Abstract
The use of suspensions of nanoparticles of titanium dioxide in photocatalytic degradation of dye solution has disadvantages of inconvenient separation of fine particles for reuse and limited penetration of light for effective degradation. These problems can be minimized by supporting titanium dioxide on various inert supports. The present study involves the preparation of immobilized titanium dioxide films by three different techniques and characterization of the prepared films. The immobilized films of nanocrystals of titanium dioxide were prepared using sol–gel technique, polyvinyl alcohol–formaldehyde binder and acrylic emulsion. The photocatalytic performance of the prepared films for degradation of amaranth dye has also been evaluated and compared. Combination of photodegradation and adsorption processes induces strong beneficial effects on removal of dyes. Addition of high adsorption capacity activated carbon to photoactive titanium dioxide in photodegradation of dyes improves the efficiency of dye mineralization. The activated carbon has also been immobilized along with titanium dioxide in the present work to examine the dual effect of photodegradation and adsorption in the removal of amaranth. The films formed with the help of polyvinyl alcohol–formaldehyde binder showed better dye degradation capabilities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The treatment of highly colored wastewater containing hazardous industrial chemicals and dyes is one of the growing needs of the present time. Dyes are an important class of synthetic organic compounds used in the textile industry and are therefore common industrial pollutants (Jain and Sikarwar 2008). Fifteen percent of the total world production of dyes is lost during the dyeing process and is released as textile effluent (Vautier et al. 2001). There is an increased need for treating the water polluted with dyes. Conventional wastewater treatment techniques like coagulation, adsorption and chemical oxidation processes do not lead to the total mineralization of hazardous dyes and have their own disadvantages (Houas et al. 2001). There are tonnes and tonnes of textile effluent being released every single day and the most effective treatment technique is yet to be grabbed. Photocatalysis has been successfully used to oxidize many organic pollutants and particularly to remove dyes. Some organic pollutants have been shown to be degraded and ultimately mineralized completely under UV irradiation on nanosized titania catalysts. Catalytic activity of nanocrystals of titanium dioxide is based on the electron/hole pair formation upon photo excitation (Hoffmann et al. 1995; Mills and LeHunte 1997; Fujishima et al. 2000; Pirkanniemi and Sillanpää 2002; Hashimoto et al. 2005; Vilhunen et al. (2009) Vilhunen and Sillanpää 2009). A great deal of research has been conducted recently to optimize the performance of the TiO2 photocatalysts with high surface area (Habibi et al. 2007). Three basic parameters which control the photocatalytic activity of a TiO2 are (a) light absorption properties of the material; (b) rate of reduction and oxidation of the molecule by photogenerated holes and electrons; (c) rate of electron–hole recombination (Yoshiya et al. 2002). Most of the literature studies related to photodegradation have been carried out using the suspension of nanocrystals of TiO2 in aqueous solution.
However, the use of aqueous suspension is limited for industrial applications due to the inconvenient and expensive separation of nanoparticles of titanium dioxide for reuse. Moreover, suspension of fine particles limits the penetration of light leading to reduced efficiency of photodegradation. Therefore, there is a need to immobilize the photocatalyst onto an appropriate inert support in an efficient way, which eliminates the need of filtration of catalyst.
In the present work, immobilized films of nano-crystals of TiO2 have been prepared using three different methods namely sol-gel technique, using polyvinyl alcohol—formaldehyde binder and using acrylic emulsion. The films obtained have been characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and UV–vis spectroscopy. The photocatalytic degradation of aqueous amaranth solution using the prepared films was also studied to evaluate the photocatalytic activity of the films. Other decontamination methods could be efficiently combined with TiO2 photocatalysis, thus increasing its efficiency (Tryba et al. 2003a). The simultaneous effect of TiO2 photocatalysis and activated carbon adsorption has shown enhanced capabilities for the removal of organic pollutants (Tryba et al. 2003b). The activated carbon acts as adsorbing agent while TiO2 mineralizes the adsorbed pollutants photocatalytically at the same time. To see the combined effect of adsorption and photodegradation, immobilized films containing both TiO2 and activated carbon were also prepared in the present work using the above-mentioned methods. The photocatalytic degradation of aqueous solution of amaranth using the prepared TiO2-activated carbon films was also studied.
Materials and methods
Materials
Titanium dioxide powder (nanocrystals) Degussa P25 (size of crystallites = 30 nm, surface area = 55 ± 15 m2/g) was procured from Degussa, Germany. Polyvinyl alcohol LR, formaldehyde LR, amaranth (C20H11N2O10S3Na3) dye powder, nitric acid (HNO3) LR and ethyl alcohol (C2H5OH) AR were obtained from s d Fine-Chem Ltd, Mumbai. Activated carbon was obtained from Merck (India) Ltd Mumbai. Titanium (IV) isopropoxide (TIPO) was obtained from Acros, USA and Cetyltrimethyl-ammonium bromide (CTAB) AR was purchased from HiMedia Laboratories Pvt. Ltd. Mumbai. Acrylic binder was obtained from Golden Chemical Works, Delhi. The chemicals in this study were used as purchased.
Preparation of sol–gel derived TiO2 films on glass and fabric support
The sol–gel technique was used to prepare sol–gel solution (Arabatzis et al. 2002; Su et al. 2004; Valtierra et al. 2006). The sol–gel film on the microscope glass slide (75 × 50 mm) was prepared by dipping and coating them in the sol–gel solution followed by heat treatment. The glass substrates were dipped in the sol–gel solution for 60 s and withdrawn at a constant rate of 1.25 mm/s. One side of the microscope slide was covered with removable tape to avoid the deposition on it. The coated sample was dried at room temperature of 30 °C (humidity 65 %) and then heated in a muffle furnace at 500 °C for 2 h. The dipping, coating and heating process was repeated three times to get the uniform and thick film. The film prepared by this method was given an identity GS1.
To see the additional effect of adsorption along with photodegradation, the sol–gel film was also deposited on cotton cloth (50 × 75 mm) by dipping the cotton cloth into the sol–gel solution followed by hydrothermal treatment as explained by Gupta et al. (2008). The cotton cloth was used to provide additional effect of adsorption as it was difficult to incorporate activated carbon during the sol–gel process. The prepared film was given an identity GS2.
Preparation of Degussa P25 films using polyvinyl alcohol–formaldehyde binder
TiO2 suspension was prepared by adding 1 g of TiO2 in 25 mL of double distilled water followed by continuous stirring for 1 h. Similarly, TiO2-activated carbon suspension was prepared by mixing 0.5 g of TiO2 and 0.5 g of activated carbon in 10 mL of double distilled water followed by stirring for 1 h to ensure homogeneity. A 6.25 % w/v polyvinyl alcohol–formaldehyde binder was prepared under constant stirring in a 70 °C water bath until transparent, sticky polymer glue was formed. The binder was kept in a sealed bottle to prevent it from rapid hardening. The fiberglass slide (75 × 50 mm) was first applied with a thin layer of binder on one side of the slide. The TiO2 suspension was then brushed onto the layer of binder to immobilize it. The film prepared by this method was given an identity FP1. The same procedure was carried out to immobilize TiO2 along with activated carbon on fiberglass sheet to examine the combined effect of adsorption and photodegradation. This film was given an identity FP2.
Preparation of Degussa P25 films using acrylic binder
The TiO2 film on microscope glass slide (75 × 50 mm) using acrylic binder was prepared according to the method explained by Noorjahan et al. (2003). 1 g of TiO2 was added in 25 mL of water followed by addition of 1 mL of acrylic emulsion under vigorous stirring for proper mixing. The film of this solution was prepared on the microscope glass slide and given an identity GA1. In a similar way, 0.5 g of TiO2 and 0.5 g of activated carbon were added in 25 mL of double distilled water followed by the addition of 1 mL of acrylic binder under vigorous stirring for proper mixing. The resultant emulsion was then applied over one side of the microscope glass slide (75 × 50 mm) with the help of a brush. The coated film was left for air-drying. Coating was repeated twice to get a uniform film without pin holes. This prepared film was given an identity GA2.
Characterization of prepared films
The SEM analysis of GS1, FP2 and GA2 films after being coated with gold was done with the help of scanning electron microscope JSM 6100 (JEOL) operated at 25 kV.
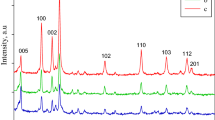
The phase composition of the GS1 film was studied by plate XRD technique (Černigoj 2007) to confirm the formation of nanocrystals of TiO2. The X-ray diffraction pattern was obtained on a Phillips PW-1710 X-ray diffractometer using Cu Kα radiation as X-ray source at an angle of 2θ ranging from 20 to 80°. The measurement was carried out at a scanning rate of 0.034 (2θ)/s. The strongest peak of TiO2 corresponding to anatase (1 0 1) was selected to evaluate the crystallinity of the samples. The mean crystallite size, L was determined from the broadening, β (full width at half maximum) of the most intense line in the X-ray diffraction pattern based on the Scherrer equation (Oskam et al. 2003; Keshmiri et al. 2004; Divya et al. 2009)
where, the Scherrer constant k was 0.9; wavelength of Cu Kα radiation λ was 1.5406 Å; θ is the Braggs diffraction angle. The FP1, FP2, GA1 and GA2 films were prepared using Degussa P25 titanium dioxide of known particle size (30 nm). Therefore, XRD analysis was not performed for these films.
UV-spectroscopy was used to record the transmittance spectra of GS1 film on HP 8453 UV–vis spectrophotometer in the wavelength range of 300–550 nm. A microscope glass slide without any film was used as a blank.
Photocatalytic activity test
All of the prepared films were used for photocatalytic degradation of amaranth dye. Photocatalytic degradation experiments were carried out in a photocatalytic chamber containing two 15W lamps (radiant flux of 16 mW cm2) as source of UV light to evaluate the photocatalytic performance of the sol–gel derived films. A dye solution of 50 mL with concentration of 10 ppm was poured into a beaker having cross-sectional area of 86.6 cm2. The slide carrying film of TiO2 was placed in the beaker in such a manner that the total available surface of photocatalyst was 50 × 75 mm. The beaker was then placed onto the working area of the photocatalytic reactor. A magnetic stirrer was used to provide mixing. Two UV light sources were then switched on and the solution was irradiated with the ultraviolet light. The concentration of the dye at different reaction times was determined by measuring the absorbance intensity at λmax = 520 nm with the help of the UV–Vis spectrophotometer. The decrease in concentration of the amaranth was plotted with respect to time for analysis. The experiment was repeated for all kind of prepared TiO2 films.
Kinetic modeling
It has been agreed that the expression for the rate of degradation of dyes with irradiated TiO2 follows the Langmuir–Hinshelwood (L–H) law of heterogeneous photocatalytic reactions (Fox and Dulay 1993; Kaur and Singh 2007; Kumar and Bansal 2010). According to L–H model, when initial concentration C 0 is very small, the following pseudo-first order rate equation is followed.
where k is pseudo-first order rate constant and C is the concentration at time t. A plot of ln (C/C 0) versus time represents a straight line, the slope of which upon linear regression equals the pseudo-first order rate constant k.
Results and discussion
Characterization of films
The SEM image of the surface of the sol–gel derived film at 5000× magnification was taken and is shown in Fig. 1a. It demonstrates the nanostructure of the film. Figure 2 depicts the XRD patterns of the sol–gel derived film GS1 with one major peak at 2θ = 25.32° which corresponds to (1 0 1) reflections of the anatase phase of TiO2. The anatase phase is supposed to be the most active one for photocatalytic reactions. Films were calcined at a temperature of 500 °C which was high enough for the formation of the active anatase phase. The use of surfactant also contributed to the formation of anatase phase. The crystallite size was determined from the (1 0 1) peak of anatase phase using the Scherrer equation. The β (full width at half maximum) obtained was 0.5018 and Braggs diffraction angle θ was 12.66°. The crystallite size was calculated using Scherrer equation and found to be 16 nm (nanosize).
The UV–vis transmittance spectrum of sol–gel derived film was measured and is represented in Fig. 3. It is obvious from the figure that the film absorbs UV radiations for activation of catalytic characteristics while almost all the visible radiations are transmitted.
The SEM images of the FP2 and GA2 films were also taken and shown in Fig. 1b, c, respectively. From the micrograph, it was observed that the TiO2 and activated carbon particles were present on the surface of the film and that they were not covered fully by the binder.
Photocatalytic activity
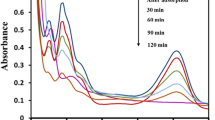
The photocatalytic degradation of amaranth was performed with films GS1, FP1 and GA1 separately for a period of 5 h in each case. The decrease in concentration of dye was recorded with respect to time and is shown in Fig. 4. The recorded data were best fitted by exponential equations with regression coefficient as high as 0.9996, 0.9904 and 0.9938 for GS1, FP1 and GA1 films, respectively. It was observed that there was 67.5 % decrease in the concentration of dye after 5 h of UV light illumination in case of GS1 film. A decrease of 83.2 and 45.2 % of dye concentration was monitored for the same period of illumination in case of FP1 and GA1 films, respectively. The value of half-life times (t 1/2) for the degradation of amaranth for GS1, FP1 and GA1 films were calculated and tabulated in Table 1.
It is obvious that the FP1 film formed by polyvinyl alcohol binder showed fastest photocatalytic degradation of amaranth among the prepared films followed by sol–gel derived film GS1. The film formed by acrylic binder depicted the slowest photocatalytic degradation among all the three types of films. The sol–gel film had considerably lower activity than film formed by using polyvinyl alcohol–formaldehyde binder. Besides the physical and chemical differences in TiO2 crystallites, one of the main reasons for the remarkable difference could be the solid–liquid interface between the TiO2 particles and the aqueous solution (Černigoj 2007). The polyvinyl alcohol–formaldehyde binder film contained slightly, physically adsorbed TiO2 aggregates on the support that is why this film was not dense and had a large contact area of TiO2. Moreover, the formaldehyde (a large portion of the binder) gets evaporated at room temperature providing more TiO2 surface area for degradation. Sol–gel film had a much denser structure, individual crystallites were connected and consequently the contact area between the TiO2 and the solution was lower (Černigoj 2007). Therefore, fewer holes and electrons reached the surface and the degradation rate was slower. The holes and electrons formed in the deeper layers of the sol–gel film were caught in the crystal lattice defects and they recombined before reaching the surface of the catalyst which lowered the photocatalytic activity of the sol–gel film. The acrylic binder film was less active because the layer of the acrylic emulsion partly covered the TiO2 particles and TiO2 was more firmly bounded to the surface resulting in less surface area available for degradation. The acrylic binder does not evaporate at room temperature.
In a similar way, the photocatalytic degradation of amaranth was also performed with films GS2, FP2 and GA2 separately for a period of 2.5 h in each case. The decrease in concentration of dye was plotted with respect to time for GS2, FP2 and GA2 films as shown in Fig. 5. The recorded data were best fitted by exponential equations; 67.8 % of the dye was photodegradad within 2.5 h of UV light illumination for GS2 film while 79.5 and 28.9 % of the dye was degraded in case of FP2 and GA2 films, respectively, for the same period. The half-life time for degradation of amaranth was calculated for GS2, FP2 and GA2 films from the respective exponential equations obtained in each case and tabulated in Table 1. It is obvious that these films gave comparatively better results than the GS1, FP1 and GA1 films due to the combined effect of adsorption and photodegradation. Activated carbon and cotton fabric acted as adsorbent for the dye molecules present in the solution. The adsorbed molecules on the surface of the activated carbon or cotton fabric then came in contact with TiO2 which caused photodegradation. TiO2-activated carbon films acted as semiconductor as well as adsorbent to give dual effect to the dye removal regardless of the characteristics of the dye. In the present case, the activated carbon adsorption worked well in combination with TiO2 photodegradation for the removal of dye. The dual effect was capable of increasing the efficiency of the whole degradation system. The film prepared using polyvinyl alcohol–formaldehyde binder (FP2) showed higher degradation rates than the film prepared by sol–gel method (GS2) and acrylic binder (GA2) due to the earlier mentioned reasons.
Kinetic modeling
The ln(C/C 0) values were calculated and plotted with respect to time for all the prepared films as shown in Figs. 6 and 7. The plotted data for all types of films were best fitted by straight lines of different slopes which indicate that the reactions followed pseudo-first order kinetics according to the L–H law. The pseudo-first order reaction rate constants were determined from the slopes of the plot ln(C/C 0) versus time for all the prepared films. Table 1 shows the values of pseudo-first order reaction rate constant (k) for GS1, FP1, GA1, GS2, FP2 and GA2 films.
Conclusion
The titania catalyst was immobilized on inert supports by three different methods, which eliminated the need of filtration of catalyst for further reuse. It is found that the TiO2 films formed with the help of polyvinyl alcohol–formaldehyde binder showed better results for photocatalytic degradation of amaranth solution than sol–gel derived and acrylic-binder films. The addition of activated carbon to the films enhanced the photocatalytic activity due to the combined effect of photodegradation and adsorption. The present results suggest that the polyvinyl alcohol–formaldehyde binder films are efficient for the treatment of aqueous solution of amaranth. The application of these films should be extended for treatment of industrial wastewater.
References
Arabatzis IM, Antonaraki S, Stergiopoulos T, Hiskia A, Papaconstantinou E, Bernard MC, Falaras P (2002) Preparation, characterization and photocatalytic activity of nanocrystalline thin film TiO2 catalysts towards 3, 5-dichlorophenol degradation. J Photochem Photobiol A 149:237–245
Černigoj U (2007) Photodegradation of organic pollutants in aqueous solutions catalyzed by immobilized titanium dioxide: Novel routs towards higher efficiency. PhD. Dissertation, University of Nova Gorica, Nova Gorica
Divya N, Bansal A, Jana AK (2009) Surface modification, characterization and photocatalytic performance of nano-sized titania modified with silver and bentonite clay. Bull Chem Reaction Eng Catal 4(2):43–53
Fox MA, Dulay MT (1993) Heterogeneous photocatalysis. Chem Rev 93:341–357
Fujishima A, Rao TN, Tryk DA (2000) Titanium dioxide photocatalysis. J Photochem Photobiol C 1:1–21
Gupta KK, Jassal M, Agrawal AK (2008) Sol–gel derived titanium dioxide finishing of cotton fabric for self cleaning. Indian J Fib Tex Res 33:443–450
Habibi MH, Esfahani MN, Egerton TA (2007) Photochemical characterization and photocatalytic properties of a nanaostructure composite TiO2 film. Int J Photoenergy 13653:1–8
Hashimoto K, Irie H, Fujishima A (2005) TiO2 photocatalysis: a historical overview and future prospects. Jpn J Appl Phys 44:8269–8285
Hoffmann MR, Martin SR, Choi W, Bahnemann DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95:69–96
Houas A, Lachheb H, Ksibi M, Elaloui E, Guillard C, Hermann JM (2001) Photocatalytic degradation pathway of methylene blue in water. Appl Catal B 31:145–157
Jain R, Sikarwar S (2008) Photodestruction and COD removal of toxic dye erioglaucine by TiO2-UV process: Influence of operational parameters. Int J Phy Sci 3(12):299–305
Kaur S, Singh V (2007) TiO2 mediated photocatalytic degradation studies of reactive red 198 by UV irradiation. J Haz Mat 141:230–236
Keshmiri M, Mohseni T, Troczynski T (2004) Development of novel TiO2 sol–gel derived composites and its photocatalytic activities for trichloroethylene oxidation. Appl Catal B 53:209–219
Kumar J, Bansal A (2010) Photocatalytic degradation of amaranth dye over immobilized nano-crystals of TiO2. In: International Conference on Energy and Environment, Cambridge, 129–133
Mills A, Le Hunte S (1997) An overview of semiconductor photocatalysis. J Photochem Photobiol A 108:1–35
Noorjahan M, Reddy MP, Kumari VD, Lavedrine B, Boule P, Subrahmanyam M (2003) Photocatalytic degradation of H-acid over a novel TiO2 thin film fixed bed reactor and in aqueous suspension. J Photochem Photobiol A 156:179–187
Oskam G, Nellore A, Lee P, Searson PC (2003) The growth kinetics of TiO2 nanoparticles from titanium (IV) alkoxide at high water/titanium ratio. J Phys Chem B 107:1734–1738
Pirkanniemi K, Sillanpää M (2002) Heterogeneous water phase catalysis as an environmental application: a review. Chemosphere 48:1047–1060
Su C, Hong BY, Tseng CM (2004) Preparation and characterization of thin film TiO2 dip coated on non-conductive substrate prepared from tetraethyl orthotitanate precursor. Catal Today 96:119–126
Tryba B, Morawski AW, Inagaki M (2003a) Application of TiO2-mounted activated carbon to the removal of phenol from water. Appl Catal B 18:281–291
Tryba B, Morawski AW, Inagaki M (2003b) A new route for preparation of TiO2-mounted activated carbon. Appl CatalB 46:203–208
Valtierra JM, Cardenas MS, Reyes CF, Calixto S (2006) Formation of smooth and rough TiO2 thin films on fiberglass by sol–gel method. J Mex Chem Soc 50(1):8–13
Vautier M, Guillard C, Herrman JM (2001) Photocatalytic degradation of dyes in water: case study of indigo and indigo carmine. J Catal 201(1):46–59
Vilhunen S, Sillanpää M (2009) Atomic layer deposited (ALD) TiO2 and TiO2-x-Nx thin film photocatalysts in salicylic acid decomposition. Wat Sci Technol 60:2471–2475
Vilhunen S, Bosund M, Kääriäinen ML (2009) Atomic layer deposited TiO2 films in photodegradation of aqueous salicylic acid. Sep Pur Technol 66:130–134
Yoshiya K, Shin-ya M, Hiroshi K, Bunsho O (2002) Design, preparation and characterization of highly active metal oxide photocatalysts. Springer, New York
Acknowledgments
The authors also wish to extend their sincere gratitude to all who assisted in promoting the present work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, J., Bansal, A. Photodegradation of amaranth in aqueous solution catalyzed by immobilized nanoparticles of titanium dioxide. Int. J. Environ. Sci. Technol. 9, 479–484 (2012). https://doi.org/10.1007/s13762-012-0064-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-012-0064-4