Abstract
Salinisation and contamination by trace elements are expected to be some of the most critical environmental and sustainability issues in the forthcoming era of global warming and human population growth. Agro-ecosystems could be increasingly influenced by salinity, given that exploitation of saline pedo/hydroresources (>20 mM in water or soil saturation extract) will have to increase across many irrigated as well as rain-fed areas, despite poor yield and low food stuff quality. Using experimental and computational (modelling) approaches it has been well established that one of crucial plant responses under salinity is increased Cd phytoextraction. In salt-affected rhizosphere environments excessive concentrations of dissolved ions can impact Cd biogeochemistry through complexation and/or competition reactions with inorganic and organic ligands. For ecologically relevant conditions (e.g. Cd-contaminated and organically-depleted salinised soil) it was estimated that under low salinity (<30 mM) free Cd2+ ion predominates across a wide range of pHs (3.5–9), whereas in moderate-to-strong salinity (135–270 mM) Cd-chloro/sulphate complexes prevail (mostly comprising Cd-Cls). Although Cd-Cl interactions are still under intensive investigation and not fully understood, complexation is likely to be one of the main mechanisms for increasing Cd transfer to plants from the salt-affected rhizosphere. Also, there is evidence that Cd uptake by plants is underpinned by additive effects of salt and Cd stresses. Great efforts have been made in elucidating Cd biochemistry after uptake, (re)translocation and its deposition in plants differing in salt/metal tolerance; it is highly likely that the role of Cd-Cl complexation in plants is of negligible importance vs. that in the rhizosphere. Sharing similar or the same routes for crossing plant membranes with essential elements (Zn, Cu, Fe, Ca), Cd-organo complexes (with S, O and N radicals) relatively easily reach transpirational tissues via the xylem, and thereafter the depositing tissues dominantly via the phloem. Genetic engineering is a promising strategy in (1) increasing plant resistance to excessive soil salinity, and (2) producing genotypes for enhanced phytoremediation of areas overloaded by metals. However, under ecologically-relevant conditions (e.g. rhizosphere soil with poor metal-buffering capacity) Cd soil-plant transfer can be enhanced by soil salinity, simultaneously interfering with nutrient (Cu, Zn, Fe) extraction. So, if salinity resistance (as a multi-gene trait) is closely associated with gene loci responsible for Cd extraction, care needs to be taken that genetically improved (e.g. salt-resistant) genotypes do not impair crops foodstuff safety/security via increased Cd accumulation and/or micronutrient deficiency.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Salinity is the most widespread abiotic constraint to higher plants, affecting metabolism of cultured and/or native vegetation on ∼ 1 billion hectares of Earth’s surface. Naturally- (sea water intrusion, groundwater fluctuation) and/or anthropogenically induced (unsustainable agricultural management practices, excessive groundwater withdrawal) environmental salinisation processes in the era of climate change and global warming may be especially pronounced and unpredictable. Recently, WMO (World Meteorological Organisation) confirmed that the last decade (2000–2009) was recorded as the warmest, and some of the recent years (e.g. 2009) are among the warmest for the past 160 years, i.e. since the beginning of official meteorological measurements in 1850 (WMO 2009). Under such conditions of global warming, salinity is expected to induce more severe phytoeffects, principally to cultivated glycophytes (crop food production), but also in long-term, exacerbate a certain interrelations (soil buffering potential to retain contaminants, provoke further soil degradation, decrease the quality of hydro-resources, reduce biodiversity etc.) in terrestrial ecosystems.
In the short-term, salinity impairs crop production and food/feed quality as a consequence of a wide range of physiological disorders known as salt stress effects (Zhu 2001; Chinnusamy et al. 2005; Zhang et al. 2012). In the long-term, particularly under specific geological (parent rock material), pedological (lower hydraulic permeability, excessive soil alkalinity and sodicity), atmospheric (high evapotranspiration demands, low precipitation) and/or hydrospheric (shallow ground water level) surroundings, salinity leads to permanent land degradation, i.e. desertification (Ondrasek et al. 2011). Topsoil crusting and salt crystallisation, dispersion of secondary clay minerals (soil aggregates), subsoil sodicity and/or toxicity of specific elements (boron, aluminium, chloride) are some of the most important long-term salinity impacts on the pedosphere.
Salt-affected areas are often situated on land used for agriculture or industrial purposes. For instance, a large part of some marine estuaries, such as Nile (Egypt) (Rashad and Dultz 2007), Murray-Darling (Australia) (Biggs et al. 2010), Ebro (Spain) (Romani et al. 2011), Neretva (Croatia) (Romic et al. 2012), developed on floodplains (alluvial soils) and used for agriculture over a long time, are subject to salinisation. Large infrastructural projects (power generation, water flow regulation, irrigation) may substantially change the hydrology of an area (e.g. decrease downstream water refreshing) and exacerbate salinisation. In the modern era, estuarine ecosystems have been shown to be some of the most vulnerable regarding environmental protection and food safety and security. Due to their naturally low topsoil fertility (e.g. light sandy soils usually with < 1% organic matter) or human-induced activities (e.g. emission of industrial/municipal effluents, intensive cultivation) aimed at retaining or even improving agricultural production, alluvial soils may gradually become overloaded by various (in)organic substances, i.e. potential contaminants. Estuarine environment thus may (1) represent an important sink for hazardous materials as a result of fluvial deposition of potentially-contaminated sediment material, and (2) become more susceptible to salinisation as a consequence of altered fresh water hydrology (river/aquifer level decreasing) and global warming (sea level rising).
Contamination of biosphere by trace elements (TEs) is expected to be one of the most critical environmental and sustainability issues of this century. Among TEs, cadmium (Cd) and its biogeochemical interactions are frequently elaborated regarding soil (rhizosphere) ecology and transfer to higher plants, i.e. into the food chain (McLaughlin and Singh 1999 and references therein; Adriano et al. 2004). Summarising the most of soil-, nutrient solution- and plant-based studies yields a several key statements for Cd: (1) its rhizosphere biogeochemistry is highly pH-dependent; (2) it is relatively easily bioavailable, especially from acidic environments; (3) it has a substantial potential for complexation/chemosorption with inorganic (salts) and organic ligands; (4) it does not any known essential biological function in plants (animals/humans), although shares some uptake/translocation mechanisms with particular essential elements; (5) it is preferentially accumulated in underground plant tissues; (6) it enters the human/animal food chain via food/feed consumption; and (7) it is toxic to a wide range of organisms, including plants and humans.
Salinisation of soil environment may substantially change Cd biogeochemistry and consequently its dynamics in the soil-plant-human continuum. For instance, by adding one of the most used agro-chemicals such as phosphatic fertilizers (e.g. diammonium phosphate, potassium dihydrogen orthophosphate) and liming salts (e.g. calcium carbonates, calcium hydroxides) can increase soil Cd adsorption, i.e. reduce its phytoavailability and/or phytotoxicity (Basta et al. 2001; Bolan et al. 2003a, b). In contrast, NaCl as the widespread salt in the nature can improve Cd mobilization by the formation of soluble inorganic chloride complexes (see Sect. 3.2.1) and thus enhance Cd phytoaccumulation, i.e. food/feed contamination. In the alkaline sodic soils Cd mobility and uptake can be enhanced due to facilitated organo-complexation with certain DOC (dissolved organic carbon) fractions (Harter and Naidu 1995). Using artificial seawater (15 g salts/L), Lores and Pennock (1998) obtained 100% desorption of Cd from dissolved organic complexes at relatively low DOC concentration (10 mg humic acid/L). Therefore, it is of great importance to elucidate Cd interactions under different salinity given that some outcomes could be highly important for improving management strategies to minimise Cd contamination and achieve food safety and security.
Plant Responses to Increased Environmental Salinity
Increased sodicity (Na) and chloride (Cl) concentration are two the most naturally widespread and intensively studied environmental types of salinity affecting plant populations worldwide. Unfortunately, in agricultural food/feed production those salinities are often accompanied by numerous environmental constrains such as: (1) water-logging or water scarcity, (2) high evapotranspirational demands, (3) shallow groundwater salinity, (4) soil acidity/alkalinity, (5) soil organic matter depletion, (6) ion toxicity (B, Al), (7) increased exchangeable sodium percentage (ESP) and/or sodium adsorption ratio (SAR), and (8) disturbed soil structure (Ondrasek et al. 2011). Complex interrelations among these variables make it almost impossible to detect which constraint is the major limitation factor in plant growth (e.g. Dang et al. 2008); hence, land management of salt-affected areas is exceedingly complicated with usually high cost-to-benefit ratio for grown crops.
In a majority of cultivated plant species, the important economic responses to increased environmental salinity (e.g. a decline in yield quality/quantity) occur even at relatively low salinity in either (1) soil (e.g. threshold salinity of ∼10 mM reduced bean and onion yield by ∼20%) (Chinnusamy et al. 2005) or (2) irrigation water (e.g. sprinkler irrigation with ∼10 and ∼30 mM salinised solution reduced watermelon yield by 50 and 100%, respectively, of the yield achieved with drip irrigation using the same salt concentrations) (Romic et al. 2008).
Many other plant metabolic functions (e.g. root/leaf membrane selectivity, photosynthesis, transpiration) (e.g. Grattan and Grieve 1999) usually are seriously compromised under excessive salinity. Moreover, using a variety of experimental approaches and many plant genotypes, it has been well established that one of crucial plant responses to increased salinity could be reduced ion selectivity resulting in enhanced Cd phytoextraction (see Sect. 3.2.1).
Cadmium in the Pedosphere
Origin of Soil Cadmium
Under natural pedosphere conditions, native soil Cd mostly has geogenic origin. Some of the most Cd-enriched natural materials are sedimentary rocks (e.g. phosphorites up to 980 and marine shales up to 219 mg Cd/kg) and sulphide minerals (e.g. sphalerite up to 50,000 and metacinnabar up to 117,000 mg Cd/kg) (Krishnamurti et al. 2005). These minerals are fairly rare in the surface lithosphere, which is the main reason for Cd naturally occurring in trace amounts, usually at several tenths of mg per kg of dry soil. Generally, soils with <0.5 mg Cd/kg (Zovko and Romic 2011), i.e. soil solution with <0.3 μM (Sanitá di Toppi and Gabbrielli 1999) can be considered as non-contaminated, whereas in contaminated environment Cd concentrations may be several magnitudes higher.
The main contributors of high Cd emission to environment during recent past have been industry, urbanisation and agriculture. Given certain physical properties of Cd (melting point 321°C), during the ore smelting and emissions from metallurgic plants, Cd relatively easily reaches atmosphere and can cause long-distance environmental contamination. From the anthropogenic Cd emission annually (∼30 million kg), about 8 million kg (27%) is emitted to the atmosphere mostly as a consequence of ore smelting and related heavy industry (metallurgic) activities, whereas a dominant portion (74%) of Cd emission (∼22 million kg) is deposited/applied to pedosphere, mostly as industrial wastes/by-products (9.5 million kg), urban wastes/effluents (4.4 million kg) and agricultural wastes/fertilisers (2.4 million kg) (adopted from Sanitá di Toppi and Gabbrielli 1999).
On salt-affected soils, which are usually deficient in phytonutrients and/or organic matter, a common practice for improving crop production is application of mineral (inorganic) and organic fertilisers/amendments. As mentioned above, sedimentary phosphorus-(P) and Cd-enriched minerals like phosphorites, as a main raw material for P fertilisers, are some of the most ubiquitous sources of Cd contamination in modern agricultural production. An application of P fertilisers may be totally avoided (extensive pastures), but on the other side additions of >500 kg/ha (double cropping in intensive horticulture) (Romic et al. 2008) may in a long-term increase soil Cd from 0.3 up to >4 g/ha annually (e.g. Singh 1994). This is in a line with recent findings by Romic et al. (2012) who in the horticultural topsoils observed high association of bioavailable P and total Cd content probably due to overuse of mineral P fertilisers.
Most soil organic amendments from industrial/urban (biosolids, composts) or agricultural (slurries, manures) sectors used for improving soil productivity are usually enriched (contaminated) by Cd even more than P-fertilisers. Allochthonous soil Cd, irrespective of whether it enters the pedosphere via previously mentioned antropogenic activities or natural processes (precipitation, flooding), is firstly deposited in the surface horizons and thereafter is transferred by soil management practices (e.g. ploughing, discing) into the rhizosphere zone of cultivated crops. However, even on such a relatively small scale (rhizosphere vs. bulk soil, soil solution vs. particulate soil), certain soil properties can be markedly different, thus variably affecting Cd chemical forms/distribution, i.e. its environmental biogeochemistry (Ondrasek and Rengel 2012).
Out of the total soil Cd content, in most pedospheres and climates, almost negligible Cd portion (<1%) (e.g. Christensen and Huang 1999) is bioavailable. However, this fact does not diminish ecological importance of Cd highlighted in recent reviews by Kabata-Pendias (2004) and Clemens (2006).
Chemical Speciation of Cd in Salinized Environment
According to Florence (1982), chemical speciation of Cd would be a function of concentrations of its various chemical forms (species) that together make up the total Cd concentration (CdTOT) in a given sample. However, mobility, bioavailability and thus toxicity of Cd in soil media in most cases cannot be related only to CdTOT. Particular forms in the CdTOT pool differ in their physico-chemical properties, from the readily available to the highly unavailable, and thus have varied environmental impacts. Accordingly, CdTOT content comprises three main physico-chemical Cd forms: (1) dissolved in soil solution (the most bioavailable and potentially the most toxic), (2) adsorbed onto soil in/organic particles (less bioavailable but still potentially toxic), and (3) Cd precipitates (inactive, i.e. non-toxic).
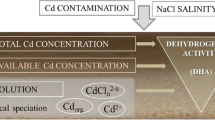
Chemical speciation as well as chemisorption (adsorption) and complexation of Cd with particular inorganic (chlorides, carbonates, Al/Fe/Mn hydroxides and organic (low-/high-molecular weight) ligands in soil environment can be detected, i.e. predicted (modelled) by appropriate analytical/computational procedures (Fig. 17.1). Formation of Cd precipitates (salts) is very rare in most naturally-occurring environmental conditions; however, it may be induced in controlled experiments and simulated by modelling. For instance, speciation-solubility geochemical modelling is a convenient concept for studying Cd (TEs) mobility and bioavailability, i.e. assessing a potential risk from metal contamination under a wide range (pH, texture, redox potential) of natural conditions. Certain methods/techniques for controlled (e.g. rhizoboxes, soil columns, chelate-buffered nutrient solution cultures) or non-controlled field (e.g. lysimeters) conditions can be useful tools for quantifying Cd in the soil solution pool and its mobility to other surrounding interfaces, as well to plants (food) (Fig. 17.1).
The soil (rhizosphere) solution generally contains most of the readily bioavailable (dissolved) and potentially the most toxic Cd forms such as free ionic hydrated Cd2+. Consequently, the potential Cd toxicity is mostly related to specific Cd2+ concentrations and physico-chemical properties of aqueous soil media, particular pH as a soil master variable (e.g. Rengel 2002). Even though dispersed inorganic (e.g. metal hydroxioxides) and organic (e.g. humic acids) colloids cannot be technically considered soil solution constituents (Helmke 1999), their active interfaces (can be altered by sampling techniques and filtration) are highly relevant in biogeochemistry of the dissolved Cd pool (Fig. 17.1). In salt-affected environments, excessive concentrations of dissolved salts, particularly anions (Cl−, SO4 2−, HCO3 −) have a large influence on Cd chemical speciation through complexation (McLaughlin and Singh 1999). Also, presence of cationic metals (Na+, Mg2+, Ca2+) is important for (1) competition with in/organic rhizosphere matrix constituents (Ondrasek and Rengel 2012), thus increasing Cd solubility and mobility in the rhizosphere solution, and (2) possibly influencing pH reaction of the media (e.g. Lores and Pennock 1998).
The predominant salt forms in salinised agro-exploited natural resources (e.g. soils, water used for irrigation) are those of sodium (Na+), chloride (Cl−) and sulphate (SO4 2−) (Ondrasek et al. 2011), and they may influence Cd bioavailability in the rhizosphere. In the next section, an emphasis will be on Cd biogeochemistry and Cd transfer into food/feed plants under salinity caused by Cl−, Na+ and SO4 2−. To create a supporting material for further discussion, a Cd “pre-screening modelling” (i.e. Cd speciation assessment) was performed for realistic salt-affected aqueous environments (e.g. soil solutions of salt-affected sandy soils, salinised channel waters, sea-intruded aquifers) over a wide range of pH levels. For instance, the modelled pH range 3.5–9.5 covers the most naturally-occurring conditions (e.g. Tipping 2005), whereas under strong acidity (pH < 3.5) or alkalinity (pH > 10.5) it is almost impossible to characterise in full the proton-affinity distribution because of a lack of reliable data (Kinniburgh et al. 1999). Inorganic speciation modelling in the Visual MINTEQ framework (Gustafsson 2006) was done using a default thermodynamic database and settings (e.g. Davies equation at 25°C), whereas organic complexation was performed by incorporated NICA-Donnan model (Kinniburgh et al. 1999) as one of the most advanced models for competitive Cd binding to dissolved high-molecular-weight organics (DOC) (Weng et al. 2001). Assumed constant concentrations of Cd (1 μM) and DOC (1 mg/L) in all models correspond well to Cd-contaminated and organically-depleted salinised soil environments.
Free Cd2+ pool was estimated from the modell to predominate in most of tested pHs but only under low salinity, whereas in moderate-to-strong salinity conditions the most prevalent pool was Cd-chloro-/sulphate complex (Cd-Cl/Cd-SO4) (Fig. 17.2). Inorganic Cd-complexes mostly comprised CdCl+, CdCl2(aq), Cd(SO4)2 −2 and CdSO4(aq) species (data not shown), although concentration of Cd in the chloro-complex (vs. Cd-SO4) was multi-fold higher (Fig. 17.2). In all three models, inorganically-complexed and free Cd2+ pools decreased with pH rising, contrary to organically-complexed pool (Cd-ORG) whose concentration positively responded to increasing pH, but predominated only under high alkalinity (pH > 9.0) and low salinity (Fig. 17.2).
Concentration distribution of Cd species in particular pools (uncomplexed cationic Cd2+; chloro-complexed Cd-Cl; sulphate-complexed Cd-SO4 and organo-complexed Cd-ORG) modelled by Visual MINTEQ under different pHs and salinities (in mM): (a) low (Na+ 15, Cl- 12, SO4 2− 1.5), (b) moderate (Na+ 75, Cl- 45, SO4 2− 15) and (c) strong (Na+ 150, Cl− 90, SO4 2− 30). In all models, concentrations of Cd (1 μM) and DOC (1 mg/L) were constant
Cl-induced Salinity and Cd Soil (Rhizosphere) Solution-plant Transfer
Over the last several decades it has been well documented that Cl salinity may be of crucial importance for food/feed safety and security (quality) with respect to Cd (trace metals) contamination, because of Cl effects on Cd mobility (Fig. 17.2) and thus soil-plant transfer (Table 17.1). In a survey study with 124 paired topsoil and durum wheat grain samples collected under highly variable field conditions (e.g. pH 5.95–8.07; Cl2–1696 mg/kg; CdDTPA 0.064–0.155 mg/kg) Norvell et al. (2000) confirmed that Cd phytoaccumulation in grains (Cdgrain) was strongly positively correlated (P < 0.01) with water-extractable soil Cl (Clw). Although Cd-Cl interactions are still under intensive investigation and not fully understood, complexation is likely to be one of the main mechanisms for enhancing Cd uptake from Cl-affected rhizospheres and deposition in plant tissues (Table 17.1). Generally, it is accepted that cadmium free cationic form (Cd2+) is the most efficient in crossing the root membranes (Sect. 3.3). Most studies listed in Table 17.1 as well as others (Bingham et al. 1984; Boukhars and Rada 2000; Li et al. 1994; McLaughlin et al. 1994, 1997; Smolders and McLaughlin 1996a, b; Smolders et al. 1998; Weggler-Beaton et al. 2000; López-Chuken and Young 2005; López-Chuken et al. 2012) indicated possible root extraction of certain Cd forms from the Cd-Cl pool, although with low efficiency (in comparison with Cd2+). Based on various experimental approaches (e.g. nutrient solution, soils) and a wide range of plant species (differing in salinity and Cd tolerance) and conditions (pH, Cl concentrations/length of exposure), it was suggested that certain Cd-Cl forms could be phytoavailable, i.e. enter roots (1) directly as a Cd-Cl-complex and/or (2) serving as a proxy form for facilitated diffusion to the root-binding sites, and afterwards dissociating and entering cells as uncomplexed Cd2+ (Cl−) (McLaughlin et al. 1994, 1997; Smolders and McLaughlin 1996a, b) (Fig. 17.3 in Sect. 3.3).
A conceptual representation of root uptake and translocation of Cd and characterisation of transport systems principally for plants with aerial fruiting bodies (adopted from references cited in the text). *Chemical composition of xylem/phloem sap in wild tobacco (Nicotiana glauca Grah.) (Hocking 1980), **Cd concentration in xylem/phloem sap collected during 3 stages [1st (at 10th), 2nd (at 14th) and 3rd (at the early grain-filling) stage] from rice (Oryza sativa L.) grown in flooded-soil containing 1.55 mg Cd/kg (extracted with 0.1 M HCl). **Fe and **Zn concentration represent their average concentration obtained during 3 stages (Yoneyama et al. 2010). ***Cd concentration in xylem sap of Indian mustard (Brassica juncea L.) exposed to 0.6 μg/mL Cd for 10 h or 7 days (in parenthesis) (Salt et al. 1995), ****Cd concentration in phloem sap of rice (Oryza sativa L.) treated with a nutrient solution containing 10 μM Cd for 2 days (Tanaka et al. 2007)
In interpreting results from Table 17.1, it is impossible to consider all variables and their influence to Cd phytoavailability. Soils (especially clayic, organic) usually have a complex matrix, composed of different organic (inorganic) metal sorbents that interact with Cd and decrease its concentration (activity) in the dissolved pool (Ondrasek et al. 2009a; Ondrasek et al. 2012). Similarly to some other metals (e.g. Cu), Cd has a strong potential for complexation with organic ligands (Romic et al. 2012). Also, a soil background and applied Cd concentrations varied considerably, from inorganic (e.g. CdCl2, Cd(NO3)2, fertilisers) to organically-enriched sources (histosols, biosolids) (Table 17.1). Phytoavailability of applied Cd is generally higher from inorganic vs. organic sources, particularly under salinity (NaCl) (Weggler et al. 2004). For instance, biosolids and some other organically-enriched materials (ORG) (e.g. peats, composts) are frequently used for improving fertility of organic matter- and nutrient-depleted soils, although simultaneously may induce contamination by Cd (TEs). Due to a substantial portion of negatively-charged reactive interfaces, ORG substances strongly compete with Cl− (SO4 2−) ligands in complexing positively-charged Cd forms, and therefore affect Cd availability and phytoextraction (Ondrasek and Rengel 2012). Bolan et al. (2003c) indicated that the addition of ORG (biosolid compost) to soil decreased the concentration of the soluble/exchangeable Cd pool at the expense of the Cd-ORG pool. As a consequence, the same authors observed decreased Cd phytoaccumulation (i.e. alleviation of Cd phytotoxicity) by ORG addition. Similar results were reported by others (e.g. Shuman et al. 2002; Pinto et al. 2004) and are mostly due to the formation of poorly phytoextractable Cd forms in the Cd-ORG pool.
However, ORG application (up to 18% of biosolids) to sandy soil (Weggler et al. 2004) and increasing Cl salinity in predominantly (>90%) ORG soil (Ondrasek et al. 2009a) resulted in (1) increased Cd (Cd-Cls) concentration in soil solutions, (2) enhanced Cd phytoaccumulation in wheat shoots or muskmelon leaves (Table 17.1), and (3) closer correlation of Cd concentration in wheat shoots with CdCl+ than with Cd2+. Both studies indicated that even in the presence of relatively high ORG content, Cd-Cl complexation was of crucial importance for the soil-plant Cd transfer.
Norvell et al. (2000) obtained a curvilinear relationship among Cdgrain and soil Cl, with an increased rate of Cd phytoaccumulation being most pronounced at marginal Cl concentrations (∼10 mM) and relatively low Cd concentration in the topsoil (CdDTPA ranged from 0.064 to 0.155 mg/kg). Though specific mechanisms responsible for Cd acquisition under low Clw salinity from uncontaminated soil are still unclear, possible influence of particular pedosphere and/or plant variables was tested. Fig. 17.2a (low Cl salinity/Cd) shows that one of possible explanation could be Cd biogeochemistry in the soil solution pool, whereby in the pH range ∼6–8 (similar to Norvell et al. 2000), the most bioavailable Cd2+ and thereafter Cd-Cl forms predominated. However, in study by Norvell et al. (2000), topsoils (0–15 cm) were quite enriched with dissolved ORGs (i.e. forming poorly available Cd-ORG pool), given that organic C was ∼20–40 g/kg. Another explanation for better Cd than CdCl uptake by plants at relatively low soil background concentration was suggested by Oporto et al. (2009), whereby Cd-Cl-complexation was of negligible importance for plant uptake under higher Cd supply.
By employing the technique of diffusive gradients in thin-films (DGT; in which a Chelex resin induces a diffusive flux of Cd2+ from a labile complexes such as dissolved Cd-Cls and/or sorbed forms on soil solids) (see Zhang and Davison 1995, 2001), fluxes of Cd (in soil/soil solution) were quantified together with root Cd uptake and shoot Cd concentration at variable Cl (0–120 mM) and Cd supply (0.4–10.5 mg/kg). They observed that (1) rising Cl salinity significantly increased shoot Cd at all soil Cd levels, but it was relatively more pronounced at the low (background) than at high soil Cd, (2) Cd uptake flux into root increased significantly (> 5-fold) due to addition of Cl at the lowest (but not at the highest) soil Cd concentration (Table 17.1), whereas DGT fluxes increased to the same extent but at all Cd levels. Correlation between the fluxes measured based on DGT and fluxes calculated based on root uptake at the background soil Cd suggested that at low Cd supply Cd phytoextraction was controlled (limited) by diffusion of Cd2+ and its replenishment from labile (e.g. Cd-Cl) pools (e.g. Oporto et al. 2009). This is in a line with recent observations by López-Chuken et al. (2012), whereby CdCl+ complexes appeared to saturate root sorption sites even at low activities and, therefore, CdCl+ activities greater than this saturation level do not cause any increase in Cd phytoextraction. With high concentration of Cd in the contaminated rhizosphere environment, the kinetics of other Cd-borne forms should also be considered, given that precipitation-dissolution reactions may significantly control element speciation (e.g. Khoshgoftar et al. 2004) and thus uptake.
With respect to possible genotypic influence on the Cd-Cl interaction, it is well known that durum is more effective in Cdgrain accumulation than bread wheat (Norvell et al. 2000). Increasing Cl-salinity exacerbated Cd accumulation in durum wheat shoots more than in four bread wheat cultivars (Khoshgoftarmanesh et al. 2006). More recently, Ozkutlu et al. (2007) confirmed that even short (<2.5 min) foliar application of Cl may be important for soil Cd extraction and/or Cd (re)mobilisation in durum wheat, i.e. Cdgrain deposition. In experiment 1, where soil was spiked with 1.0 mg Cd kg (i.e. with a background concentration totally contained 1.27 mg Cd/kg) they observed increases in Cdgrain concentrations by up to 41% with 167 mM Cl foilar application (vs. H2O treatment). In experiment 2 (with unspiked soil) foliar application of Cd-contaminated solution (8.8 mM with different Cd salts) significantly enhanced Cdgrain concentration but it was even more pronounced (∼45% in case with CdCl2) by applying of Cl salinity (Table 17.1). Above studies suggesting that durum (vs. bread wheat) genotypes could be more effective, not only in Cd root extraction, but also in Cd root-shoot (i.e. leaf-fruit) translocation under exposure to Cl salinity.
Although in many studies (Table 17.1), Cd phytoaccumulation positively correlates with salinity, there are certain exceptions. Fritioff et al. (2005) detected that accumulation of Cd in submergent spp. (Elodea canadensis L. and Potamogeton natans Michx.) significantly decreased with NaCl, without any influence of salinity on growth (Table 17.1). However, they used relatively low NaCl (up to 5 mM), corresponding well to the modelled situation in Fig. 17.2a where the proportion of Cd2+ appeared to increase with increasing salinity.
Na-induced Salinity
Na is the most frequent causative agents of naturally-induced soil alkalinity and is accompanied with salinity induced by Cl. As a consequence, similar to Cl, Na might also correlate well with phytoextracted Cd, although it seems unlikely to play any significant role in enhancing Cd uptake (e.g. Smolders et al. 1998; Mühling and Läuchli 2003); such relationships are attributed mostly to multi-colinearity with Cl (Norvell et al. 2000). Indirectly, it is possible that excessive Na+ substantially impacts Cd biogeochemistry in the rhizosphere via (1) competition with Cd cationic free/complexed species for (in)organic exchange interfaces, root transport mechanisms, and thereafter (re)translocation/deposition in the plant (Sect. 3.3), (2) deprotonation from (in)organic soil matrix (e.g. humics) and thus acidification of surrounded (unbuffered) solution (e.g. Lores and Pennock 1998), and/or (3) induced osmotic effects in the rhizosphere solution (e.g. water stress, Na toxicity, disruption of integrity/selectivity of root plasma membrane and the plant homeostasis) (reviewed by Tester and Davenport 2003; Munns and Tester 2008). Na+ may become harmful for many plants if its cytosolic concentration exceeds 10 mM (e.g. Munns and Tester 2008). This is relatively easily achievable in natural saline environments, causing common plant responses to Na-toxicity such as growth inhibition (Table 17.1) and in extreme cases early senescence and plant mortality (e.g. Romic et al. 2008).
When associated with anions other than Cl−, Na salts may induce relatively moderate plant (environmental) responses. An addition of NaNO3 can be sevenfold less effective than NaCl in increasing CdTOT content (Table 17.1) and without influence on Cd2+ and CdCl+ concentrations in soil solution (Khoshgoftar et al. 2004). In comparison to non-salinised control, NaNO3 salinity did not increase concentration of soil CdTOT, as in case of NaCl, but it significantly reduced wheat growth/yield, and marginally (but significantly at P < 0.05) improved shoot Cd accumulation (Table 17.1). Similarly, the relative increase in CdTOT in soil solution was fivefold at 120 mM NaNO3 and 20-fold at 120 mM NaCl, which was attributed to formation of Cd-Cls (Oporto et al. 2009). The twofold greater Cd accumulation in shoots was obtained with NaCl than NaNO3 application at the same molar rates (Table 17.1), probably as a result of the reduced plant yield at increasing ionic strength (Oporto et al. 2009). Both salinity types imposed similar negative effects on shoot yield at low but not at high Cd supply, where NaCl depressed the yield more severely (12-fold) than NaNO3 (4-fold) compared to non-salinised control (Table 17.1), probably due to additive NaCl/Cd toxicities, i.e. stresses (Sect. 3.3).
SO4-Induced Salinity
Sulphate is a relatively abundant anion in agro-exploited pedo/hydrosphere resources affected by salinity (Rhoades et al. 1999). In such circumstances, and in sufficient magnitude of Cd2+ pool, Cd-sulphate complexes were common, although orders of magnitude less abundant (Fig. 17.2) and less stable compared to Cd-Cls (cf. Norvell et al. 2000). The Cd-sulphate complexes might also influence Cd chemistry in the rhizosphere (e.g. McLaughlin et al. 1998b). From negatively-charged Cd(SO4)2 2− to neutral CdSO4 0 complexes, these complexes can enhance soil-plant Cd transport by either (1) diffusive transport of CdSO4 0 through the lipophilic plasma membrane bilayers as shown for HgCl2 0 by Gutknecht (1981), and suggested for H2BO species by Welch (1995) and recently in durum wheat leaf epiderms for CdCl2 0 by Ozkutlu et al. (2007), or (2) through facilitated diffusive transport of charged Cd-SO4 complexes to absorption sites at the root interface as suggested above for Cd-Cl (Fig. 17.3). In support of the first assumption, McLaughlin at al. (1998a) did not observe better plant uptake of Cd by increasing dissolved calcium (Ca) concentration in solution to compensate for CaSO4 complexation, confirming that CdSO4 0 could be phytoextracted as efficiently as Cd2+. Direct crossing of negative Cd-SO4 forms across the plasma membrane via channel/transport proteins seems less likely given possible size restriction of their ionic radii (see discussion by Smolders and McLaughlin 1996a).
Compared to studies focused on Cd-Cl interactions and their plant responses, those including Cd-SO4 are rare and less instructive. Cd-SO4 complexation in nutrient solutions (up to 58 mM SO4) has been shown as having an insignificant effect on Cd phytoextraction (McLaughlin et al. 1998a), whereas in approximately double SO4-concentrated soil solution, Cd shoot deposition increased marginally but significantly (P < 0.05) (McLaughlin et al. 1998b). Comparing some other studies that used low Cl salinity and other crop species (Table 17.1), and also earlier similar approaches and conditions (e.g. salinity duration, chemical environment in the rhizosphere) with the same tested plant species (Smolders and McLaughlin 1996a; Smolders et al. 1998), obviously a substantially lower effects of Cd-SO4 complexation may be expected in Cd transfer to crops than in Cd-Cl complexation. Norvell and associates (2000) also obtained (1) highly positive correlation among concentrations of Cdgrain and water-extractable sulphates (SO4w) (Pearson correlation coefficient was even higher then with Clw), and (2) explained high variability (around 60%) in Cdgrain by attributing such a relationship mostly to SO4 2− association (i.e. multi-colinearity) with Cl, rather than to direct causality.
Soil-Plant Transfer of Cd from Saline Environment
As one of the most soluble and bioavailable trace metals in the rhizosphere, and due to low selectivity for root extraction in majority of plant species, Cd relatively easily overcomes the soil-plant barriers. Although concentration as well as distribution of Cd decreases with the upstream transpirational flow (hence, it is higher in belowground than aboveground plant parts, Table 17.1, Fig. 17.3), consumption of crops produced in Cd-enriched environment is the principal route of Cd “intoxication” for humans. Relatively enhanced soil-plant transfer of Cd and its biotoxicity are main causes for strictly defined acceptable limits of Cd intake from foodstuff (e.g. 1 μg Cd per kg body weight per day) (WHO 1992). In Australia and New Zealand for example, Cd is recognised as the most common trace metal reaching the food chain through animal transfer in pastoral agriculture (Bolan et al. 2003a). Also, for the same populations consumption of crop foodstuffs represents predominant route (>80%) in total daily Cd intake of ∼9 μg, with >60% originating from potatoes (∼4.3 μg) and wheat (∼1.5 μg) consumption (Ondrasek and Rengel 2012), whereas ∼50% of the total Cd intake by Japanese comes from white rice (Yoneyama et al. 2010). A similar situation could be expected across other Cd-noncontaminated areas worldwide, given that potatoes, wheat and rice are among the top five agricultural crop commodities in the world (FAO 2009). In irrigated potato production, but under highly contaminated (e.g. Cd in soil up to 80 mg/kg and in irrigated water up to 240 μg/L) and salinised conditions [e.g. in soil solution (mM) 107 Cl−, 90 Na+ and 42 SO4 2−], it was estimated that daily Cd intake from potato consumption could reach ∼100 μg (Oporto et al. 2007).
Similarly to other metals, the Cd species most capable of entering root is soluble Cd2+, though under certain conditions Cd uptake is better explained by the activity of the Cd-Cl complexes than Cd2+ (López-Chuken et al. 2012). Evidence on Cd-complexes being extracted by plant root is relatively scarce and based mostly on assumptions. Besides, in the presence of Cd-Cl (Table 17.1), increased Cd uptake was confirmed under increasing concentrations of low- (Chiang et al. 2011) and high-molecular-weight organic acid (L/HMWOA) ligands (Evangelou et al. 2004; Bandiera et al. 2009). Undoubtedly, metal complexation with dissolved ligands would enhance Cd desorption from the (in)organic solid interfaces in the rhizosphere, and improve the transfer of Cd complexes into plants, although complete mechanisms are not fully explained. Also, Cd-Cl+ complexes (vs. Cd2+) are less strongly sorbed onto rhizosphere interfaces, and thus formation of Cl-complexes tends to shift Cd from solid to dissolved phase, thereby enhancing its solubility/mobility (López-Chuken et al. 2012). The most logical explanations of Cd uptake from the rhizosphere would rely on well characterised Cd2+ and CdCl+ forms (Fig. 17.3).
Due to many similarities between Cd and some divalent metal phytonutrients in physical (e.g. ionic radii in pm: Cd2+ 97, Ca2+ 99, Mn2+ 80, Fe2+ and Zn2+ 74) or chemical (e.g. redox-activity, Lewis acidity) properties, non-essential Cd would most probably enter roots via routes specific for similar essential elements. Cadmium uptake across the root cell plasma membrane was shown to occur via a concentration-dependent mechanism exhibiting saturable kinetics (Salt et al. 1995; Hart et al. 1998), with confirmed Cd-Zn; Cd-Mn and Cd-Cu competition (Hart et al. 2002; Salt et al. 1995) or Fe-Cd complementarity (Nakanishi et al. 2006). Cadmium uptake by plants could be facilitated under salt- (Na, Cl) (Ondrasek and Rengel 2012; Ondrasek et al. 2012) and/or Cd-stressed (e.g. Salt et al. 1995; López-Chuken and Young 2005; Ahmad et al. 2011) conditions. For instance, Mühling and Läuchli (2003) showed that a combined NaCl/Cd stress (vs. their separate influence) can enhance Cd phytoextraction, probably by exacerbating oxidative stress (i.e. production of O2 − radicals and H2O2) which could increase plasma membrane permeability and/or inhibit activities of antioxidant enzymes involved in the oxidative defense mechanism (Ahmad et al. 2011). Also, Mühling and Läuchli (2003) highlighted that salt-sensitive genotype exposed to salinity showed higher Cd accumulation than salt-resistant ones. Similarly, López-Chuken and Young (2005) confirmed Cl-enhanced uptake of Cd (at 100 mM NaCl) from highly contaminated soil (58 mg Cd/kg) by salt-tolerant genotypes (Brassica juncea G32192 and Zea mays hybrid W23/L317), with some seed not germinating probably due to salt and/or Cd toxicity. Both studies indicate large challenges faced by genetic engineering for improved food safety and security (e.g. Ahmad et al. 2012), especially if salinity tolerance (as multi-gene trait) is closely associated with gene loci responsible for Cd phytoextraction.
The likely Cd uptake routes from the rhizosphere are via channel (CP) and/or transport proteins (TP) (e.g. Ca2+CP; Zn2+TP; Fe2+TP), and less probably via specific Cd2+CP or Cd2+TP embedded into the plasma membrane of root cells (Clemens 2006; Lee and An 2009; Yoneyama et al. 2010) (Fig. 17.3). It remains unclear whether Cd enters the roots as CdCl+ and is then translocated in the same form via the xylem to shoot (discussed in above sections).
To moderate high reactivity/toxicity of Cd2+ in the symplast caused by its strong complexation with cytosolic metabolites (e.g. organic acids, amino acids, peptides etc.; Kato et al. 2010 and refs therein), Cd must be immobilised. Salt et al. (1995) and recently Karlsson et al. (2005) confirmed by EXAFS (Extended X-ray Absorption Fine Structure) experiments that reduced organic S ligands could be involved in Cd complexation in the root tissues. After entering root cytoplasm, possible initial Cd complexation could be with glutathion (Gtn) to form Cd-Gtn complex as a precursor of Pht-Cd (Phytochelatin-Cd complex) (Fig. 17.3). Both Gtn and Pht are LMW cistein-enriched peptides involved in maintenance of low activity of free forms of cytoplasmic metals. As a Pht-Cd complex, Cd can cross the tonoplast (via certain TPs) and create Phytochelatin-based HMW-Cd complexes in the vacuole (Salt and Rauser 1995); however, it remains unclear whether the same form can be loaded into the xylem. To some extent free cytosoilic Cd can be anti-ported into the vacuole (Salt and Wagner 1993) and sequestered to LMWOA-Cd compounds, whereas a fraction of Cd from the LMW vacuolar pool may be remobilised into the cytosol (over AtNramp3 proteins) and chelated with S-enriched ligands (Cd-S-L) (Fig. 17.3). The physiological mechanisms responsible for differential movement of Cd in roots and from roots to shoots (i.e. Cd xylem/phloem loading) still remain unclear (Hart et al. 2006; Yoneyama et al. 2010).
Due to slight acidity of the xylem sap (pH ∼ 6) (Fig. 17.3) there is a possibility that a large proportion of Cd is complexed. It is presumed that the same or similar transport and deposition routes (as in root) of Cd complexation occur in the aboveground tissues, as shown in tobacco cell leaves, where cytoplasmic Pht-Cd is directly transported to vacuole (Vögeli-Lange and Wagner 1990). Cadmium deposition could specifically be targeted to specific leaf cells such as trichomes, i.e. leaf hair/gland cells derived from specialized epidermal layer on the leaf or stem surfaces. Compared to concentration within leaves (without trichomes), Salt et al. (1995) observed a 43-fold greater accumulation of Cd in trichomes of Indian mustard leaves after 24-h exposure to 0.1 μg Cd/mL, explaining one of possible detoxification strategies in plants.
Under slightly basic conditions and prevalence of organic assimilates (e.g. S-, O-, N-rich compounds) that exist in the phloem sap, dominant Cd forms in the phloem could be those from Cd-ORG pool, but Cd-Cl complexation cannot be excluded because of Cl abundance (Fig. 17.3). During last decades, biochemistry of Cd transport in phloem was studied in potato tubers (Dunbar et al. 2003; Reid et al. 2003), peanuts seeds (Popelka et al. 1996), soybeans (Yada et al. 2004), wheat (Harris and Taylor 2001) and rice (Tanaka et al. 2007; Yoneyama et al. 2010); however, it is likely that Cd-Cl complexation inside the vascular system is less relevant than in the rhizosphere. Recently, Kato et al. (2010) reported that major Cd chelators in phloem rice sap are proteinous/S-rich (e.g. sulfhydryl) substances of ∼ 13 kDa, which distinguishes it from similar metals (Fe, Zn, Cu, Mn, Ni, Co), probably complexed with other organics (e.g. nicotianamine, 2’-deoxymugineic acid, citrate and histidine). Although in both vascular systems (xylem and phloem) Cd has similar and relatively high mobility (Reid et al. 2003; Dunbar et al. 2003), and pose similar fluctuation pattern during vegetation period (from 10th leaf to early grain rice filling stage) in Cd concentration (Yoneyama et al. 2010), retranslocation and redistribution of Cd from leaves to fruits in cereals could predominantly (>90%) occur in phloem (Tanaka et al. 2007).
Conclusions and Future Perspective
It is expected that in the forthcoming era of global warming and human population growth agro-exploited resources could be dramatically impacted by salinity and metal contamination. During last decades a significant research effort with different genotypes has been devoted to elucidation of Cd biogeochemistry in the rhizosphere, its (re)translocation and deposition in plants. The importance of Cd complexation with inorganic salt ligands (particularly Cl) in the rhizosphere was established. Cadmium taken up is transported (notably in organo-complexed forms) to the transpirational tissues via the xylem, and thereafter to the depositing tissues dominantly via the phloem. Recently, genetic engineering combined with traditional breeding has been highlighted as a promising strategy for improving (1) agri-food production in saline environment, and (2) phytoremediation of metal-contaminated areas. Soil salinity can increase the soil-plant transfer of Cd under ecologically-relevant conditions (e.g. soils with poor metal-buffering capacity), but can also interfere with phytoextraction of nutrients (Zn, Cu, Fe). Thus, growing salt-resistant crops might impact food safety and security by exacerbating Cd accumulation and decreasing micronutrient content, especially if salinity tolerance (as a multi-gene trait) is closely associated with gene loci responsible for Cd phytoextraction.
References
Adriano DC, Wenzel WW, Vangronsveld J, Bolan NS (2004) Role of assisted natural remediation in environmental cleanup. Geoderma 122:121–142
Ahmad P, Nabi G, Ashraf M (2011) Cadmium-induced oxidative damage in mustard [Brassica juncea (L.) Czern. & Coss.] plants can be alleviated by salicylic acid. S Afr J Bot 77:36–44
Ahmad P, Ashraf M, Younis M, Hu X, Kumar K, Akram NA, Al-Qurainy F (2012) Role of transgenic plants in agriculture and biopharming. Biotechnol Adv 30(3):524–540
Bandiera M, Moscaa G, Vamerali T (2009) Humic acids affect root characteristics of fodder radish (Raphanus sativus L. var. oleiformis Pers.) in metal-polluted wastes. Desalination 246:78–91
Basta NT, Gradwohl R, Snethen KL, Schroder JL (2001) Chemical immobilisation of lead, zinc and cadmium in smelter contaminated soils using biosolids and rock phosphate. J Environ Qual 30:1222–1230
Biggs AJW, Watling KM, Cupples N, Minehan K (2010) Salinity risk assessment for the Queensland Murray-Darling region. Queensland Department of Environment and Resource Management, Toowoomba
Bingham FT, Sposito G, Strong JE (1984) The effect of chloride on the availability of cadmium. J Environ Qual 13:71–74
Bolan NS, Adriano DC, Mani P, Duraisamy A, Arulmozhiselvan S (2003a) Immobilization and phytoavailability of cadmium in variable charge soils: I. Effect of phosphate addition. Plant Soil 250:83–94
Bolan NS, Adriano DC, Mani P, Duraisamy A, Arulmozhiselvan S (2003b) Immobilization and phytoavailability of cadmium in variable charge soils: II. Effect of lime addition. Plant Soil 250:187–198
Bolan NS, Adriano DC, Duraisamy P, Mani A (2003c) Immobilization and phytoavailability of cadmium in variable charge soils III. Effect of biosolid compost addition. Plant Soil 256:231–241
Boukhars L, Rada A (2000) Plant exposure to cadmium in Moroccan calcareous saline soils treated with sewage sludge and waste waters. Agrochimica 44:641–652
Chiang PN, Chiu CY, Wang MK, Chen BT (2011) Low-molecular-weight organic acids exuded by Millet (Setaria italica (L.) Beauv) roots and their effect on the remediation of cadmium-contaminated soil. Soil Sci 176:33–38
Chinnusamy V, Jagendorf A, Zhu JK (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448
Christensen TH, Huang PM (1999) Solid phase cadmium and the reactions of aqueous cadmium with soil surfaces. In: McLaughlin MJ, Singh BR (eds) Cadmium in soils and plants. Kluwer Academic Publ, Dordrecht\The Netherlands, pp 65–96
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719
Dang YP, Dalal RC, Mayer DG, McDonald M, Routley R, Schwenke GD, Buck SR, Daniells IG, Singh DK, Manning W, Ferguson N (2008) High subsoil chloride concentrations reduce soil water extraction and crop yield on Vertosols in north-eastern Australia. Aust J Agric Res 59:321–330
Dunbar KR, McLaughlin MJ, Reid RJ (2003) The uptake and partitioning of cadmium in two cultivars of potato (Solanum tuberosum L.). J Exp Bot 54:349–354
Evangelou MWH, Dagan H, Schaeffer A (2004) The influence of humic acids on the phytoextraction of cadmium from soil. Chemosphere 57:207–213
FAO (2009) http://faostat.fao.org/site/339/default.aspx. Accessed 07 Jan 2012
Florence TM (1982) The speciation of trace elements in waters. Talanta 29(5):345–364
Fritioff A, Kautsky L, Greger M (2005) Influence of temperature and salinity on heavy metal uptake by submersed plants. Environ Pollut 133:265–274
Grattan SR, Grieve CM (1999) Salinity-mineral nutrient relations in horticultural crops. Sci Hortic-Amsterdam 78(1):127–157
Gustafsson JP (2006) Arsenate adsorption to soils: modelling the competition from humic substances. Geoderma 136:320–330
Gutknecht J (1981) Inorganic mercury (Hg2+) transport through lipid bilayer membranes. J Membr Biol 61:61–66
Harris NS, Taylor GJ (2001) Remobilization of Cd in maturing shoots of near isogenic lines of durum wheat that differ in grain Cd accumulation. J Exp Bot 52:1473–1481
Hart JJ, Welch RM, Norvell WA, Sullivan LA, Kochian LV (1998) Characterization of cadmium binding, uptake, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiol 116:1413–1420
Hart JJ, Welch RM, Norvell WA, Kochian LV (2002) Transport interactions between cadmium and zinc in roots of bread and durum wheat seedlings. Physiol Plantarum 116:73–78
Hart JJ, Welch RM, Norvell WA, Kochian LV (2006) Characterization of cadmium uptake, translocation and storage in near-isogenic lines of durum wheat that differ in grain cadmium concentration. New Phytol 172:261–271
Harter RDR, Naidu R (1995) Role of metal-organic complexation in metal sorption by soils. Adv Agron 55:219–264
Helal HM, Upenov A, Issa GJ (1999) Growth and uptake of Cd and Zn by Leucaena leucocephala in reclaimed soils as affected by NaCl salinity. J Plant Nut Soil Sci 162:589–592
Helmke PA (1999) Chemistry of cadmium in soil solution. In: McLaughlin MJ, Singh BR (eds) Cadmium in soils and plants. Kluwer Academic Publ, Dordrecht\The Netherlands, pp 39–64
Hocking PJ (1980) The composition of phloem exudates and xylem sap from tree tobacco (Nicotiana glauca Grah). Ann Bot-London 45:633–643
Kabata-Pendias A (2004) Soil-plant transfer of trace elements – an environmental issue. Geoderma 122:143–149
Karlsson T, Persson P, Skyllberg U (2005) Extended X-ray absorption fine structure spectroscopy evidence for the complexation of cadmium by reduced sulfur groups in natural organic matter. Environ Sci Tech 39:3048–3055
Kato M, Ishikawa S, Inagaki K, Chiba K, Hayashi H, Yanagisawa S, Yoneyama T (2010) Possible chemical forms of cadmium and varietal differences in cadmium concentrations in the phloem sap of rice plants (Oryza sativa L.). Soil Sci Plant Nutr 56:839–847
Khoshgoftar AH, Shariatmadari H, Karimian N, Kalbasi M, van der Seatm Z, Parker DR (2004) Salinity and Zn application effects on phytoavailability of Cd and Zn. Soil Sci Soc Am J 68:1885–1889
Khoshgoftarmanesh AH, Shariatmadari H, Karimian N, Kalbasi M, van der Seatm Z (2006) Cadmium and zinc in saline soil solutions and their concentrations in wheat. Soil Sci Soc Am J 70:582–589
Kinniburgh DG, van Riemsdijk WH, Koopal LK, Borkovec M, Benedetti MF, Avena MJ (1999) Ion binding to natural organic matter: competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloid Surface A151:147–166
Krishnamurti GSR, McArthur DFE, Wang MK, Kozak LM, Huang PM (2005) Biogeochemistry of soil cadmium and the impact on terrestrial food chain contamination. In: Huang PM, Gobran GR (eds) Biogeochemistry of trace elements in the rhizosphere. Elsevier, Amsterdam, pp 197–257
Lee S, An G (2009) Over-expression of OsIRT1 leads to increased iron and zinc accumulation in rice. Plant Cell Environ 32:408–416
Li YM, Chaney L, Schneiter AA (1994) Effect of soil chloride level on cadmium concentration in sunflower kernels. Plant Soil 167:275–280
López-Chuken UJ, Young SD (2005) Plant screening of halophyte species for cadmium phytoremediation. Z Naturforsch 60:236–243
López-Chuken UJ, López-Domínguez U, Parra-Saldivar R, Moreno-Jimánez E, Hinojosa-Reyes L, Guzmán-Mar JL, Olivares-Sáenz E (2012) Implications of chloride-enhanced cadmium uptake in saline agriculture: modeling cadmium uptake by maize and tobacco. Int J Environ Sci Technol 9:69–77
Lores EM, Pennock JR (1998) The effect of salinity on binding of Cd, Cr, Cu and Zn to dissolved organic matter. Chemosphere 37:861–874
McLaughlin MJ, Singh BR (1999) Cadmium in soils and plants. Kluwer Academic Publishers, Dordrecht\The Netherlands
McLaughlin MJ, Palmer LT, Tiller KG, Beech TA, Smart MK (1994) Increased soil salinity causes elevated cadmium concentrations in field grown potato tubers. J Environ Qual 23:1013–1018
McLaughlin MJ, Tiller KG, Smart MK (1997) Speciation of cadmium in soil solutions of saline/sodic soils and relationships with cadmium concentrations in potato tubers (Solanum tuberosum L). Aust J Soil Res 35:183–198
McLaughlin MJ, Andrews SJ, Smart MK, Smolders E (1998a) Effects of sulfate on cadmium uptake by Swiss chard I. Effects of complexation and calcium competition in nutrient solutions. Plant Soil 202:211–216
McLaughlin MJ, Lambrechts RM, Smolders E, Smart MK (1998b) Effects of sulfate on cadmium uptake by Swiss chard: II. Effects due to sulfate addition to soil. Plant Soil 202:217–222
Mühling KH, Läuchli A (2003) Interaction of NaCl and Cd stress on compartmentation pattern of cations, antioxidant enzymes and proteins in leaves of two wheat genotypes differing in salt tolerance. Plant Soil 253:219–231
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakanishi H, Ogawa I, Ishimaru Y, Mori S, Nishizawa NK (2006) Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci Plant Nutr 52:464–469
Norvell WA, Wu J, Hopkins DG, Welch RM (2000) Association of cadmium in durum wheat grain with soil chloride and chelateextractable soil cadmium. Soil Sci Soc Am J 64:2162–2168
Ondrasek G, Rengel Z (2012) The role of soil organic matter in trace elements bioavailability and toxicity. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants: metabolism productivity and sustainability. Springer, New York, pp 403–423
Ondrasek G, Romic D, Rengel Z, Romic M, Zovko M (2009a) Cadmium accumulation by muskmelon under salt stress in contaminated organic soil. Sci Tot Environ 407:2175–2182
Ondrasek G, Rengel Z, Romic D, Poljak M, Romic M (2009b) Accumulation of non/essential elements in radish plants grown in salt-affected and cadmium-contaminated environment. Cereal Res Commun 37(1):9–12
Ondrasek G, Rengel Z, Veres S (2011) Soil salinisation and salt stress in crop production. In: Shanker AK and Venkateswarlu B (eds.) Abiotic stress in plants: mechanisms and adaptations. In Tech, Rijeka, pp 171–190. Available from http://www.intechopen.com/articles/show/title/soil-salinisation-and-salt-stress-in-crop-production. Accessed on 20 Dec, 2012
Ondrasek G, Rengel Z, Romic D, Savic R (2012) Salinity decreases dissolved organic carbon in the rhizosphere and increases trace elements phyto-accumulation. Eur J Soil Sci. doi:10.1111/j.1365-2389.2012.01463.x
Oporto C, Vandecasteele C, Smolders E (2007) Elevated cadmium concentrations in potato tubers due to irrigation with river water contaminated by mining in Potosí, Bolivia. J Environ Qual 36:1181–1186
Oporto C, Smolders E, Degryse F, Verheyen L, Vandecasteele C (2009) DGT-measured fluxes explain the chloride-enhanced cadmium uptake by plants at low but not at high Cd supply. Plant Soil 318:127–135
Ozkutlu F, Ozturk L, Erdem H, McLaughlin MJ, Cakmak I (2007) Leaf-applied sodium chloride promotes cadmium accumulation in durum wheat grain. Plant Soil 290:323–331
Pinto AP, Mota AM, de Varennes A, Pinto FC (2004) Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. Sci Tot Environ 326:239–247
Popelka JC, Schubert S, Schulz R, Hansen AP (1996) Cadmium uptake and translocation during reproductive development of peanut (Arachis hypogoea L.). Angew Bot 70:140–143
Rashad M, Dultz S (2007) Decision factors of clay dispersion in alluvial oils of the Nile River Delta – a study on surface charge properties. American-Eurasian J Agric Environ Sci 2(3):213–219
Reid RJ, Dunbar KR, McLaughlin MJ (2003) Cadmium loading into potato tubers: the roles of the periderm, xylem and phloem. Plant Cell Environ 26:201–206
Rengel Z (2002) Role of pH in availability of ions in soil. In: Rengel Z (ed) Handbook of plant growth. pH as the master variable. Marcel Dekker, Inc, New York, pp 323–350
Rhoades JD, Chanduvi F, Lesch S (1999) Soil salinity assessment. Methods and interpretation of electrical conductivity measurements. FAO Irrig and Drain Paper 57, Rome, p 165
Romani AM, Sabater S, Munoz I (2011) The physical framework and historic human influences in the Ebro River. In: Barcelo D, Petrovic M (eds) The Ebro River basin, Hdb. Env. Chem. 13. Springer, New York, pp 1–20
Romic D, Ondrasek G, Romic M, Borosic J, Vranjes M, Petosic D (2008) Salinity and irrigation method affect crop yield and soil quality in watermelon (Citrullus lanatus L.) Growing. Irrig Drain 57:463–469
Romic D, Romic M, Rengel Z, Zovko M, Bakic H, Ondrasek G (2012) Trace metals in the coastal soils developed from estuarine floodplain sediments in the Croatian Mediterranean region. Environ Geochem Health 34:399–416. doi:10.1007/s10653-012-9449-z
Salt DE, Rauser WE (1995) MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiol 107:1293–1301
Salt DE, Wagner GJ (1993) Cadmium transport across tonoplast of vesicles from oat roots. Evidence for a Cd2+/H + antiport activity. J Biol Chem 268:12297–12302
Salt DE, Prince RC, Pickering IJ, Raskin I (1995) Mechanisms of cadmium mobility and accumulation in Indian Mustard. Plant Physiol 109:1427–1433
Sanitá di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Shuman LM, Dudka S, Das K (2002) Cadmium forms and plant availability in compost-amended soil. Commun Soil Sci Plant Anal 33:737–748
Singh BR (1994) Trace element availability to plants in agricultural soils, with special emphasis on fertilizer inputs. Environ Rev 2:133–146
Smolders E, McLaughlin MJ (1996a) Effect of Cl and Cd uptake by Swiss chard in nutrient solution. Plant Soil 179:57–64
Smolders E, McLaughlin MJ (1996b) Chloride increases Cd uptake in Swiss chard in a resin-buffered nutrient solution. Soil Sci Soc Am J 60:1443–1447
Smolders E, Lambregts RM, McLaughlin MJ, Tiller KG (1998) Effect of soil solution chloride on cadmium availability to Swiss chard. J Environ Qual 27:426–431
Tanaka K, Fujimaki S, Fujiwara T, Yoneyama T, Hayashi H (2007) Quantitative estimation of the contribution of the phloem in cadmium transport to grains in rice plants (Oryza sativa L.). Soil Sci Plant Nutr 53:72–77
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot-London 91(5):503–527
Tipping E (2005) Modelling Al competition for heavy metal binding by dissolved organic matter in soil and surface waters of acid and neutral pH. Geoderma 127:293–304
Vögeli-Lange R, Wagner GJ (1990) Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves. Implication of a transport function for cadmium-binding peptides. Plant Physiol 92:1086–1093
Weggler K, McLaughlin MJ, Graham RD (2004) Effect of chloride in soil solution on the plant availability of biosolid-borne cadmium. J Enviro Qual 33:496–504
Weggler-Beaton K, McLaughlin MJ, Graham RD (2000) Salinity increases cadmium uptake by wheat and Swiss chard from soil amended with biosolids. Aust J Soil Res 38:37–45
Welch RM (1995) Micronutrient nutrition of plants. Crit Rev Plant Sci 14:49–82
Weng L, Temminghoff EJM, van Riemsdijk WH (2001) Contribution of individual sorbents to the control of heavy metal activity in sandy soil. Environ Sci Techn 35:4436–4443
World Health Organisation (WHO) (1992) Cadmium. Environmental health criteria 134. World Health Organization, Geneva
World Meteorological Organization (WMO) (2009) Statement on the status of the global climate in 2009, Press No 869. World Meteorological Organization, Geneva
Yada S, Oda H, Kawasaki A (2004) Uptake and transport of Cd supplied at early growth stage in hydroponically cultured soybean plants. Biomed Res Trace Elements 15:292–294
Yoneyama T, Gosho T, Kato M, Goto S, Hayashi H (2010) Xylem and phloem transport of Cd, Zn, and Fe into the grains of rice plants (Oryza sativa L.) grown in continuously flooded Cd-contaminated soil. Soil Sci Plant Nutr 56:445–453
Zhang H, Davison W (1995) Performance-characteristics of diffusion gradients in thin-films for the in-situ measurement of trace-metals in aqueous-solution. Anal Chem 67:3391–3400
Zhang H, Davison W (2001) A new method to measure effective soil solution concentration predicts copper availability to plants. Environ Sci Technol 35:2602–2607
Zhang H, Han B, Wang T, Chen SX, Li HY, Zhang YH, Dai SJ (2012) Mechanisms of plant salt response: insights from proteomics. J Proteome Res 11(1):49–67
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6(2):66–71
Zovko M, Romic M (2011) Soil contamination by trace metals: geochemical behaviour as an element of risk assessment. In: Ahmad DI and Ahmad DM (eds.) Earth and environmental sciences. InTech Rijeka, pp 437–456. Available from http://www.intechopen.com/articles/show/title/soil-contamination-by-trace-metals-geochemical-behaviour-as-an-element-of-risk-assessment. Accessed on 20 Dec, 2012
Acknowledgments
The author is grateful to Winthrop Professor Zed Rengel (University of Western Australia) for valuable discussion, comments and text improvement.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Ondrasek, G. (2013). The Responses of Salt-Affected Plants to Cadmium. In: Ahmad, P., Azooz, M.M., Prasad, M.N.V. (eds) Salt Stress in Plants. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6108-1_17
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6108-1_17
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6107-4
Online ISBN: 978-1-4614-6108-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)