound form in the red mud is due to the desilication step carried out in the Bayer process for removal of kaolinitic silica in bauxite. Bound soda is in the form of sodalite complex which can be stated as “NAS” phases: 3(Na2OAl2O32SiO2)Na2X (X = CO3 2−, 2OH−, SO4 2−, 2Cl−) (Kurdowski and Sorrentino 1997). In red mud, about 20–25 % is the free soda while the rest is in the form of sodalite complex.
Seawater
Seawater was collected from Dadar seashore (19°3′30″N,72°56′31″E), Mumbai, India and its average analysis is given in Table 1. The analysis of seawater was carried out at Regional Public Health laboratory, Nagpur, India.
Experimental set up
Figure 1 shows the schematic experimental set up for the study. Red mud slurry was prepared by mixing red mud and seawater and was taken in 1 L stainless steel vessel with stirrer. The vessel was placed in a temperature-controlled water bath (Galaxy Scientific Equipment, Dombivali, Mumbai, India) and agitated at a constant speed (speed regulator from REMI Motors). The pH of the slurry was measured after each test using LabX Light Titrator (Potentiometric Titrator), Mettlor Toledo GmBH, Switzerland.
Taguchi’s design of experimental methodology
A well-planned set of experiments, in which all parameters of interest are varied over a specified range, is a much better approach to obtain optimized process parameters. Mathematically speaking, such a complete set of experiments ought to give desired results. Dr. Taguchi of Nippon Telephones and Telegraph Company, Japan has developed a method based on “ORTHOGONAL ARRAY” experiments which gives much reduced “variance” for the experiment with “optimum settings” of control parameters. Taguchi methods (Peace 1993) use orthogonal array distribution to design an experiment producing smaller, less costly experiments that have a high rate of reproducibility.
In neutralization of red mud with seawater, it is important to identify important factors affecting the process and the way that they interact in order to neutralize the red mud. However, one of the main problems is the difficulty in designing experiments to compare the effects and interactions of multiple variables. For example, a traditional experimental design to compare four independent variables at three different levels each requires a large number of individual experiments (81 experiments). The logistical and resource implications of this experimental design make these experiments very difficult to carry out. Using L 9 orthogonal array with Taguchi method to design an experiment, a study involving four factors at three different levels can be conducted with only nine individual experiments. Taguchi method consist of three phases: designing the experiment, running and analyzing, and confirming and validating the experimental results. After selecting the variables to be studied, Taguchi method depends on distributing the factors under study in an orthogonal array, which distributes the variables (factors) in a balanced manner. Each array can be identified by the form L A (B C), the subscript of L, which is designated by A, represents the number of experiments that would be conducted using this design, B denotes the number of levels within each column which denotes how many levels could be investigated, while the letter C indicates how many factors or variables could be included in the experiment (Peace 1993).
L 9 orthogonal array which consists of four control parameters (factors) such as weight of red mud (factor A), volume of seawater (factor B), stirring time (factor C) and temperature (factor D) which could have their effect on final pH of red mud slurry when neutralized with seawater at three levels in Taguchi method was used for the experimental layout given in Table 2. The levels were set at minimum, middle and maximum value (i.e., levels 1, 2 and 3). The parameters were decided based on the preliminary experimentation carried out. In all, nine experiments had to be conducted. Each experimental trial was repeated thrice. The pH values (replicated thrice as R 1, R 2 and R 3) obtained for each experiment of the L 9 array are shown in Table 3. Subsequently, ANOVA based on the Taguchi method was carried out using the values to determine the contribution of each parameter in the process of neutralization.
Neutralization studies
The initial pH of the red mud slurry was 10.7 (50 g of red mud was stirred with 100 mL of distilled water for 30 min). Different quantity of red mud was mixed with various volume of seawater as per Taguchi’s experimental design, and the slurry was agitated at a constant speed for a fixed duration of time and at the desired temperature in the temperature-controlled water bath. After each experimental run, pH of the slurry was measured. The slurry sample with the lowest pH value and close to 7.0 was filtered using Whatman paper no. 40. The filtrate was analyzed for various parameters and the red mud was dried and analyzed.
Red mud obtained after neutralization at the optimized condition was analyzed using wet chemical method for its chemical constituents. It was analyzed mineralogically for determination of phases using X-ray diffractometer (PAnalytical X-Pert Pro) using Cu Kα radiation (λ = 1.54060 Å). Scanning electron microscopy (SEM) was conducted to study the morphology of neutralized red mud using Electron Probe Analyzer (MAKE: JEOL, Japan, JXA-840A). To observe the improvement in physical characteristic of red mud, filtration rate of two red mud slurry samples was studied. The change in color of filtrate was also observed. Two samples of red mud slurry were prepared by mixing red mud with distilled water (sample 1), and the other sample was prepared by mixing red mud with the same quantity of seawater (sample 2). Filtration rate was measured for both the samples.
The decanted (entrained) liquor after neutralization at optimized parameters was analyzed and compared with the liquor without neutralization (keeping all the parameters same, red mud was washed thoroughly with distilled water and liquor generated).
pH rebound phenomena was studied with two samples of red mud slurry (red mud mixed with seawater) prepared at the optimized condition of parameters, and investigations were carried out to check whether the pH of the red mud slurry rebounds. The phenomenon was observed for 2 months duration time.
Analysis of experimental data
The experimental data generated were analyzed by ANOVA, and Taguchi excel sheet was used for all the analysis which is based on Taguchi method for determination of main effects of the process parameters.
Neutral value of pH (i.e., 7.0) was divided by experimental pH values to get R 1′, R 2′ and R 3′. The value close to 7.0 will have the highest ratio, and on this basis, the “higher-is-better” quality characteristic was used in the analysis of experimental data. The plot of response curves, ANOVA for raw data and S/N ratio data was used for the analysis of results. The mean of the response characteristic pH (Y opt) at the optimal condition was estimated as
where T/N is the overall mean of the response in which T is the grand total of all results and N is the number of experiments and A 1avg, B 3avg, C 1avg and D 3avg represent average values of response at the first, third, first and third levels of parameters A, B, C and D, respectively.
In order to confirm that the optimal parametric values using Taguchi’s methodology are valid, selected confirmatory experiments were carried out under optimal conditions. The average of the results of the confirmation experiment is then compared with the anticipated average based on the optimal parameters and levels tested by Taguchi’s methodology. The Y opt value obtained is 0.86 and pH value of which comes out to be 8.11 which is very close to the optimized experimental value of 8.09–8.15 in the run 7. The deviation between actual and determined pH by Taguchi’s methodology is within 5 %, and hence it is confirmed that the optimal parametric values determined by Taguchi’s design are valid.
Results and discussion
Effect of the process parameters
Table 4 provides the raw data for the average value ratios of pH and S/N ratio for each parameter at levels 1, 2 and 3. The effects of various parameters (A, B, C, D) are also shown in this table. The weight of red mud at level 1 and volume of seawater at level 3 have the greatest influence on value of pH. The difference between the levels also shows the same trend.
The effects of process parameters on the observed values (pH) based on Taguchi methodology are being discussed. The analysis is used to study the trend of the effects of each of the factors at three levels and is shown in Fig 2. Figure 2 shows the response in curves of the individual parameters on the pH values (7/experimental pH value) and S/N ratio. From figure, it can be seen that the pH of the neutralized slurry increases (i.e., the ratio of 7/experimental pH value decreases) with the increase in weight of red mud (factor A). It is due to the increase in free caustic soda in the slurry with the increase in quantity of red mud. There is a sharp decrease in pH ratio when factor A changes from level 1 to 2 and also when the quantity of red mud changes from level 2 to 3. Seawater addition (factor B) definitely decreases the pH ratio of the slurry as a sharp incline in pH ratio is observed. An increase in quantity of seawater decreases the pH of red mud slurry and hence the ratio increases. An increase of factor B from level 1 to 2 decreases the pH much below 8.8. Further increase in factor B to the level 3 decreased the value to about 8.4 due to more availability of calcium and magnesium ions for the reaction to take place between sodium hydroxide and sodium aluminate to form weaker hydroxides. A slight variation in pH value is observed with the increase in stirring/contact time of seawater with red mud from level 1 to 2 and subsequently from level 2 to 3. Similarly, a very marginal change in pH values is observed with the increase in temperature from 30 to 50 °C which shows that the temperature (factor D) at which the neutralization process is taking place in the neutralization of red mud with seawater, which is not temperature dependant. The overall results convey that the pH of the slurry is affected by the quantity of red mud and the other major parameter, i.e., volume of seawater. The lowest pH of the neutralized slurry is obtained with the weight of red mud at level 1, volume of seawater to be at level 3, stirring time at level 1 and temperature at level 1 (A 1 B 3 C 1 D 1).
The contribution of individual parameters was weighted as shown in Table 5 with ANOVA to see their effect on desired response characteristic (pH). The most significant parameters are found to be the weight of red mud and volume of seawater with percent contribution of 44.92 and 53.59 %, respectively. The stirring time has a much lesser effect of about 0.29 %. As the temperature (factor D) has a negligible effect on the value of pH achieved, it has a smaller variance and is of small significance. Hence, it was pooled to increase the statistical significance of other important factors.
Confirmation experiment
From Table 3, it can be seen that the experimental condition (A 1 B 3 C 3 D 3) was the one in which the pH value achieved (8.09–8.15) which was close to the neutral condition of 7 in all the 3 replications. The optimized condition obtained from Fig. 2 was A 1 B 3 C 1 D 3 having pH value of about 8.0 which is the lowest of all the values. Hence, these conditions were fine tuned accordingly as given in Table 6 by increasing the volume of seawater. The pH values could not be lowered down below 8.0 in spite of increasing the volume of seawater.
Hence, the final optimized conditions to achieve a pH value of about 8.0 were: 25 g of red mud, 150 mL of seawater (solid to liquid ratio of 1:6), 30 min stirring time and 30 °C temperature. The stirring time can be lowered and the process can be carried out at ambient temperature as these are the two parameters having sub-significant effect.
Red mud analysis after neutralization
Chemical analysis
The chemical analysis of red mud before and after neutralization is shown in Table 7. For the other constituents in red mud remaining nearly the same, about 20 % of total caustic soda in the red mud has been reduced. Almost all of the adhered soda to the red mud (free soda) reacted with the calcium and magnesium carbonates of seawater to form their hydroxides, and get precipitated in red mud which is shown by the increase in percentage of calcium in it. The sodium carbonate and residual sodium as sodium chloride along with other salts formed in the liquor were removed by rinsing the seawater-neutralized red mud before disposal.
Formation of different phases
To investigate the phase structures of the red mud, the X-ray diffraction (XRD) pattern was measured. Figure 3 shows the XRD pattern with the peaks showing the phases which indicate the formation of brucite (magnesium oxide, Mg(OH)2 [JCPDS no: 00-001-1169] and magnesium aluminum oxide (MgAl2O47·9H2O) [JCPDS no: 00-022-0432]. The other phases seen were of hematite (Fe2O3), magnetite (Fe3O4), gibbsite (Al(OH)3), sodalite, halite, calcite and ileminite. Characterisation of seawater-neutralized bauxite residue using XRD has been studied in detail by researchers (Palmer and Ray 2009).
Morphology of red mud
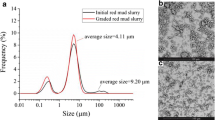
Figure 4 shows the morphological structure of “as it is” red mud which indicate scattered fine particles of about 1 μm size, whereas Fig. 5 of neutralized red mud shows that large agglomerates of size of about 80–150 μm are formed after neutralization which has improved the physical characteristic of red mud. The agglomerates are circular as well as elongated and rectangular. The agglomerates have fine particles of iron, aluminum and aluminosilicates attached to it as shown in Fig. 6. Figure 7 shows insoluble compounds having hexagonal structure being trapped inside the dense mass. The hexagonal morphology is of brucite and hydrotalcite formed during the neutralization process, precipitated and got entrapped inside the dense mass.
Physical characteristics of red mud
Seawater neutralization improved the physical characteristics of red mud as can be seen from the filtration rate measured with two samples of red mud slurry with and without neutralization (Table 8). Filtration rate of sample 2 increases tremendously as the time required to filter the same amount of slurry is only half the time required to filter sample 1. Above all, the filtrate obtained with sample 1 is reddish in color and highly turbid which may require another filtration. But the filtrate with sample 2 is clear and devoid of any color. This is due to the fact that when unneutralized red mud comes in contact with seawater, the fine mineral particles flocculate into large agglomerates with the multivalent exchange cations, Ca and Mg, forming electrostatic bridges (Mcbride 1994). These sites then act as nucleation sites for the precipitation of magnesium and calcium hydroxides, taking hydroxide ions from solution and lowering the pH (McConchie et al. 2000). Precipitates of hydroxycarbonates of aluminum, calcium and magnesium and hydrotalcite are formed once the original hydroxide concentration is reduced by the precipitation of hydroxides such as brucite.
Entrained liquor analysis after neutralization
As per the Guidelines of Australian and New Zealand Environment and Conservation Council (ANZEX) and Agriculture and Resource Management Council of Australia and New Zealand (ARMCANZ), the liquor being strongly alkaline with a high pH, requires neutralization to a pH below 9 with an optimum value of 8.5–8.9 before becoming environmentally benign (Hanahan et al. 2004). At this pH, chemically adsorbed Na is released, alkaline buffer minerals are neutralized and toxic metals become insoluble.
The entrained liquor analysis after neutralization is shown in Table 9. It can be seen from Table 9 that the water after washing red mud (without neutralization) has high pH, high alkalinity and high turbidity. If seawater is used to neutralize the red mud, the entrained liquor will have a pH which is within disposal limit (pH 8.0) with a much lower alkalinity and turbidity. If the seawater obtained after neutralization is compared with the “as it is” seawater, the chloride content has decreased as some of the chlorine remains in the red mud along with residual sodium as sodium chloride. The hardness of the seawater also decreases up to about 50 % with an increase in slight alkalinity and turbidity as compared to original seawater (refer Table 1). The seawater pH also increases slightly from 7.07 to about 8.10 and this water can very well be disposed off in the sea without creating hazard to flora and fauna of sea. The increased turbidity can be reduced by employing better filtration facility.
pH rebound
The increase in pH and aluminum concentration after the neutralization of bauxite refinery residues is commonly known as reversion which can be minimized by seawater neutralization. This is unlike the case of CO2 sequestration in red mud. A study observed pH rebound with red mud samples neutralized with CO2. It was hypothesized that dissolution of tricalcium aluminate (C3A) in the neutralized slurry causes this reversion. As pH rebound was observed with CO2-neutralized red mud samples (Khaitan et al. 2009), the phenomena was studied with seawater-neutralized red mud also. With the neutralization of red mud with seawater at the optimized condition, very slight increase in pH is observed in the initial days but the pH remains constant subsequently for the prolonged duration of 2 months without any pH rebound (Fig. 8). This is due to the formation of hydrotalcite by removing the hydroxide and aluminum ions from the solution, aragonite and calcite which are less soluble and weakly alkaline solids in the neutralized red mud slurry. Magnesium hydroxide (brucite) also has a low solubility in water with a K sp of 1.5 × 10−11 and can be precipitated by the metathesis reaction or double decomposition reaction between magnesium salts and sodium, potassium, or ammonium hydroxide.
Conclusion
The paper has discussed in detail the feasibility of use of seawater in neutralization of alkaline red mud. It is found that the method of neutralization of red mud with seawater would be a highly successful and effective process for treatment of red mud. The study shows that neutralization of red mud with seawater reduces alkalinity, lowers down the pH of red mud slurry permanently, without pH rebound within acceptable and disposable limits to a value of about 8.0 and subsequently improves the physical characteristics of red mud. The Taguchi’s experimental methodology is found to be a valuable tool for the investigation of the effects of multiple variables in obtaining the response (pH) values. The optimum process conditions were found to be as 25 g of red mud, 150 mL of seawater (i.e., solid to liquid ratio of 1:6), 30 min stirring time and 30 °C temperature to get a pH of about 8.0. Weight of red mud and volume of seawater was found to be the most significant factors affecting the neutralization process with nearly equal percentage contribution. The stirring time and the temperature have nearly a negligible effect on the value of pH achieved. The volume of seawater may be required in large amount for neutralization, but this volume can be decreased if the concentration of calcium and magnesium ions could be increased in it. Neutralization with seawater modifies the physical characteristics of red mud and decreases the viscosity of the red mud, thus improving settling and filterability of red mud. Thus, red mud can be treated with seawater and the entrained liquor after filtration can be disposed off with a much reduced pH and alkalinity thus rendering it safe for disposal. Considering the abundance of seawater, it would also be a cost effective method and also not create any hazards for aquatic life and for the fauna of sea. The studies would be very much useful for future alumina plants in India which may come up on the seashore and may utilize seawater for its red mud treatment. The neutralized red mud may be used for vegetation or as an adsorbent for which further studies need to be carried out.
References
Barker T (1990) Engineering quality by design. Marcel Deter, New York
Brunori C, Cremisini C, Massanisso P, Pinto V, Torricelli L (2005) Reuse of a treated red mud bauxite waste: studies on environmental compatibility. J Hazard Mater B117:55–63
Fuhrman G, Jens C, McConchie D (2004) Adsorption of arsenic from water using activated neutralized red mud. Environ Sci Technol 38(8):2428–2434
Hanahan C, McConchie D, Pohl J, Creelman R, Clark M, Stocksiek C (2004) Chemistry of seawater neutralization of bauxite refining residues (red mud). Environ Eng Sci 21:125–138
Khaitan S, David A, Gegory V (2009) Mechanisms of neutralization of bauxite residue by carbon dioxide. J Environ Eng 135(6):433–438
Kurdowski W, Sorrentino F (1997) Waste materials used in concrete manufacturing. In: Satish C (ed) William Andrew Publishing, Noyes, pp 290–308
Lataye D, Mishra L, Mall I (2008) Multicomponent sorptive removal of toxins pyridine, 2-picoline and 4-picoline from aqueous solution by Bagasse fly ash: optimisation of process parameters. Ind Eng Chem Res 47:5629–5635
Lataye D, Mishra L, Mall I (2009) Multicomponent sorption of pyridine and its derivatives from aqueous solution into rice husk ash and granular activated carbon. Practice Periodical of hazardous, toxic and radioactive waste management 13(4):218–228
Mcbride M (1994) Environmental chemistry of soils. Oxford University Press, New York
McConchie D, Clark M, Hanahan C (2000) The use of seawater neutralized bauxite refinery residues in the management of acid sulphate soils, sulphidic mine tailings and acid mine drainage. In: 3rd Queensland Environmental Conference on Sustainable Solutions for Industry and Government, Brisbane, pp 201–208
Palmer S, Ray L (2009) Characterisation of bauxite and seawater neutralized bauxite residue using XRD and vibrational spectroscopic techniques. J Mater Sci 44(1):55–63
Palmer S, Nothling M, Bakon K, Frost R (2010) Thermally activated seawater neutralised red mud used for the removal of arsenate, vanadate and molybdate from aqueous solutions. J Colloid Interface Sci 342(1):147–154
Paramguru R, Rath P, Misra N (2005) Trends in red mud utilization—a review. Mineral Process Extractive Metall Rev 26:1–29
Peace GS (1993) Taguchi methods: a hands-on approach. Addison-Wesley, Boston
Rai S, Wasewar K, Mukhopadhaya J, Chang K, Uslu H (2012) Neutralization and utilization of red mud for its better waste management. Arch Environ Sci 6:13–33
Ross PJ (1996) Taguchi techniques for quality engineering. McGraw Hill Book Co, New York
Roy RK (1990) A primer on the Taguchi method. Soc Manufact Eng, Dearborn
Srivastava V, Mall I, Mishra I (2007) Multicomponent adsorption study of metal ions onto Bagasse fly ash using Taguchi’s design of experimental methodology. Ind Eng Chem Res 46:5697–5706
Taguchi G (1986) Introduction to quality engineering. Quality resources, NewYork
Taguchi G, Yu-in W (1979) Off-line quality control. Central Japan Quality Control Association Nagoya, Japan
Acknowledgments
Authors acknowledge Mr. M. J. Chaddha, Mr. R. S. Mishra and Mr. P. Mahinderan (Scientists, JNARDDC, INDIA) for their technical and analytical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rai, S., Wasewar, K.L., Lataye, D.H. et al. Feasibility of red mud neutralization with seawater using Taguchi’s methodology. Int. J. Environ. Sci. Technol. 10, 305–314 (2013). https://doi.org/10.1007/s13762-012-0118-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-012-0118-7