Abstract
Immobilization of photocatalyst particles on the surface of inert supporting materials is a technique that has the potential to improve the efficiency of photocatalytic processes. The five TiO2/activated carbon composites were synthesized from a titanium (III) sulfate onto the Norit SAE SUPER activated carbon. Three different ways were used for this: the sol–gel method through low-temperature hydrolysis, without calcination; the sol–gel method through low-temperature hydrolysis with fluorine doping in a molar Ti:F ratio which was 2:1, 7:1, and 15:1 but without further calcination; and homogeneous precipitation without hydrolysis and calcination. X-ray diffraction confirmed the exclusively amorphous phase formation during homogeneous precipitation without hydrolysis and calcination; a mixed structure of brookite, rutile, and amorphous TiO2 formation as a result of the low-temperature sol–gel method without calcination; and a pure anatase crystallization by the sol–gel method through low-temperature hydrolysis with fluorine doping without calcination. The adsorption removal degree of the orange-yellow S of an initial concentration of 225 mg∙L−1 by synthesized composites ranged from 40 to 90%, which is lower than that of the initial active carbon. The photocatalytic decomposition of this dye in the presence of synthesized composites was 32–65% at UV irradiation of 8 W, and 65–98% at UV irradiation of 24 W. The data obtained convincingly prove the high photocatalytic efficiency of the synthesized composites, which shows their perspective for practical application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photoelectrochemical properties and photoactivity of TiO2 and other metal oxides are determined mainly by two types of surface properties (Akakuru et al. 2020; Terescenco et al. 2019; Korde et al. 2023; Wang et al. 2023; Chen et al. 2021; Millán-Franco et al. 2023; Ren et al. 2023). The first is macroscopic properties, which are represented by a solid surface as a continuous medium. A typical characteristic that is associated with macroscopic properties is the electronic structure and Fermi energy (Akakuru et al. 2020; Terescenco et al. 2019; Korde et al. 2023; Wang et al. 2023). The second is local microscopic properties, which are presented in specific places on the surface, such as surface-active centers, which are formed due to defects on the surface. The disorder of surface layer and bulk phase defects might be completely different, which leads to the formation of a potential barrier (Chen et al. 2021; Millán-Franco et al. 2023; Ren et al. 2023). The movement of charges across the interface between the electrode and the electrolyte is determined by the distributed electric potential. The potential barrier includes a barrier through the Helmholtz layer, which is formed in the layer of liquid adjacent to the interphase of the solid phase and the liquid; and the Goya layer, which is located between the Helmholtz layer and the volume of the liquid (Lei et al. 2022; Franz and Bestetti 2023; Surovčík et al. 2022; Mingmuang et al. 2022; Rao et al. 2023). The last one is the reason solar energy is absorbed mainly not by the bulk phase of the particle, but by the surface layer with a thickness of 1 μm. The use of composite photocatalytic active materials on the base of the porous matrix is capable to overcome those obstacles.
Due to their small size, dispersed TiO2 particles tend to rapid aggregation processes, and as a result of that, the effective surface area and catalytic activity of the photocatalyst are significantly reduced. Taking into account the crystal structure and non-porous surface of the particles, titanium (IV) oxide shows generally low adsorption activity, especially toward organic molecules; as a result, the molecules in the system compete for the pre-limited adsorption centers of the photocatalyst. Moreover, there is a tendency to form intermediates and degradation products in the process of photocatalysis, which can be potentially more toxic, even in comparison with the initial compounds of the photocatalytic process (Wang et al. 2020; Wei et al. 2023; Zhang et al. 2021).
Immobilization of photocatalyst particles on the surface of inert supporting materials is a technique that has the potential to improve the efficiency of photocatalytic processes. A fundamental concept of this approach is that inert supporting materials are able to adsorb target pollutant molecules, which are generally difficult to adsorb onto the surface of photocatalyst particles. A zone of increased concentration of pollutants is formed at a relatively short distance from the photocatalytic active center. After the adsorption of the pollutant molecule, as a result of the phenomenon of surface diffusion, the migration of molecules straight to the photocatalytic center is possible (Lei and Robertson 2023; Tian et al. 2023; Davarikia et al. 2022).
Carbon materials (Ali et al. 2022; Batista et al. 2022; Ye et al. 2022; Li et al. 2023; Escamilla-Mejía et al. 2023; Ivanenko et al. 2019; Kukh et al. 2018), polymers (Gonzalez et al. 2018; Kusworo et al. 2023), clays (Cardona et al. 2023; Dlamini et al. 2021; Rind et al. 2023; Justin et al. 2023), silica gel (Zandonà et al. 2022; Dawngliana et al. 2023; Ma et al. 2023; Rincón and Motta 2019), etc. can be used as inert supporting materials for TiO2-based photocatalysts. The combination of polymeric materials with TiO2 has significant potential for use, but at the same time, there are many limitations, including the tendency to destroy the inert supporting material in the process of photocatalysis (Gonzalez et al. 2018). A variety of carbon materials are the most widely used, among them: graphene (Ali et al. 2022), fullerenes (Guo et al. 2018; Gakhar et al. 2022), carbon nanotubes (Ivanenko et al. 2019; Kukh et al. 2020), and others. But activated carbon is the biggest group of supporting materials, due to exceptional adsorption properties, highly developed surface, ability to adsorb various types of toxic pollutants, and the ability to modify. The combination of activated carbon and titanium (IV) oxide shows a synergistic effect, which leads to an overall increase in the photocatalytic process efficiency; even the physical mixture of activated carbon and TiO2 demonstrates the synergistic effect of the components. From this point of view, the synthesis of TiO2 on an extensive porous surface of active carbon with a resulting composite obtaining and the study of its adsorption capacity and photocatalytic activity are very relevant. Therefore, the aim of the presented paper was the synthesis and investigation of a new composite adsorbent-photocatalyst titanium oxide on the surface of activated carbon.
Experimental part
Composites synthesis
A titanium (III) sulfate solution (15% mass. in sulfuric acid) was used as a TiO2 precursor and powdered activated carbon Norit© SAE Super was used as an inert porous supporting material. For composites synthesis by low-temperature sol–gel method, pH of the precursor solution was adjusted to 1.2 and a sample of activated carbon, theoretically calculated to obtain a 10% TiO2 content in the composite, was added with constant stirring. After its complete wetting and distribution in the volume, a control pH measurement was performed and it was adjusted in case of deviation from the value of 1.2. The resulting suspension of activated carbon in the precursor solution was heated to a temperature of 50 °C in a water bath and kept for 30 min for hydrolysis. Then, it was left for aging for 7 days at room temperature, and the obtained precipitate was washed on a vacuum filter until the reaction to sulfate ions disappeared. The separated wet solid phase of the composite was dried at 50 °C in the air without further calcination (Kukh et al. 2018). Thus, the TiO2(lth)/AC composite was synthesized.
Chemical transformations occurring during the sol–gel process can be described by the following sequence of chemical equations. As a result of the course of hydrolysis, titanium (III) hydroxide is formed:
The formed titanium (III) hydroxide is unstable and at the stage of aging, under the influence of oxygen, it quickly changes into the form of hydrated titanium (IV) oxide of variable composition:
Further drying of hydrated titanium (IV) oxide leads to the removal of water.
In order to synthesize the TiO2/activated carbon composites doped with fluor in different amounts, a calculated portion of KF dopant was added simultaneously with activated carbon to the precursor solution before the hydrolysis stage. Similar actions were performed further with the resulting suspension as it is described for TiO2(lth)/AC composite. Thus, the molar ratio of Ti to F in the TiO2(2F)/AC composite was 2:1; in the TiO2(7F)/AC composite, it was 7:1; and in the TiO2(15F)/AC composite, it was 15:1.
While adding a KF dopant at the aging stage, modification of TiO2 with fluorine occurs:
The TiO2(pr)/AC composite was synthesized by the direct precipitation of the precursor solution onto the active carbon surface without the hydrolysis stage. For this, the resulting suspension was immediately precipitated by dropwise addition of NH4OH solution to pH 9,4 with constant stirring and left for aging for 1 h. The formed precipitate was washed on a vacuum filter until the reaction with sulfate ions disappeared and dried at 50 °C in the air.
Measurement procedures
XRD analysis was performed using a diffractometer Ultima-IV, Rigaku (Japan), equipped with a high-intensity emitter operating in the range of 20–60 kV and 2–60 mA. The scan was performed in a wide range of Bragg angles (-3° < 2θ < 162°). The obtained XRD diffraction patterns were analyzed using standard ICDD files. Quantitative analysis of the phase composition was performed with the help of a PDF-2 database and software. The regions of coherent scattering were calculated from the extensions of the radiograph lines according to the Debye–Scherrer equation. The size of composite crystallites was calculated by the Scherrer formula.
Scanning electron microscopy (SEM) was performed on a SELMI REM-106I at the accelerating voltage of 10 keV.
The porous structure of synthesized samples was studied by the method of low-temperature adsorption–desorption of nitrogen on a Quatachrome Nova 1000e apparatus. The distribution of pore radius was calculated by the method of Density Functional Theory (DFT method). BET specific surface area (S, m2·g−1), total pore volume for pores with a radius less than 185 nm at P/Po = 0.99 (VΣ, mL·g−1), mesopores volume (Vmeso, mL·g−1), micropores volume (Vmicro, mL·g−1), average pores radius (r, nm) were calculated by mathematical processing of obtained nitrogen adsorption–desorption isotherms.
A study of the photocatalytic activity of synthesized composites was performed using a solution of orange-yellow S and UV irradiation of wavelength 254 nm and two different powers, 8 and 24 W for 5 min. Investigation of the adsorption capacity of synthesized composites was performed using a solution of orange-yellow S for 15 min. The volume of dye constituted 25 mL; an initial concentration was 225 mg∙L−1 and was investigated by the photoelectro-colorimetric method. Each experiment was carried out at least three times and the measurements were averaged. The removal degree (X, %) was calculated as X = ((co-ct)/c0)·100, where co – initial dye concentration; ct – current dye concentration; m – composite mass; V – solution volume.
Results and discussion
SEM images of the composites, which are shown in Fig. 1, indicated that TiO2 particles were deposited on the surface of activated carbon either in the form of uneven flat flakes or rounded uniform particles with a tendency to agglomerate. The size of TiO2 particles in composites ranged from several tens to several hundreds of nanometers. Particles of TiO2 had different shapes and sizes and were attached to the activated carbon surface individually, without strong adhesion in the composites obtained by the sol–gel method (see Fig. 1a–h). A strong agglomeration of uniform spherical TiO2 particles of bigger size was observed in the composite synthesized by the precipitation method (see Fig. 1i, j).
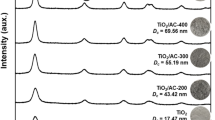
The results XRD analysis of synthesized composites, which are shown in Fig. 2, made it possible to establish differences in their phase composition. The phase composition of TiO2 in the TiO2(lth)/AC composite was represented by a mixture of brookite, rutile, and amorphous phase, with a significant predominance of the latter. The size of the TiO2(lth)/AC sample crystallites was 10,6 nm (Fig. 2a). In the case of composites doped with fluorine in various ratios, the formation of a pure anatase phase was observed (Fig. 2b–d) and there were no other peaks that could be associated with the presence of other crystalline phases, in particular rutile. The size of TiO2 crystallites in doped with fluorine composites was in the range 18–26 nm and increased with the Ti:F ratio increasing. TiO2 in the TiO2(pr)/AC composite was represented only by an amorphous phase and did not show any other TiO2 crystalline phases, as can be seen from Fig. 2e.
From our point of view, the differences in the phase composition of the synthesized samples are explained by the differences in their synthesis. The fluorine ions adding in the process of obtaining of TiO2(2F)/AC, TiO2(7F)/AC, TiO2(15F)/AC composites shifts the equilibrium in the system toward the formation of the anatase crystalline phase (see Fig. 2b–d). The presence of fluorine ions allows to obtain the crystalline phase of pure anatase by sol–gel method without calcination. The shift of the peaks in relation to the data in the standard ICDD card (25.28°) with increasing angles indicates the presence of fluoride ions in the TiO2 crystal lattice.
Direct precipitation without aging and heat treatment in the TiO2(pr)/AC sample expectedly led to the formation of a TiO2 amorphous phase (see Fig. 2e). At the initial stage, the significant availability of water molecules in the solution provided sufficient conditions for the hydrolysis of the precursor titanium (III) sulfate. A greater contribution to the formation of TiO2 was made by the exchange reaction with ammonium hydroxide solution:
It is known that titanium (III) hydroxide (Ti(OH)3) is unstable and oxidizes rapidly in oxygen presence. This process can be described by the following equation very approximately:
While deposition by ammonium hydroxide solution structures of variable composition, so-called hydrated TiO2 formed, which can be presented by the general formula: TiO(2-x)(OH)2x∙yH2O, where y is usually close to 1. It is known that in such polyionic structures (hydrated amorphous titanium (IV) oxides), water molecules are located on the surface of the structure, while OH− groups bind to titanium ions. In addition, some part of these groups in the polyions can be replaced by structural groups, forming a polymerization product in the form of chains of different lengths, which include groups OH−, HSO4− and/or SO42− and water.
The porous structure of the initial active carbon and the obtained composites was investigated by the low-temperature nitrogen adsorption–desorption method and it was found that they were mixed porous adsorbents with a fairly developed surface area, an average of about 1100 m2∙g−1 and a branched system of micro- and mesopores. Nitrogen adsorbed volumes achieved by composites were lower than that of the initial active carbon by an average of 150–180 mL·g−1; as a result, their specific surface area was also reduced by 235–380 m2·g−1. The greatest loss of surface and porosity occurred in the TiO2(7F)/AC composite, as can be seen in Table 1. The pore radius distribution was also changed as a result of the doping. In fluorine-doped composites total porosity decreased by 25–45%, the volume of micropores decreased by 17–23%, and mesoporosity decreased by 33–50% (see Table 1). Such a reduction of mesopores amount occurred due to the fact that the TiO2 particles formed in the process of doping were deposited on the inner surface of the transition and the micropores of activated carbon, thereby taking up some of their internal volume, in our opinion. The average pore size of the composites was half of the initial activated carbon, which also indicated the localization of the TiO2 particles on the inner surface of its transition and micropores.
In order to evaluate the adsorption activity of the synthesized samples of composites based on TiO2 and activated carbon, a model solution of orange-yellow S dye with an initial concentration of 225 mg∙L−1 was used. The results of the determination of the dependence of the residual concentration and the degree of adsorption removal of dye on the mass of the applied adsorbent showed that the mass of 0.04 g was optimal for most composites and it was used in subsequent investigations.
The results of the study of the obtained composites adsorption activity in the orange-yellow S dye adsorption, which is given in Fig. 3, show that all composites had some activity in the adsorption of this dye with quite high concentration, and their efficiency ranged from 40 to 90%. The highest adsorption removal degree was demonstrated by the TiO2(15F)/AC composite synthesized by the sol–gel method through low-temperature hydrolysis with fluorine doping in a molar Ti:F ratio of 1:15 without calcination, and the lowest one by the TiO2(2F)/AC composite. The adsorption degree of dye by composites did not exceed the degree reached by the initial activated carbon. The industrial photocatalyst Evonik AEROXIDE® TiO2 P25 showed a very low adsorption capacity expectedly.
Investigation of the photocatalytic activity of synthesized composites was performed using a solution of orange-yellow S with an initial concentration of 225 mg∙L−1 and UV irradiation of wavelength 254 nm and two different powers, 8 and 24 W for 5 min. The results of this investigation in Fig. 4 show that the photocatalytic decomposition of dye using composite photocatalysts at a UV irradiation of 8 W was quite high and ranged from 32 to 65%. While increasing the UV irradiation intensity to 24 W, there was a significant rise of the dye decomposition degree for all samples to 65–98%. The largest increase in the degree of dye decomposition was observed for TiO2(2F)/AC, and TiO2(lth)/AC composites, which was 55% and 45%, respectively. The composites synthesized by the low-temperature sol–gel method without calcination and by homogeneous precipitation without hydrolysis and calcination demonstrated the highest photocatalytic activity, 95 and 98%, respectively, at UV irradiation of 24 W. Evonik AEROXIDE TiO2 P25 demonstrated extremely low photocatalytic activity at both investigated irradiation intensities.
Almost complete removal of dye with a high concentration in 5 min of the experiment occurs as a result of a sum of the adsorption and photocatalysis effect in our opinion. The process of photocatalytic degradation is one of the variants of the heterogeneous catalytic process, the initial stage of which is the adsorption of molecules on the active centers of the catalyst. Due to the developed surface and adsorption capacity of activated carbon, a zone of increased concentration of dye molecules is formed around the photocatalytic center, i.e. TiO2, which cannot adsorb them on its own. In addition, the high porosity of activated carbon prevents diffusion back into the solution of semi-products, as well as free radicals. Thus, the number of elementary acts of the photocatalytic process increases, the intensity and depth of dye destruction increases. On the other hand, the depletion of the adsorption capacity of activated carbon occurs very slowly or does not occur at all due to the immobilization of TiO2 on the surface of the adsorbent and the course of the photocatalytic reaction, since pollutants are not only adsorbed on the surface but also undergo mineralization. TiO2 particles deposited in the pores of active carbon also serve as additional adsorption centers, the presence of which ensures the retention of free radicals and half-life products from drift in the solution. All this demonstrates and explains the synergistic effect of TiO2 photocatalyst and active carbon supporting material in our opinion.
Conclusions
The five composites of titanium oxide and Norit SAE SUPER activated carbon have been synthesized and three different ways were used for this: the sol–gel method through low-temperature hydrolysis, without calcination; the sol–gel method through low-temperature hydrolysis with fluorine doping in a molar Ti:F ratio which was 2:1, 7:1, and 15:1 but without further calcination; and homogeneous precipitation without hydrolysis and calcination.
XR diffraction showed that titanium oxide in the composite synthesized by the low-temperature sol–gel method without calcination consisted of a mixture of brookite, rutile, and amorphous phase with a significant predominance of the latter. In the composite obtained by the sol–gel method through low-temperature hydrolysis with fluorine doping, but without calcination, titanium oxide crystallized in pure anatase modification with fluoride ions in the TiO2 crystal lattice. The composite synthesized by homogeneous precipitation without hydrolysis and calcination contained only the amorphous phase of titanium oxide.
The initial activated carbon used as a porous supporting material lost its internal surface area by 10–30% and total porosity by 20–40% due to the deposition of titanium oxide on its surface. Despite this, all synthesized composites had a developed surface area (on average ~ 1100 m2∙g−1) and wide total porosity (on average 0.93 mL·g−1).
All synthesized composites were less active adsorbents than the initial activated carbon toward the orange-yellow S dye. The photocatalytic activity of obtained composites depended very strongly, but disproportionately and nonlinearly, on the intensity of ultraviolet irradiation, and it was higher than that of the well-known industrial photocatalyst EVONIK AEROXIDE TiO2 P25 in all experiments. The photocatalytic decomposition of the orange-yellow S dye by composite photocatalysts at UV irradiation of 8 W was in the range of 32–65% and rose in 10–55% while increasing the irradiation intensity to 24 W.
The results obtained convincingly prove the high photocatalytic efficiency of the synthesized composites, which shows their perspective for practical application.
References
Akakuru OU, Iqbal ZM, Wu A (2020) TiO2 Nanoparticles: Properties and Applications. In: TiO2 Nanoparticles. https://doi.org/10.1002/9783527825431.ch1
Ali A, Shoeb M, Li B, Khan MA (2022) Photocatalytic degradation of antibiotic drug and dye pollutants under visible-light irradiation by reduced graphene oxide decorated MoO3/TiO2 nanocomposite. Mater Sci Semicond. https://doi.org/10.1016/j.mssp.2022.106974
Batista CC, Cunha R, Santos AC, Reis PM, at all, (2022) Synthesis of a reusable magnetic photocatalyst based on carbon xerogel/TiO2 composites and its application on acetaminophen degradation. Ceram Int. https://doi.org/10.1016/j.ceramint.2022.08.018
Cardona Y, Węgrzyn A, Miśkowiec P, Korili SA, Gil A (2023) Heterogeneous Fenton- and photo-Fenton-like catalytic degradation of emerging pollutants using Fe2O3/TiO2/pillared clays synthesized from aluminum industrial wastes. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2023.103494
Chen Q, Wang K, Gao G, Ren J, Duan R, Fang Y, Xun HuX (2021) Singlet oxygen generation boosted by Ag–Pt nanoalloy combined with disordered surface layer over TiO2 nanosheet for improving the photocatalytic activity. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2020.147944
Davarikia Y, Aroujalian A, Salimi P (2022) Immobilization of TiO2 nanoparticles on PES substrate via dopamine and poly (vinyl alcohol) for long-term oil/water purification. Process Saf Environ Prot. https://doi.org/10.1016/j.psep.2022.08.067
Dawngliana KMS, Fanai AL, Rai S (2023) Structural and optical studies of Sm3+-doped silica glass along with TiO2 nanoparticles for photonic applications. J Non Cryst Solids. https://doi.org/10.1016/j.jnoncrysol.2023.122226
Dlamini MC, Maubane-Nkadimeng MS, Moma JA (2021) The use of TiO2/clay heterostructures in the photocatalytic remediation of water containing organic pollutants: a review. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.106546
Escamilla-Mejía JC, Hidalgo-Carrillo J, Martín-Gómez J, López-Tenllado FJ, Estévez-Toledano RC, Marinas A, Urbano FJ (2023) Pt Preferential incorporation onto TiO2 in TiO2-carbon composites for hydrogen production from glycerol photoreforming. Catal Today. https://doi.org/10.1016/j.cattod.2022.11.004
Franz S, Bestetti M (2023) 5 - Kinetic models in photoelectrocatalysis. In: Palmisano L, Yurdakal S (ed) Photoelectrocatalysis, Elsevier. https://doi.org/10.1016/B978-0-12-823989-6.00009-6
Gakhar T, Rosenwaks Y, Hazra F (2022) Fullerene (C60) functionalized TiO2 nanotubes for conductometric sensing of formaldehyde. Sens. Actuators B Chem. https://doi.org/10.1016/j.snb.2022.131892
Gonzalez ES, Olmos D, Lorente MA, Velaz I, Gonzalez-Benito J (2018) Preparation and characterization of polymer composite materials based on PLA/TiO2 for antibacterial packaging. Polymers. https://doi.org/10.3390/polym10121365
Guo X, Zhang B, Lin Z et al (2018) Interface engineering of TiO2/perovskite interface via fullerene derivatives for high performance planar perovskite solar cells. Org Electron. https://doi.org/10.1016/j.orgel.2018.08.039
Ivanenko I, Voronova A, Astrelin I, Romanenko Y (2019) Structural and catalytic properties of Ni–Co spinel and its composites. Bull Mater Scie. https://doi.org/10.1007/s12034-019-1854-9
Justin BTD, Blaise N, Valery HG (2023) Investigation of the photoactivation effect of TiO2 onto carbon-clay paste electrode by cyclic voltammetry analysis. Heliyon. https://doi.org/10.1016/j.heliyon.2023.e13474
Korde SA, Thombre PB, Dipake SS, Sangshetti JN, Rajbhoj AS, Gaikwad ST (2023) Neem Gum (Azadirachta indicia) facilitated green synthesis of TiO2 and ZrO2 nanoparticles as antimicrobial agents. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2023.110777
Kukh AA, Ivanenko IM, Astrelin IM (2018) TiO2 and its composites as effective photocatalyst for glucose degradation processes. Appl Nanoscie. https://doi.org/10.1007/s13204-018-0691-2
Kukh A, Ivanenko I, Asterlin I (2020) Composite titanium dioxide photocatalytically active materials: review. In: O. Fesenko, L. Yatsenko (ed) Nanooptics and Photonics, Nanochemistry and Nanobiotechnology, and Their Applications Cham, Switzerland: Springer proceedings in physic. https://doi.org/10.1007/978-3-030-52268-1_28
Kusworo TD, Yulfarida M, Kumoro AC, Utomo DP (2023) Purification of bioethanol fermentation broth using hydrophilic PVA crosslinked PVDF-GO/TiO2 membrane. Chin J Chem Eng. https://doi.org/10.1016/j.cjche.2022.04.028
Lei B, Robertson N (2023) TiO2 mesocrystals: Immobilisation, surface fluorination and application in photocatalytic water treatment. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2023.156487
Lei L, Sang L, Gao Y (2022) Pulse electrodeposition of Ag, Cu nanoparticles on TiO2 nanoring/nanotube arrays for enhanced photoelectrochemical water splitting. Adv Powder Technol. https://doi.org/10.1016/j.apt.2022.103511
Li Y, Liu S, Lu X, Zhao H, Cui J, Zhang Y, He W (2023) Hydrothermal synthesis of carbon-coated mixed crystalline phase TiO2 nanoparticle carbon microsphere composites as high performance anode materials for Li-ion batteries. Diam Relat Mater. https://doi.org/10.1016/j.diamond.2023.109913
Ma H, Liu H, Xu Y, Chang Y, Zhou X (2023) Energy aggregation properties of TiO2-silica composite aerogel under ultra-high-energy (7 kW·cm-2) continuous-wave laser irradiation. Ceram Int. https://doi.org/10.1016/j.ceramint.2023.03.247
Millán-Franco MA, Rodríguez-Castañeda CA, Moreno-Romero PM, Prias-Barragán JJ, Jaramillo-Quintero OA, Hu H (2023) A direct correlation between structural and morphological defects of TiO2 thin films on FTO substrates and photovoltaic performance of planar perovskite solar cells. Mater Sci Semicond. https://doi.org/10.1016/j.mssp.2023.107452
Mingmuang Y, Chanlek N, Moontragoon P, Srepusharawoot P, Thongbai P (2022) Effects of Sn4+ and Ta5+ dopant concentration on dielectric and electrical properties of TiO2: Internal barrier layer capacitor effect. Results Phys. https://doi.org/10.1016/j.rinp.2022.106029
Rao VN, Sairam PK, Kim MD et al (2023) CdS/TiO2 nano hybrid heterostructured materials for superior hydrogen production and gas sensor application. J Environ Manag. https://doi.org/10.1016/j.jenvman.2023.117895
Ren L, Ma S, Shi Y, Zhao C, Wang XL, Gao Z, Xie H (2023) Insights into the pivotal role of surface defects on anatase TiO2 nanosheets with exposed 001 facets for enhanced photocatalytic activity. Mater Res Bull. https://doi.org/10.1016/j.materresbull.2023.112255
Rincón GJ, La Motta EJ (2019) A fluidized-bed reactor for the photocatalytic mineralization of phenol on TiO2-coated silica gel. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e01966
Rind IK, Tuzen M, Sarı A, Lanjwani MF, Memon N, Saleh TA (2023) Synthesis of TiO2 nanoparticles loaded on magnetite nanoparticles modified kaolinite clay (KC) and their efficiency for As(III) adsorption. Chem Eng Res Des. https://doi.org/10.1016/j.cherd.2023.01.046
Surovčík J, Medvecká V, Greguš J, Gregor M, Roch T, Annušová A, Ďurina P, Vojteková T (2022) Characterization of TiO2 nanofibers with enhanced photocatalytic properties prepared by plasma assisted calcination. Ceram Int. https://doi.org/10.1016/j.ceramint.2022.08.309
Terescenco D, Hucher N, Savary G, Picard C (2019) From interface towards organised network: Questioning the role of the droplets arrangements in macroscopically stable O/W emulsions composed of a conventional non-ionic surfactant, TiO2 particles, or their mixture. Physicochem Eng Asp, Colloids Surf A. https://doi.org/10.1016/j.colsurfa.2019.123630
Tian Z, Song Y, Tao K, Liu N, Qin S, Yang J, Li J, Cui Z (2023) Preparation of TiO2-Ag heterostructure via tannic acid-assistance and its immobilization on PVDF membrane for the degradation of dye under visible light. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2023.157195
Wang M, Jin C, Luo Q, Kim EJ (2020) Sol-gel derived TiO2–carbon composites with adsorption-enhanced photocatalytic activity and gas sensing performance. Ceram Int. https://doi.org/10.1016/j.ceramint.2020.04.171
Wang L, Qiu J, Wu N, Yu X, An X (2023) TiO2/ CsPbBr 3 S-scheme heterojunctions with highly improved CO2 photoreduction activity through facet-induced Fermi level modulation. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2022.08.120
Wei P, Zhang Y, Huang Y, Chen L (2023) Structural design of SiO2/TiO2 materials and their adsorption-photocatalytic activities and mechanism of treating cyanide wastewater. J Mol Liq. https://doi.org/10.1016/j.molliq.2023.121519
Ye X, Li Z, Sun H, Wu M, An Z, Pang Y, Yang J, Zheng S (2022) Incorporating TiO2 nanoparticles into the multichannels of electrospun carbon fibers to increase the adsorption of polysulfides in room temperature sodium-sulfur batteries. New Carbon Mater. https://doi.org/10.1016/S1872-5805(22)60607-3
Zandonà A, Chesneau E, Helsch G, Canizarès A, Deubener J, Montouillout V, Fayon F, Allix M (2022) Glass-forming ability and structural features of melt-quenched and gel-derived SiO2-TiO2 glasses. J Non Cryst Solids. https://doi.org/10.1016/j.jnoncrysol.2022.121967
Zhang Y, Xiong M, Sun A, Shi Z, Zhu B, Macharia DK, Li F, Chen Z, Liu J, Zhang L (2021) MIL-101(Fe) nanodot-induced improvement of adsorption and photocatalytic activity of carbon fiber/TiO2-based weavable photocatalyst for removing pharmaceutical pollutants. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.125782
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ivanenko, I., Kukh, A., Fedenko, Y. et al. Adsorptive-photocatalytic removal of orange–yellow dye with titanium oxide–activated carbon composites. Appl Nanosci 13, 7135–7143 (2023). https://doi.org/10.1007/s13204-023-02867-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-023-02867-6