Abstract
The present study deals with the assessment of different physicochemical parameters (pH, electrical conductivity (E.C.), turbidity, total dissolved solids (TDS), and dissolved oxygen) in different surface water such as pond, river, and canal water in four different seasons, viz. March, June, September, and December 2023. The research endeavors to assess the impact of a cationic polyelectrolyte, specifically poly(diallyl dimethyl ammonium chloride) (PDADMAC), utilized as a coagulation aid in conjunction with lime for water treatment. Employing a conventional jar test apparatus, turbidity removal from diverse water samples is examined. Furthermore, the samples undergo characterization utilizing X-ray diffraction (XRD) and scanning electron microscopy (SEM) techniques. The study also conducts correlation analyses on various parameters such as electrical conductivity (EC), pH, total dissolved solids (TDS), turbidity of raw water, polyelectrolyte dosage, and percentage of turbidity removal across different water sources. Utilizing the Statistical Package for Social Science (SPSS) software, these analyses aim to establish robust relationships among initial turbidity, temperature, percentage of turbidity removal, dosage of coagulant aid, electrical conductivity, and total dissolved solids (TDS) in pond water, river water, and canal water. A strong positive correlation could be found between the percentage of turbidity removal and the value of initial turbidity of all surface water. However, a negative correlation could be observed between the polyelectrolyte dosage and raw water’s turbidity. By elucidating these correlations, the study contributes to a deeper understanding of the effectiveness of PDADMAC and lime in water treatment processes across diverse environmental conditions. This research enhances our comprehension of surface water treatment methodologies and provides valuable insights for optimizing water treatment strategies to address the challenges posed by varying water sources and seasonal fluctuations.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The well-being of mankind mainly depends upon the availability of clean water. Despite the great effort, human beings in the present era continue to face water pollution challenges. A large quantity of particles are picked up by surface water as it flows over the ground to streams and then through rivers and canals. Industrial discharge and human activities carry significant contaminants to the surface water. The quality of surface water gets polluted because it is highly used by humans for drinking, bathing, agriculture, and other needs (Sánchez-Martín et al., 2010; Soros et al., 2019). Turbidity, a measure of suspended and colloidal matter in water, is a key indicator of water quality, affecting both aesthetics and functionality (Asthana et al., 2017). High turbidity levels not only diminish water purity but also introduce unpleasant tastes and odors, impacting water treatment processes and increasing treatment costs (Muthuraman & Sasikala, 2014). Moreover, turbid water inhibits respiratory processes and reduces visibility, underscoring the importance of minimizing turbidity levels in surface water (Frieder et al., 2012; Gautam, 2011; Hargreaves & Tucker, 2002; Li et al., 2013; Paul et al., 2019).
In India, the pollution of rivers has reached alarming levels due to rapid urbanization and industrial growth, posing significant threats to aquatic ecosystems and human health (Roy & Shamim, 2020). Pollution stems from various sources, including industrial and sewage waste discharge, agricultural runoff, and solid waste deposition (Singh et al., 2005; Wang et al., 2019). The influx of untreated and treated suspensions further exacerbates water quality degradation, necessitating effective suspension removal strategies (Voulvoulis, 2018).
Ahmed et al. (2022) have done a Pearson correlation study for the physicochemical parameters of Rawal water shed. They concluded that there is a positive correlation between turbidity and temperature. However, dissolved oxygen could not show any significant correlation with lithological parameters. Panda et al. (2018) studied the correlation of 16 different parameters, including biological oxygen demand (BOD), dissolved oxygen, chromium, iron, chlorides, and pathogenic bacteria of river Salandi, Odisha, and revealed both positive and negative correlations among specific parameters. Sahu et al. (2024) used K means to determine the correlation study of different physicochemical parameters of groundwater of Raipur, India. The positive correlation could be found by the researchers among TDS, hardness, alkalinity, and EC ( Sahu et al., 2024; https://doi.org/10.1016/j.measen.2024.101278).
Polyelectrolytes have emerged as crucial agents for water treatment, offering enhanced flocculation capabilities and versatility in controlling properties such as charge units and molecular weight (Yadav & Goyal, 2022). When used in conjunction with metal coagulants, polyelectrolytes aid in destabilizing suspended particles and enhancing flocculation processes (Jabin et al., 2021, 2023). Poly(diallyl dimethyl ammonium chloride) (PDADMAC), a high-charge density cationic polyelectrolyte, is particularly effective as a secondary coagulant for suspension removal (Jabin & Kapoor, 2020).
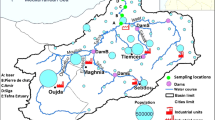
In this study, surface water samples were collected from various sources in India, including ponds, rivers, and canals, to assess turbidity levels and water quality parameters. Pond water, sourced from Thanthri, Palwal District, Haryana, river water from the Yamuna River, and canal water from the Kheri canal in Greater Faridabad, Haryana, were analyzed. The Yamuna River, a major tributary of the Ganga River, faces substantial pollution from treated and untreated waste, highlighting the urgency of effective water treatment measures. A view of the area and collection site of all three sources of surface water has been shown in Fig. 1.
The objective of this study was to determine the optimum dosage of lime and PDADMAC for minimizing turbidity in pond, river, and canal water in all four seasons, viz., March, June, September, and December 2023. Additionally, correlation analyses were conducted to understand the relationships among various water quality parameters including initial turbidity, electrical conductivity, total dissolved solids, pH, polyelectrolyte dosage, and percentage removal of turbidity. By elucidating these correlations, the study aims to provide insights into effective water treatment strategies and facilitate informed decision-making for water quality management. The X-ray diffraction analysis of the sludge-lime and sludge-lime-polymer composite was conducted to explore the interaction mechanism among sludge, lime, and polymer. SEM analysis was also done in this study to explore the surface topography and adsorption behavior of lime as a primary coagulant and polymer as a secondary coagulant in different kinds of surface water (pond, river, and canal).
To the best of our knowledge, no research work has been done to date on physicochemical parameters assessment and its correlation study on the pond of the village Thanthri, Palwal District, Haryana, India, and Kheri Canal, Faridabad, India. The research work on turbidity removal was also not done earlier on this Pond water and Kheri Canal water of Faridabad. This research will be helpful in the long run for the people of village Thanthari and those in the vicinity of Kheri Canal, Faridabad, because they are a significant water source for the people of a particular area. Extensive research has been done on the water samples of the Yamuna River. However, the comparative study on the water of Yamuna River, pond water of village Thanthari, and Kheri Canal water has been done for the first time to the best of our knowledge. Based on the Pearson correlation study in all three water samples, it can be understood that this result will be helpful in the long run for the researchers. Hence, it is significant and different from conventional studies.
Materials and methods
Water samples were collected throughout the year 2023 across four distinct seasons: March (spring), June (summer), September (autumn), and December (winter). Physicochemical parameters, including pH, electrical conductivity (E.C.), turbidity, total dissolved solids (TDS), and dissolved oxygen, were determined using standard methods outlined by the American Public Health Association (APHA, 1995) (Association, 1926).
The samples were collected in clean polyethylene bottles, rinsed with de-ionized water to avoid contamination, and stored in a refrigerator before analysis. This study provides insights into surface water quality and anthropogenic activities’ impacts on water resources.
Inorganic coagulant lime was sourced from CDH (India) and prepared in distilled water to obtain a 10 mg/L concentration. Poly(diallyl dimethyl ammonium chloride) (PDADMAC), obtained from Sigma Aldrich, was utilized as a coagulant aid.
Water pH was measured using a pH meter (Anna HI 8314, USA), while electrical conductivity was assessed with a Philips conductivity bridge and dip-type cell at a temperature of 27 °C ± 3 °C. Turbidity measurements were conducted using a turbidity meter (Hanna HI93703, USA), and TDS values were calculated using a tubular TDS meter.
Tables 1, 2, and 3 present the turbidity, temperature, E.C., TDS, and pH values of pond water, river water, and canal water across different seasons, along with a summary of basic statistical analysis. These tables offer comprehensive insights into the variations of key water quality parameters across different seasons and water sources.
Raw pond water characteristics
The E.C. of pond water exhibited notable variations throughout the investigation, reaching its peak in September and lowest point in December. This fluctuation in E.C. mirrored the trends observed in TDS. Additionally, the pH levels of pond water displayed seasonal variations. In March, June, and December 2023, the mean pH was recorded at 8, whereas in September 2023, it decreased to 7.0. This pH variation is significant as it influences subsequent turbidity treatment processes. The pond water temperature was found to be 30 °C, 39 °C, 27 °C, and 20 °C in March, June, September, and December 2023, respectively. As per the literature, pond water’s temperature should be between 10 and 40 °C ( Mehta & Kumari, 2022). However, a temperature of more than 25 °C is found to be objectionable for the survival of flora and fauna present in aquatic medium.
The turbidity levels of pond water also varied across different seasons. In March, June, September, and December 2023, the turbidity measurements were recorded at 175 NTU, 189 NTU, 96 NTU, and 145 NTU, respectively. These fluctuations in turbidity levels underscore the dynamic nature of water quality in ponds and emphasize the importance of continuous monitoring and treatment measures. Both EC and TDS were found to be highest in the month of September 2023 for pond water. A linear correlation could be observed between EC and TDS in pond water. The result is in close agreement with the work of Sarang et al. (2018).
Raw river water characteristics
Upon evaluating the raw water from the Yamuna, it was observed that the turbidity levels peaked at 249 NTU in June and reached their lowest point at 94 NTU in March. Concurrently, the temperature of the water samples exhibited fluctuations ranging from 19 to 38 °C, with the highest temperatures recorded in June and the lowest in March.
Similarly, the E.C. demonstrated its highest values in June and its lowest in March. The elevated E.C. values in June indicate significant dissolved inorganic substances in the ionized water. TDS indicates water’s overall salinity, with the highest TDS recorded in June at 849 mg/L and the lowest in September at 595 mg/L.
The pH levels of the water samples ranged from 7.4 to 8.6, showcasing variations in acidity and alkalinity. These findings highlight the dynamic nature of water quality parameters in the Yamuna, emphasizing the importance of continued monitoring and management strategies to ensure water safety and purity.
Raw canal water characteristics
The turbidity levels in canal water were notably higher than other surface water sources. In June 2023, the average turbidity reached 333 NTU, decreasing to a minimum of 198 NTU in December 2023. It could be observed from the results that turbidity is highest in the summer season in pond, river, and canal water. It may be because of water loss in the summer season in all kinds of surface water due to high temperatures, which leads to an increase in the concentration of siliceous materials in the water bodies. Siliceous materials and colloidal particles present in surface water cause turbidity. Colloidal matter can be small organisms and mud. Small organisms grow at a faster rate in the summer season (Dey et al., 2021).
Concurrently, the temperature of the water samples exhibited fluctuations ranging from 22 to 39 °C, with June 2023 recording the highest temperatures and December 2023 the lowest. Similarly, the E.C. values fluctuated between 0.5 and 1.4 mho, mirroring the trend observed in TDS. Notably, the E.C. peaked in December 2023 and was at its lowest in June 2023, demonstrating seasonal variations in water quality parameters.
Removal of turbidity using polyelectrolyte
The raw water underwent filtration using a stainless sieve followed by filter paper with a 7–8 µm pore size. Subsequently, a jar test procedure was conducted, a widely adopted laboratory method for assessing coagulation and flocculation processes at a bench scale.
This experiment utilized a conventional jar test apparatus featuring the Phipps and Bird six-paddle stirrer with an illuminated base within 2 L square Plexiglas containers. An RPM (rotation per minute) gauge at the top Centre of the device is present for uniformly controlling the mixing speed in all containers. Each jar was filled with one L of water. Desired lime was added to each jar and agitated for one minute at 100 RPM. Then, the desired dosage of PDADMAC as a coagulant aid was added after mixing the lime. The mixing speed was reduced to 50 rpm for 7.5 min, followed by 20 RPM for another 7.5 min. After a 30-min sedimentation period, a 10-ml aliquot was extracted from the mid-depth of the beaker, and the residual turbidity was measured (Chiavola et al., 2023; Haghiri et al., 2018). This process was repeated for all types of water samples. Variations in results were observed due to disparities in the physicochemical properties of water quality. The optimum dosage of lime and polymer has been tabulated and discussed in the results and discussion section.
X-ray diffraction (XRD) analysis
The studies of X-ray diffraction were carried out using a Rigaku D/Max-2500 X-ray diffractometer. This instrument utilized a Cu-Kα X-ray tube with a wavelength of 1.540538 angstroms, operating at a current of 20 mA and an input voltage of 40 kV. Throughout the study, two diffractograms were generated to investigate the interactions within the sludge-lime-polymer system-one for sludge with lime and another for sludge with lime and polyelectrolyte. These analyses aimed to provide insights into the complex interactions occurring within the composite material.
Scanning electron microscopy (SEM) analysis
Materials were imaged using an SEM apparatus manufactured by Jeol (Japan), specifically the JSM 6510Lv model. Operating at a 15 kV accelerating voltage, this SEM enabled detailed visualization of the specimens. A typical SEM allows for scanning areas ranging from 1 to 5 μm, offering magnifications between 20x and 30,000 × with a spatial resolution of 50–100 nm. In SEM analysis, a focused beam of electrons interacts with the specimen, producing images that unveil the sample’s surface topography.
Result and discussion
Result of different surface water with basic statistics
Sixteen water samples were collected from various surface water sources over 1 year. Table 4 summarizes the optimization of lime dosage for four different samples.
The optimal lime dosage varied across different water sources and seasons, with specific dosages identified for each scenario. In March, the optimal lime dosage was 7.5 mg/L for pond water and 10 mg/L for river water. Conversely, canal water consistently required 5 mg/L of lime dosage throughout all seasons due to its consistently high turbidity compared to other surface water sources. Notably, an inverse relationship was observed between turbidity levels and optimal lime dosage, with higher turbidity necessitating lower lime dosages. This trend was consistent across all four seasons. These results are in close agreement with Kapoor et al. (2015). To address residual lime concentration in treated water and further reduce turbidity, cationic polyelectrolyte PDADMAC was employed in conjunction with lime for turbidity removal from various water samples. Enhanced performance was achieved when the coagulant aid was added to the water sample after proper lime mixing, in contrast to the simultaneous addition of polyelectrolyte and lime.
PDADMAC, characterized by its quaternary ammonium salt nature and synthetic polyelectrolyte properties, demonstrated efficacy in turbidity removal. Its high charge density and molecular weight facilitated the flocculation of negatively charged suspended particles through adsorption and charge neutralization mechanisms. This effectiveness was particularly notable in high turbidity water compared to low turbidity water (Kapoor et al., 2015; Piaskowski et al., 2023).
The treatment results for pond water across four different seasons are depicted in Fig. 2A–B. Following treatment, the average residual turbidity of pond water ranged from 4.76 to 9.0 NTU for March, June, September, and December 2023, respectively. Interestingly, the lowest residual turbidity was observed when the raw water exhibited the highest turbidity levels. This underscores PDADMAC’s superior performance in highly turbid water conditions compared to situations with lower turbidity levels.
The efficacy of PDADMAC in turbidity removal from river water is illustrated in Fig. 3A–D across the months of March, June, September, and December 2023. Notably, the combined action of lime and PDADMAC proved notably more effective at higher initial turbidity levels compared to lower turbidity conditions. This heightened effectiveness can be attributed to the presence of ample colloidal suspensions during periods of maximum turbidity, facilitating adsorption and charge neutralization processes. June 2023 exhibited the highest turbidity levels in river water, as depicted in Fig. 3B.
In canal water, using polyelectrolyte as a coagulant aid in the flocculation process resulted in reduced lime dosage and residual turbidity, as shown in Fig. 4A–B. Since canal water exhibited the highest raw turbidity in June compared to other surface water sources, the removal of turbidity peaked during this period (97.99%), as illustrated in Fig. 4. Following jar testing, minimal changes in pH (within ± 0.1) were observed across all treated water samples.
Table 5 summarizes the optimal polyelectrolyte dosage alongside the percentage of turbidity removal across various surface water sources. Polyelectrolyte dosage emerged as a critical factor in turbidity removal, with the highest removal rates achieved at minimal polyelectrolyte dosages across all water samples. In the presence of suspended solids, low molecular mass polymers primarily react with soluble organics, while high molecular mass polymers, at low dosages, preferentially interact with suspended solids (Kapoor et al., 2015; Piaskowski et al., 2023). Consequently, PDADMAC, a high molecular weight polyelectrolyte, operates optimally at a dosage of 2 mg/L for highly turbid canal and river water in June. Maintaining an optimal dosage of coagulant aid is crucial to prevent excessive adsorption, which could lead to poor adsorption site accessibility.
Moreover, the optimal polyelectrolyte dosage was identified as 4 mg/L in river water for the March, June, and December seasons. This optimized dosage strategy balances effective turbidity removal while minimizing excess polyelectrolyte adsorption, ensuring optimal treatment outcomes.
Due to the significant variation in turbidity of different raw water, it is very challenging to predict the dosage of polyelectrolyte to be used in a particular kind of water. Determination of optimum dosage of polyelectrolyte can only be determined by jar tests, because there is no set formula for defining polymer dosage in the coagulation-flocculation process. Based on the results obtained, it can be concluded that treating surface water with lime as a primary coagulant decreases the required dosage of PDADMAC as a coagulant aid because a high dosage of polymer covers the particle to a more significant extent and does not give sufficient space for adsorption. It is also noted that a dose more than the optimum polymer dosage does not enhance turbidity removal.
X-ray powder diffraction (XRD) analysis
The XRD spectra obtained for the samples containing sludge with lime and sludge with lime with polyelectrolyte provide valuable insights into these components’ structural composition and interaction.
The XRD spectra obtained for the sludge with lime combination reveal distinctive diffraction peaks corresponding to the crystalline phases present in the sample. Typically, the XRD pattern for sludge with lime exhibits peaks corresponding to the crystalline phases of lime, including calcium hydroxide (Ca(OH)2), calcium carbonate (CaCO3), and calcium oxide (CaO). These peaks align well with the characteristic peaks of calcite at 2θ ~ 29.20°, indicative of the (111) plane, as observed in Card No. 5–586 from the International Center for Diffraction Data (ICDD) (Galván-Ruiz et al., 2009; Pires, 2015). However, the intensity of the peak at 39.02° corresponding to the (200) plane is comparatively low due to the overlay of sludge samples onto the lime (Nasrazadani & Eureste, 2008) (Fig. 5A). These peaks facilitate the identification of crystalline phases formed upon the addition of lime to the sludge, providing insights into the degree of crystallinity and phase composition of the sample. Moreover, shifts or changes in peak intensity suggest potential interactions or reactions between the sludge and lime components.
In contrast, the XRD spectra obtained for the sludge with lime plus polyelectrolyte combination exhibit alterations in peak intensity, position, or appearance compared to the sludge with lime alone. These changes indicate potential modifications in the crystalline structure or phase composition induced by adding polyelectrolyte. Specifically, a sharp peak near 2θ ~ 30° becomes more intense and undergoes a slight shift in angle after interaction with polyelectrolyte, while a peak observed at 2θ ~ 17 disappears upon the addition of polyelectrolyte. Furthermore, a new peak emerges at 2θ ~ 31.68°, characteristic of PDADMAC, suggesting an increase in the semicrystalline nature of the sample following the addition of polyelectrolyte (Tyagi & Sharma, 2016). The disappearance of the peak at 2θ ~ 34° from the spectra indicates the dominance of polyelectrolyte in the material over lime (Fig. 5B). These alterations in peak characteristics signify interactions between the polyelectrolyte and the sludge-lime matrix, potentially leading to new crystalline phases or changes in crystallographic parameters. Overall, XRD analysis provides valuable insights into the structural modifications induced by the incorporation of polyelectrolyte into the sludge-lime composite, shedding light on its influence on the material's overall properties.
Scanning electron microscopy (SEM) analysis
SEM analysis was conducted on sludge samples (after filtration) treated with lime alone and combined with polyelectrolyte. The impact of lime as a standalone metal coagulant on sludge was examined at magnification scales of 500X (Fig. 6A), 25KX (Fig. 6B), and 50KX (Fig. 6C). Similarly, sludge treated with lime in conjunction with polyelectrolyte was studied at the same magnification scales of 500X (Fig. 6D), 25KX (Fig. 6E), and 50KX (Fig. 6F).
Figure 6A presents the original sludge particles treated with lime alone. These particles exhibit a smaller size, an unevenly dispersed surface arrangement, and strong adhesion to water. Figure 6B and C depict the unadjusted spaces observable on the surface without uniform consistency. These observations provide insight into the structural changes induced by applying lime as a solo metal coagulant.
Following the addition of polyelectrolyte, molecules permeate and occupy spaces within the structure, indicating the formation of a sludge-lime-polyelectrolyte composite (Fig. 6E and F). Analysis of the sludge treated with lime and polyelectrolyte confirms the development of dense and smooth floc structures during treatment (Fig. 6D). Under SEM examination, strong bonding within the sludge-lime-polyelectrolyte composite is evident, showcasing unique and well-defined features (Fig. 6D–F).
Polyelectrolytes are crucial in enlarging sludge particles and facilitating strong aggregation through charge neutralization mechanisms (Fig. 6E and F). This phenomenon enhances water separation from sludge, leading to improved water purification. Furthermore, adsorption and charge neutralization transform destabilized particles into larger aggregates and flocs, aiding in effective water cleaning.
SPSS Pearson correlations
Monitoring water quality is facilitated through correlation studies among various parameters (Chekkala et al., 2023; Shroff et al., 2015). Using SPSS, a correlation matrix for different variables is constructed to establish correlations between different aspects of surface water. The degree of correlation between variables is assessed through the Pearson correlation coefficient. This coefficient, denoted as r, indicates the strength and direction of linear relationships between pairs of continuous variables. The analysis determines whether variables are strongly correlated with each other. Essentially, the Pearson correlation assesses whether statistical evidence supports a linear relationship among the parameters.
Analysis of pond water
The Statistical Package for Social Science (SPSS) software was employed to analyze certain factors' influence on pond water characteristics. Table 6 presents the Pearson correlation coefficient, illustrating the relationship between turbidity, temperature, E.C., TDS, pH, percentage removal of turbidity, and initial turbidity.
The correlation analysis of pond water revealed several significant relationships. There was a strong positive linear correlation between the percentage removal of turbidity and initial turbidity (r = 0.887), which was statistically significant (p = 0.000). Additionally, the polyelectrolyte dosage exhibited a strong negative correlation with the percentage of removal (r = − 0.881), which is also statistically significant.
Furthermore, the turbidity of raw water displayed a strong positive correlation with the removal percentage. Higher initial turbidity corresponded to a more significant percentage of turbidity removal. Moreover, the E.C. showed a significant positive correlation with TDS (r = + 0.977), indicating a direct relationship between the two parameters. Lastly, a direct correlation was observed between temperature and the initial turbidity of raw water (r = + 0.570).
Analysis of river water
The correlation analysis of river water variables was conducted using IBM SPSS software, and the Pearson correlation coefficients are presented in Table 7. The Pearson correlation coefficient indicates the strength and direction of the relationship between different parameters.
The correlation matrix revealed several significant relationships. Initial turbidity exhibited a strong positive correlation (r = 0.740) with the temperature of river water, which was statistically significant. Furthermore, the dosage of polyelectrolyte displayed a strong negative linear relationship with initial turbidity (r = − 0.879), which was also statistically significant (p = 0.000). Additionally, initial turbidity showed a strong negative correlation (r = − 0.914) with E.C., also statistically significant (p = 0.000).
Moreover, the correlation matrix indicated a strong negative linear relationship between the polyelectrolyte dosage and initial turbidity (r = − 0.977), with statistical significance (p = 0.000). Furthermore, the percentage removal of turbidity showed a strong positive linear relation with initial turbidity (r = 0.851), which was statistically significant (p = 0.000). Additionally, there was a statistically significant relationship between the percentage of removal and the dosage of polyelectrolyte (p = 0.000), with both parameters negatively correlated (r = − 0.825) with each other.
Analysis of canal water
The correlation analysis for various variables was conducted using SPSS software, revealing the relationships between different parameters. Table 8 presents the Pearson correlation coefficients for other parameters of canal water.
The correlation matrix indicated several significant findings. Initial turbidity demonstrated a statistically significant (p = 0.002) strong positive correlation (r = 0.712) with the temperature of canal water. Furthermore, initial turbidity exhibited a strong negative correlation with E.C. (r = − 0.917) and TDS (r = − 0.945), both of which were statistically significant (p = 0.000). However, no significant correlation was observed between initial turbidity and pH.
Moreover, the polyelectrolyte dosage displayed a strong negative linear relationship with initial turbidity (r = − 0.910), which was statistically significant (p = 0.000). Additionally, the correlation matrix showed a strong positive linear relationship between the percentage removal of turbidity and initial turbidity (r = 0.690), which was statistically significant (p = 0.003). Furthermore, a negative correlation (r = − 0.584) was observed between the dosages of polyelectrolyte and the percentage of turbidity removal.
The correlation analysis across different surface water types revealed consistent trends. Initial turbidity showed a strong correlation with temperature across all surface water types, and the polyelectrolyte dosage exhibited a strong correlation with initial turbidity across all surface water types.
Conclusion
The results from the SPSS correlation analysis revealed a robust and interesting correlation between initial turbidity with removal percentage of turbidity, dosage of polyelectrolyte, and temperature. Specifically, a notable negative correlation was observed between the polymer dosage and initial turbidity in all kinds of surface water. However, a positive correlation could be concluded between initial turbidity and temperature as well as turbidity of raw water with a percentage of turbidity removal. Moreover, in pond and canal water, E.C. and TDS were found to be strongly correlated with each other, whereas they exhibited a moderate correlation in river water. Notably, the performance of lime in conjunction with polyelectrolyte was significantly more effective at high initial turbidity levels than at low turbidity levels. Adding PDADMAC alongside lime led to an enhancement in flocculation size, indicating improved treatment efficiency. Thus, the findings support the successful application of polyelectrolytes as a coagulant aid for turbidity removal in various surface water contexts. Generally, the fluctuating water turbidity levels at different stages further complicate the treatment process. However, the usage of lime in conjunction with PDADMAC has given remarkable results in this study, which can be recommended in the long run as a reference for further research in the field of water engineering. In addition, insights into the interaction of impurities with lime and polyelectrolyte were gained through XRD studies of sludge with lime and polyelectrolyte. Additionally, SEM analysis confirmed that treating sludge with lime and polyelectrolyte resulted in the formation of dense flocs, aiding in water purification via charge neutralization.
Recommendations
Overall results showed that adding a low dosage of lime significantly improved suspended particles and colloid removal by polyelectrolyte. The combined coagulant and coagulant aid removes the turbidity in different kinds of surface water efficiently. The study also showed that the specific dosage of PDADMAC is significantly low in all kinds of surface water when used as a coagulant aid. Pearson correlation helped to conclude a strong relationship between initial turbidity with a dosage of polymer and removal percentage. This correlation can be helpful in the long run for further research in water engineering.
Limitations and future work
This work is limited to chemical methods for removing turbidity from different kinds of surface water. The chemical method is a sustainable solution for different surface water (ponds, rivers, canals, etc.). In the future, novel polyelectrolytes can be synthesized and applied to the same water sources. Evaluation of the correlation of different parameters can be explored further on the same water samples by using discretization.
Data availability
Data will be made available on request.
References
Ahmed, M., Mumtaz, R., Baig, S., & Zaidi, S. M. H. (2022). Assessment of correlation amongst physico-chemical, topographical, geological, lithological and soil type parameters for measuring water quality of Rawal watershed using remote sensing. Water Supply, 22(4), 3645–3660.
Association, A. P. H. (1926). Standard Methods for The Examination of Water and Wastewater (Vol. 6). American Public Health Association.
Asthana, M., Kumar, A., & Sharma, B. S. (2017). Wastewater treatment. Principles and Applications of Environmental Biotechnology for A sustainable Future, 173–232.
Chekkala, A., Atasoy, M., Williams, C., & Cetecioglu, Z. (2023). Statistical analysis of SARS-CoV-2 using wastewater-based data of Stockholm, Sweden. International Journal of Environmental Research and Public Health, 20(5), 4181.
Chiavola, A., Di Marcantonio, C., D’Agostini, M., Leoni, S., & Lazzazzara, M. (2023). A combined experimental-modeling approach for turbidity removal optimization in a coagulation–flocculation unit of a drinking water treatment plant. Journal of Process Control, 130, 103068.
Dey, S., Botta, S., Kallam, R., Angadala, R., & Andugala, J. (2021). Seasonal variation in water quality parameters of Gudlavalleru Engineering College pond. Current Research in Green and Sustainable Chemistry, 4, 100058.
Frieder, C. A., Nam, S. H., Martz, T. R., & Levin, L. A. (2012). High temporal and spatial variability of dissolved oxygen and pH in a nearshore California kelp forest. Biogeosciences, 9(10), 3917–3930.
Galván-Ruiz, M., Hernández, J., Baños, L., Noriega-Montes, J., & Rodríguez-García, M. E. (2009). Characterization of calcium carbonate, calcium oxide, and calcium hydroxide as starting point to the improvement of lime for their use in construction. Journal of Materials in Civil Engineering, 21(11), 694–698.
Gautam, D. K. (2011). Effect of pollution on dissolved oxygen concentration in stream of Shivalik Himalayas: A case study. International Journal of Life Science & Pharma Research, 1(1), L-77–80.
Haghiri, S., Daghighi, A., & Moharramzadeh, S. (2018). Optimum coagulant forecasting by modeling jar test experiments using ANNs. Drinking Water Engineering and Science, 11(1), 1–8.
Hargreaves, J. A., & Tucker, C. S. (2002). Measuring Dissolved Oxygen Concentration in Aquaculture. Southern Regional Aquaculture Center Stoneville, MS, USA, SRAC Publication No. 4601.
Jabin, S., Gupta, P., & Sharma, M. (2021). Polyelectrolytes as a material of value in water treatment: A review. Asian Journal of Water, Environment and Pollution, 18(3), 109–115.
Jabin, S., & Kapoor, J. K. (2020). Role of polyelectrolytes in the treatment of water and wastewater BT - Sustainable green chemical processes and their allied applications. In Inamuddin & A. Asiri (Eds.), (pp. 289–309). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-030-42284-4_10
Jabin, S., Kapoor, J. K., Jadoun, S., Chandna, N., & Chauhan, N. P. S. (2023). Synthesis and characterization of polyamine-based polyelectrolytes for wastewater treatment in the sugar industry. Journal of Molecular Structure, 1275, 134573. https://doi.org/10.1016/J.MOLSTRUC.2022.134573
Kapoor, J. K., Jabin, S., & Bhatia, H. S. (2015). Optimization of coagulation-flocculation process for food industry waste water treatment using polyelectrolytes with inorganic coagulants. Journal of the Indian Chemical Society, 92, 1697–1703.
Li, H. Y., Xu, J., & Xu, R. Q. (2013). The effect of temperature on the water quality of lake. Advanced Materials Research, 821, 1001–1004.
Mehta, N. K., & Kumari, A. (2022). Study of water quality using of physico-chemical parameters of two perennial fish ponds of Darbhanga. International Journal of Fishries and Aquatic Studies, 10(3), 128–132.
Muthuraman, G., & Sasikala, S. (2014). Removal of turbidity from drinking water using natural coagulants. Journal of Industrial and Engineering Chemistry, 20(4), 1727–1731.
Nasrazadani, S., & Eureste, E. (2008). Application of FTIR for quantitative lime analysis. www.ntis.gov. Accessed 27 February 2024
Panda, P. K., Panda, R. B., & Dash, P. K. (2018). The study of water quality and pearson’s correlation coefficients among different physico-chemical parameters of River Salandi, Bhadrak, Odisha. India. American Journal of Water Resources, 6(4), 146–155.
Paul, M. J., Coffey, R., Stamp, J., & Johnson, T. (2019). A review of water quality responses to air temperature and precipitation changes 1: Flow, water temperature, saltwater intrusion. JAWRA Journal of the American Water Resources Association, 55(4), 824–843.
Piaskowski, K., Świderska-Dąbrowska, R., & Dąbrowski, T. (2023). Impact of cationic polyelectrolytes on activated sludge morphology and biological wastewater treatment in a Sequential Batch Reactor (SBR). Journal of Water Process Engineering, 52, 103500.
Pires, J. (2015). Simple analysis of historical lime mortars. Journal of Chemical Education, 92(3), 521–523. https://doi.org/10.1021/ed500336p
Roy, M., & Shamim, F. (2020). Research on the impact of industrial pollution on River Ganga: A Review. International Journal of Prevention and Control of Industrial Pollution, 6(1), 43–51.
Sahu, M., Shrivastava, A., Jhariya, D. C., Diwan, S., & Subhadarsini, J. (2024). Evaluation of correlation of physicochemical parameters and major ions present in groundwater of raipur using discretization. Measurement: Sensors, 34, 101278.
Sánchez-Martín, J., Ghebremichael, K., & Beltrán-Heredia, J. (2010). Comparison of single-step and two-step purified coagulants from Moringa oleifera seed for turbidity and DOC removal. Bioresource Technology, 101(15), 6259–6261.
Sarang, A., Parsa, S., Ahmadi, A., & Azarnivand, A. R. (2018). Analysis of the relationship between EC and TDS and their changes in the Karaj River. In 11th international Congress on Civil Engineering at: University of Tehran, Tehran, Iran (Vol. 7).
Shroff, P., Vashi, R. T., Champaneri, V. A., & Patel, K. K. (2015). Correlation study among water quality parameters of groundwater of Valsad district of south Gujarat (India). Journal of Fundamental and Applied Sciences, 7(3), 340–349.
Singh, K. P., Malik, A., & Sinha, S. (2005). Water quality assessment and apportionment of pollution sources of Gomti river (India) using multivariate statistical techniques—a case study. Analytica Chimica Acta, 538(1–2), 355–374.
Soros, A., Amburgey, J. E., Stauber, C. E., Sobsey, M. D., & Casanova, L. M. (2019). Turbidity reduction in drinking water by coagulation-flocculation with chitosan polymers. Journal of Water and Health, 17(2), 204–218.
Tyagi, C., & Sharma, A. (2016). Optimization of structural and dielectric properties of CdSe loaded poly (diallyl dimethyl ammonium chloride) polymer in a desired frequency and temperature window. Journal of Applied Physics, 119(1).
Voulvoulis, N. (2018). Water reuse from a circular economy perspective and potential risks from an unregulated approach. Current Opinion in Environmental Science & Health, 2, 32–45.
Wang, Y., Yang, J., & Chang, J. (2019). Development of a coupled quantity-quality-environment water allocation model applying the optimization-simulation method. Journal of Cleaner Production, 213, 944–955.
Yadav, S., & Goyal, V. C. (2022). Current status of ponds in India: A framework for restoration, policies and circular economy. Wetlands, 42(8), 107.
Acknowledgements
The authors thank the School of Engineering and Technology, Manav Rachna International Institute of Research & Studies, for providing lab facilities for practical work. The corresponding author, Sapana Jadoun, is grateful for the support of the National Research and Development Agency of Chile (ANID) for FONDECYT project 3200850 and ANID/FONDAP/1523A000, Solar Energy Research Center, SERC-Chile.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. S.J. performed lab work, data collection, and analysis. J.K.K. guided her during the work. A.C. and A.G. analyzed SPSS. S.J. wrote the first draft of the manuscript. S.J. reviewed, edited, and finalized the manuscript for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to participate
Consent was obtained from all participants included in the study.
Consent for publication
The author confirms that the work described has not been published before and is not under consideration for publication elsewhere. All co-authors have approved the work.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The submitted work is original and has not been published or submitted elsewhere in any form or language.
Highlights
• Studied physicochemical properties of surface water across seasons.
• Employed PDADMAC polyelectrolyte as a coagulation aid.
• Analyzed using XRD and SEM peaks to explore sludge-lime-polyelectrolyte interaction.
• Utilized SPSS for parameter analysis.
• Identified a significant correlation between polyelectrolyte dosage, initial turbidity, and turbidity removal percentage.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jabin, S., Kapoor, J.K., Chadha, A. et al. Assessment of poly(diallyl dimethyl ammonium chloride) and lime for surface water treatment (pond, river, and canal water): seasonal variations and correlation analyses. Environ Monit Assess 196, 874 (2024). https://doi.org/10.1007/s10661-024-13004-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-024-13004-3