Abstract
The relationship between Parkinson’s disease (PD) and risk of hip fracture yielded inconsistent results. Therefore, we conducted the present systematic review and meta-analysis of published observational studies to assess the association between PD and risk of hip fracture. PubMed, ISI, EMBASE, and Cochrane databases were searched systematically to identify studies assessing the relationship between PD and the risk of hip fracture up to July 01, 2017. In addition, to find related articles, the reference section of retrieved articles was checked. Random-effects model was used for calculation of pooled hazard ratio (HR) and 95% confidence intervals (CI). Thirteen independent studies containing 564,947 participants were included in the meta-analysis. The overall results of included studies showed PD to be associated with the risk of hip fracture (HRoverall = 3.13, 95% CI 2.53–3.87) in women 3.11 (2.51–3.86) and men 2.60 (2.19–3.09). Our meta-analysis showed the direct association between PD and the risk of hip fracture in both men and women. However, due to the limitations of this study, further well-designed studies are required to confirm our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a chronic neurological disease that mostly affects the age group of > 50 years of age, and is not prevalent in the age group of < 40. This disease is one of the most prevalent neurodegenerative diseases that its worldwide prevalence increases with age, from 0.6% in ages of 56–69 years to 3% in elderlies of above 80 [1, 2]. In this condition, the loss of dopaminergic neurons, leading to symptoms such as tremor, muscle rigidity, and situational imbalance, increases the risk of falling in patients [3]. Several studies have shown that PD increased the risk of fractures [4,5,6]. A part of the association between PD and fractures can be related to the increased risk of falling in patients suffering from this disease [7]. On the other hand, PD is associated with other factors that indirectly have an adverse effect on bones [8, 9]; in a way that decrease in the level of 25-hydroxy vitamin D and compensation for high levels of thyroid hormone have been observed in PD patients, and it is possible for these factors to decrease bone density [8]. Both of these factors (falling and decrease in bone density) increase the risk of bone fracture. Besides these factors, due to the side-effects of usual medicines (such as levodopa and dopamine agonist) used for the treatment of PD, including situational blood pressure and reduction of attention or confusion, as well, patient do not have proper protective responses in falling, and thus, the risk of bone fracture increases [10, 11]. A study showed that about 50% of bone fractures happened in PD patients were hip fractures [12].
In addition, studies that have shown the direct association between PD and risk of hip fracture have had a great growth in recent years [6, 13, 14]. Despite this epidemiological evidence, the relationship between PD and the risk of hip fracture has not been systematically assessed; therefore, this relationship has been studied in the present systematic review and meta-analysis.
Methods
To conduct the current meta-analysis, MOOSE guideline (guideline for meta-analysis of observational studies) was used [15]. PubMed, ISI, EMBASE, and Cochrane databases were searched systematically to identify studies assessing the relationship between PD and risk of hip fracture up to July 01, 2017. In addition, the reference section of retrieved articles was checked to find related articles. The search was performed using medical subheadings such as Parkinson disease or PD, and hip fracture or fracture, in a systematic manner. This search was limited to the studies performed on humans and there were no lingual restrictions.
Inclusion and exclusion criteria of studies
Studies that possessed the following criteria were entered into the meta-analysis: (1) original article; (2) clinical trial, cohort, case–control, and cross-sectional studies; (3) adult human population; (4) relationship index: odds ratio (OR), hazard ratio (HR), or relative risk (RR), reported together with confidence interval (CI) of 95%. Out of 20 epidemiological studies [6, 13, 14, 16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] that had the inclusion criteria, 18 were cohort studies, 2 were case–control studies, and one was researching news. Studies that had not reported necessary information for calculation of standard error were excluded from the meta-analysis. In addition, in the event that several studies were reported from one population or cohort, only data from the latest study were entered. Two studies were excluded, because they had only reported the point estimate (without CI and standard error) [28, 31], as well as two other studies due to reporting the results of one cohort [13, 14], one study because of ambiguity in reporting the extent of relationship [6], and finally, two studies were excluded from the meta-analysis because of inexplicitness of their report on the extent of relationship [30, 33].
Quality assessment of studies and data extraction
The following details were extracted and recorded for each of the included studies using a pre-designed data extraction form: publication source (surname of the first author, year of publication of the article, and country of the studied population), name of the study, study design, sample size, follow-up period for cohort studies, age, sex, point estimate together with CI, and variables controlled for in the multivariate model. For each study, we extracted the risk estimations that reflected the greatest degree of control for potential confounders. Study design, participants characteristics, adjustment for potential confounders, and estimates of associations were independently extracted by two reviewers (AH and MKH); disagreements between reviewers were resolved by referring to a third author (AAH).
Eight studies [17,18,19, 24, 26, 29, 32, 34] had not performed their analyses for men and women separately. However, since estimates were made in presence of adjustment for age and sex, as well as HR and 95% CI, these studies were used in the meta-analysis for men and women. Two studies [23, 24] had reported RR instead of HR, and thus, RR was used in place of HR.
To review the quality of the included studies, 9-Star Newcastle Ottawa Scale (NOS) was used [35]. In a way that 9 points were given to a study with the highest methodological quality, points 4, 2, and 3 were allocated to “choosing study groups”, “comparability of study groups”, and “outcome assessment and adequacy follow-ups” respectively. Studies with a total score of 0–3, 4–6, and 7–9 were recognized as studies with poor, moderate, and high qualities, respectively.
Statistical analysis
HR was used as the common relationship index for all studies. To assess multivariate-adjusted HRs and its 95% CIs, a forest plot was drawn. In the present meta-analysis, HR logarithm with its standard error was utilized. Summary of HR estimates and its corresponding 95% CIs were calculated using the method of DerSimonian and Laird [36] as well as fixed-effects model and random-effects model. The summary HR estimates from random-effects model were used to investigate the variability between the studies. Statistical heterogeneity of HR between the studies was investigated using Cochran’s Q test and I2 statistic [37]. In case I2 ≥ 50% and P ≤ 0.05, heterogeneity was considered statistically significant [38]. The random-effects model was used for pooling the results of the study [36], since this model takes into account study’s sample size and between studies variation [39].
Meta-regression and subgroup analysis were done for detection of heterogeneity source. Subgroup analysis was done based on sex, study location, number of participants in a study, age, and quality of the study. In addition, to investigate the potential influence of each of the studies on the overall results, sensitivity analysis was performed.
Publication bias was assessed by the funnel plot [40]. In the funnel plot, HRs were plotted against the inverse of the square of the standard error (a measure of precision). In addition, Begg’s test [41] and Egger’s test [42] were used to investigate publication bias. All analyses were done using STATA 12.0 software (StataCorp, College Station, TX, USA) [14]. All P values were two sided with a significant level of less than 0.05.
Results
Study characteristics
Based on pre-defined criteria, out of 13 independent studies containing 564,947 participants, a range of 364–498,849 participants was qualified to be included in the meta-analysis. Table 1 shows the characteristics of the included studies in summary [17,18,19,20,21,22,23,24,25,26,27, 29, 32]. Out of the above- mentioned 13 studies, four studies were conducted in the USA [20, 21, 25, 27], five in Europe [17, 18, 23, 26, 29], three in Asia [19, 22, 24], and one in Australia [32]. Two studies [25, 27] were only performed on women and one study [21] only on men, and ten studies [17,18,19,20, 22,23,24, 26, 29, 32] were performed on both men and women. From all the studies, 12 [17,18,19, 21,22,23,24,25,26,27, 29,30,31,32] had used HR or RR adjusted for potentially confounding variables as the measure of association, and only in one study [20], adjusted variables were not reported for calculated HR. Table 2 illustrates the results of the quality assessment of studies based on NOS. Ten studies had high quality and three had moderate quality.
Main analysis
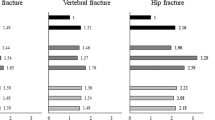
The results of each study together with their overall results were presented in the forest plot (Fig. 1). Out of 13 studies, 12 studies [17, 18, 20,21,22,23,24, 26, 27, 29, 32] had reported a positive significant relationship between PD and the risk of hip fracture, and one study [25] had reported a positive but not significant relationship between PD and the risk of hip fractures (Table 1). The overall results of included studies showed that based on the random-effect model; PD has a direct association with the risk of hip fracture (HRoverall = 3.13, 95% CI 2.53–3.87). However, heterogeneity was statistically significant (Phetrogenisity = 0.00 I2 = 93.4%). Based on meta-regression results, the sample size was the most important factor in creating heterogeneity. With regard to the results of subgroup analysis, studies conducted by Walker et al. [23] and Jorgensen et al. [29] had the most part in creating heterogeneity due to having large sample sizes. In addition, the study of Paul et al. [32], which was done in Australia, also caused heterogeneity. In the analysis conducted after excluding the above-mentioned studies, there was no major change in the relationship between PD and hip fracture (HRoverall = 2.87, 95% CI 2.43–3.38); and the heterogeneity test was not statistically significant (I2 = 5.2%; Pheterogeneity = 0.39) (Fig. 2).
Forest plot of the association between Parkinson’s disease and risk of hip fracture. Random-effects model was used to pool the overall hazard ratios (HRs) and 95% confidence intervals (CIs). The diamond represents the pooled HR, and the squares and the horizontal lines, respectively, represent the HR and 95% CI of each individual study
Forest plot of the association between Parkinson’s disease and risk of hip fracture. Random-effects model was used to pool the overall hazard ratios (HRs) and 95% confidence intervals (CIs). The diamond represents the pooled HR, and the squares and the horizontal lines, respectively, represent the HR and 95% CI of each individual study
Sensitivity and subgroup analyses
Subgroup analysis was performed based on sex, study location, number of participants in the study, age, follow-up period, and quality of performed studies (Table 3). Based on the obtained results, it can be observed that the effect of PD on hip fracture in women 3.11 (2.51–3.86), is slightly higher than that of men 2.60 (2.19–3.09). However, regarding study location, the effect of PD on hip fracture is considerably higher in the studies performed in Europe 4.85 (1.79–13.17) than those performed in the USA 2.96 (1.79–4.89), as well as in Asia 2.63 (2.17–3.19), and in Australia 2.06 (1.95–2.17). In addition, the effect of PD on hip fracture in the group of above 60 years of age old 4.16 (1.81–9.57) was considerably higher than the group of below 60 years old 2.68 (1.96–3.68). The analysis that was performed based on the duration of the follow-up period showed that the highest value of HR was in the study group that had the follow-up period of fewer than 5 years 5.48 (1.78–16.88); and in the studies with follow-up periods of between 5–10 years and above 10 years, HR were, respectively, 2.44 (1.98–2.99) and 2.79 (1.66–4.69). Likewise, the effect of PD on hip fracture in the high-quality study group was 4.03 (2.52–6.43), which is almost twice the amount of the low-quality study group 1.92(1.63–2.26).
In the sensitivity analysis, that in it, one study was omitted each time, and overall results were then calculated, there were no significant changes in the overall results of HR. The scope of results in the sensitivity analyses was between HR = 2.41 (95%, CI 2.06–2.80) and HR = 3.65 (95%, CI 2.50–5.33).
Publication bias
Begg’s test and Egger’s test showed some evidence for the existence of publication bias in the studies on PD and hip fracture (P ≤ 0.05). Therefore, to evaluate and also adjust the effect of publication bias on the results of the meta-analysis, the method of Trim and Fill was used. The overall results obtained by this method HR = 3.32 (2.23–2.7) were slightly different from the overall results obtained from the meta-analysis HR = 2.87 (2.42–3.38).
Discussion
The findings from this meta-analysis indicate that PD almost tripled the risk of hip fracture. Subgroup and sensitivity analyses showed that the overall results of this analysis had enough stability.
To explain the relationship between PD and hip fracture, numerous factors have been pointed out in different texts. One of the factors that increases the risk of hip fracture is vitamin D deficiency. In a study conducted by Dhanwal et al., it was demonstrated that about 75% of those who suffer from hip fracture had vitamin D deficiency [43]. In addition, several other studies have pointed out the relationship between vitamin D deficiency and hip fracture [43,44,45,46,47]. Vitamin D deficiency is caused by hypocalcemia and compensatory hyperparathyroidism; and if the parathyroid hormone is more than the required amount, it can cause bone loss by stimulating the activity of osteoclasts [48].
Therefore, due to the fact that vitamin D has a vital role in bone metabolism, it is clear that its deficiency increases the risk of falling and hip fracture [49]; while it has been demonstrated that prevalence of vitamin D deficiency in PD patients was significantly higher compared to healthy individuals or the Alzheimer’s disease patients. This matter shows a special relationship between vitamin D deficiency and PD [8, 50, 51]. Vitamin D deficiency decreases muscle strength [48]. Muscle strength has an indirect correlation with bone density, and it affects bone formation and reconstruction through mechanical signals which it creates [52,53,54].
In a way that isokinetic muscle strength of PD patients is decreased compared to healthy individuals, even at the beginning stages of the disease, and it is reduced even more by disease’s progression; although the reason behind this is unknown [55]. The next factor that explains the relationship between PD and hip fracture is the lack of movement or low physical activity in PD patients. Lack of movement or low physical activity causes bone loss and increases the risk of hip fracture [48, 56, 57]. Because of the low activity of PD patients [58], bone density in these patients is lower than healthy individuals [13, 20, 59].
One of the reasons which immobility causes a decrease in bone density is that the bone tissue is sensitive to its mechanical environment, and is constantly stimulated by muscle contractions and the movements that cause weight bearing. When bones are under pressure, a fluid flow is created that osteocytes are able to recognize through their dendritic connections. In response to these tensions, osteocytes create warning molecules that stimulate bone reconstruction via osteoclasts and osteoblasts [60, 61]. Therefore, unusual mechanical pressure as a result of immobility or low physical activity leads to bone loss and decrease in bone density [62]; and this matter increases the risk of hip fracture.
Another factor that has a negative effect on bone density and increases the risk of hip fracture is malnutrition [57]. The PD patients are exposed to malnutrition for several reasons including impaired hand–mouth coordination, dysphagia, decreased bowel movement, depression, cognitive impairment, and side-effects of drugs. At the same time, considering the muscle strength and involuntary movements in these patients, energy requirement tends to increase. Furthermore, malnutrition can lead to decreased levels of vitamin D, folic acid, and vitamin B12, which have negative effects on bone formation and strength [63, 64].
In addition, using different drugs for treating PD patients might be related to the risk of fracture. In a study performed by Vastergaard, it was shown that Levodopa was generally related to increased risk of fracture and in higher doses, increased the risk of hip fracture [65]. This matter could be due to the fact that Levodopa can create hyperhomocysteinemia as a result of its metabolism [66].
The risk of hip fracture in women was slightly higher than men in the subgroup analysis. This result is compatible with the results of the study of Joseph Melton III that had shown that the risk of hip fracture during lifetime was 17.5% for women and 6% for men. The bigger percentage of hip fracture in women is related to osteoporosis [67]. Osteoporosis can happen due to low bone mass or because of bone loss. Bone loss in women is increased after the menopause period due to decreased estrogen levels, and thus, the possibility of osteoporosis and hip fracture is increased [68]. Considering the fact that, in the present review study, most studies were done in ages of post menopause, therefore, it is possible that this is the reason for the observed difference between the two sexes.
In addition, based on the subgroup analysis, the greatest risk of hip fracture was in the continent of Europe, while the lowest risk was in Australia. These results are compatible with the study conducted by Dhanwal et al.; based on their results, same as the results obtained in this study, the highest rate of hip fractures had happened in European countries and the rate of such fractures in Asian countries was moderate [69]. Furthermore, a North-to-South geographical pattern has been seen, especially in Europe and USA, in a way that the most amount of hip fractures had happened in Northern regions [70]. Observed geographical differences can be related to demographical factors, race, latitude, physical activity, and vitamin D deficiency in Northern regions due to less sunlight [69]. Regarding the placement of Australia in a low latitude, the existence of the North-to-South pattern might explain the low amount of fracture risk in Australia. Although considering the fact that there was only one study from there, the results are not entirely reliable.
In addition, based on the obtained results from subgroup analysis, the risk of hip fracture in individuals of above 60 years of age was considerably higher than those of below 60 years. This finding is compatible with the findings of the previous studies [17, 19, 22, 23]. According to the World Health Organization’s declaration in 2015, one of the possible reasons for increased risk of hip fracture in individuals of above 60 years of age is osteoporosis that its prevalence is increased with aging; in a way that about 15% of Caucasians suffer from osteoporosis at the age of 50 and about 70% at the age of 80; and this matter is more prevalent in women [68, 70]. In the present study, the effect of PD on hip fracture was higher in studies with less follow-up period, and the more the duration of the follow-up period, the less the risk of fracture. We do not have an explanation for this matter, but it may be due to that the risk of PD on hip fracture is high at the beginning, but with increased follow-up period, that risk is reduced; and this reduction is not because of decreased risk of PD, but because of the increased risk in the control group.
Restrictions
There were several limitations in this study. First, there was a significant heterogeneity in the overall analysis (I2 = 93.4%); and as it was demonstrated in the subgroup analysis, sex, study location, and sample size probably had a role in the observed heterogeneity. Second, there might be uncontrolled confounders. These residual confounders such as the history of hip fracture, taking corticosteroids or other medicines used in the treatment of PD, and serum status of vitamin D may confound the obtained results. Third, participants in the studies were mainly from Europe and America, and therefore, findings of this study may not be applicable for other populations. Another limitation of this study was publication bias; although, using the Trim and Fill method showed that the obtained results from this method were not majorly different from the meta-analysis results. Although, the possibility of the existence of publication bias is omnipresent in all meta-analyses.
Conclusion
The result from this meta-analysis showed a direct association between PD and the risk of hip fracture in both men and women. Despite the observational studies which we analyzed, it does not seem that the observed relationship was due to confounder factors alone. However, due to the limitations of this study, further well-designed studies are required to confirm our findings.
References
Launer L, Berger K, Breteler M, Dartigues J, Baldereschi M, Fratiglioni L, Lobo A, Martinez-Lage J, Trenkwalder C, Hofman A (2000) Prevalence of Parkinson’s disease in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54(11 Suppl 5):S21-23
Pringsheim T, Jette N, Frolkis A, Steeves TDL (2014) The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 29(13):1583–1590
Burch D, Sheerin F (2005) Parkinson’s disease. Lancet 365(9459):622–627
Johnell O, Melton Iii LJ, Atkinson EJ, O’Fallon WM, Kurland LT (1992) Fracture risk in patients with parkinsonism: a population-based study in Olmsted County, Minnesota. Age Ageing 21(1):32–38
Johnell O, Sernbo I (1986) Health and social status in patients with hip fractures and controls. Age Ageing 15(5):285–291
Grisso JA, Kelsey JL, Strom BL, Ghiu GY, Maislin G, O’Brien LA et al (1991) Risk factors for falls as a cause of hip fracture in women. N Engl J Med 324(19):1326–1331
Fink HA, Kuskowski MA, Orwoll ES, Cauley JA, Ensrud KE (2005) Association between Parkinson’s disease and low bone density and falls in older men: the osteoporotic fractures in men study. J Am Geriatr Soc 53(9):1559–1564
Sato Y, Kikuyama M, Oizumi K (1997) High prevalence of vitamin D deficiency and reduced bone mass in Parkinson’s disease. Neurology 49(5):1273–1278
Kao CH, Chen CC, Wang SJ, Chia LG, Yeh SH (1994) Bone mineral density in patients with Parkinson’s disease measured by dual photon absorptiometry. Nucl Med Commun 15(3):173–177
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373(9680):2055–2066
Vaserman N (2005) Parkinson’s disease and osteoporosis. Joint Bone Spine 72:484–488
Williams DR, Watt HC, Lees AJ (2006) Predictors of falls and fractures in bradykinetic rigid syndromes: a retrospective study. J Neurol Neurosurg Psychiatry 77(4):468–473
Schneider JL, Fink HA, Ewing SK, Ensrud KE, Cummings SR, Study of Osteoporotic Fractures Research G (2008) The association of Parkinson’s disease with bone mineral density and fracture in older women. Osteoporos Int 19(7):1093–1097
Taylor BC, Schreiner PJ, Stone KL, Fink HA, Cummings SR, Nevitt MC et al (2004) Long-term prediction of incident hip fracture risk in elderly white women: study of osteoporotic fractures. J Am Geriatr Soc 52(9):1479–1486
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283(15):2008–2012
Mayor S (2016) Parkinson’s disease diagnosis is preceded by increased risk of falls, study finds. BMJ 352:i695
Kauppi M, Stenholm S, Impivaara O, Mäki J, Heliövaara M, Jula A (2014) Fall-related risk factors and heel quantitative ultrasound in the assessment of hip fracture risk: a 10-year follow-up of a nationally representative adult population sample. Osteoporos Int 25(6):1685–1695
Pouwels S, Bazelier MT, De Boer A, Weber WEJ, Neef C, Cooper C et al (2013) Risk of fracture in patients with Parkinson’s disease. Osteoporos Int 24(8):2283–2290
Huang Y-F, Cherng Y-G, Hsu SPC, Yeh C-C, Chou Y-C, Wu C-H et al (2015) Risk and adverse outcomes of fractures in patients with Parkinson’s disease: two nationwide studies. Osteoporos Int 26(6):1723–1732
Melton LJ, Leibson CL, Achenbach SJ, Bower JH, Maraganore DM, Oberg AL et al (2006) Fracture risk after the diagnosis of Parkinson’s disease: influence of concomitant dementia. Mov Disord 21(9):1361–1367
Cauley JA, Cawthon PM, Peters KE, Cummings SR, Ensrud KE, Bauer DC et al (2016) Risk factors for hip fracture in older men: the osteoporotic fractures in men study (MrOS). J Bone Miner Res 31(10):1810–1819
Chen Y-Y, Cheng P-Y, Wu S-L, Lai C-H (2012) Parkinson’s disease and risk of hip fracture: an 8-year follow-up study in Taiwan. Parkinsonism Relat Disord 18(5):506–509
Walker RW, Chaplin A, Hancock RL, Rutherford R, Gray WK (2013) Hip fractures in people with idiopathic Parkinson’s disease: incidence and outcomes. Mov Disord 28(3):334–340
Yamanashi A, Yamazaki K, Kanamori M, Mochizuki K, Okamoto S, Koide Y et al (2005) Assessment of risk factors for second hip fractures in Japanese elderly. Osteoporos Int 16(10):1239–1246
Schousboe JT, Paudel ML, Taylor BC, Virnig BA, Cauley JA, Curtis JR et al (2013) Magnitude and consequences of misclassification of incident hip fractures in large cohort studies: the study of osteoporotic fractures and medicare claims data. Osteoporos Int 24(3):801–810
Wiklund R, Toots A, Conradsson M, Olofsson B, Holmberg H, Rosendahl E et al (2016) Risk factors for hip fracture in very old people: a population-based study. Osteoporos Int 27(3):923–931
Cauley JA, Lui L, Genant HK, Salamone L, Browner W, Fink HA et al (2009) Risk factors for severity and type of the hip fracture. J Bone Miner Res 24(5):943–955
Abstracts of the eighteenth international congress of Parkinson’s disease and movement disorders, June 8–12, 2014, Stockholm, Sweden (2014). Mov Disord 29(Suppl 1):S1–S687
Olsen J, Nøhr EA, Thomsen RW, Støvring H (2013) Non-communicable disease epidemic: epidemiology in action (EuroEpi 2013 and NordicEpi 2013) Aarhus, Denmark from 11 August to 14 August 2013. Eur J Epidemiol 28:S1-270
Nyström H, Nordström A, Nordström P (2016) Risk of injurious fall and hip fracture up to 26 y before the diagnosis of Parkinson disease: Nested case-control studies in a nationwide cohort. PLoS Med 13(2):e1001954
Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE et al (1995) Risk factors for hip fracture in white women. N Engl J Med 332(12):767–774
Paul SS, Harvey L, Canning CG, Boufous S, Lord SR, Close JCT et al (2017) Fall-related hospitalization in people with Parkinson’s disease. Eur J Neurol 24(3):523–529
Kalilani L, Asgharnejad M, Palokangas T, Durgin T (2016) Comparing the incidence of falls/fractures in Parkinson’s disease patients in the US population. PLoS One 11(9):e0161689
Chen YY, Cheng PY, Wu SL, Lai CH (2012) Parkinson’s disease and risk of hip fracture: an 8-year follow-up study in Taiwan. Parkinsonism Relat Disord 18(5):506–509
Stang A (2010) Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Higgins J, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions. Wiley, Hoboken
Rosenthal L, Schisterman E (2010) Meta-analysis: drawing conclusions when study results vary. Methods Mol Biol 594:427–434
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Johnson AL, Smith JJ, Smith JM, Sanzone AG (2013) Vitamin D insufficiency in patients with acute hip fractures of all ages and both sexes in a sunny climate. J Orthop Trauma 27(12):e275–e280
Chalmers J, Conacher W, Gardner D, Scott P (1967) Osteomalacia—a common disease in elderly women. J Bone Joint Surg Br 49(3):403–423
Feskanich D, Willett WC, Colditz GA (2003) Calcium, vitamin D, milk consumption, and hip fractures: a prospective study among postmenopausal women. Am J Clin Nutr 77(2):504–511
Brown IRF, Bakowska A, Millard PH (1976) Vitamin D status of patients with femoral neck fractures. Age Ageing 5(3):127–131
Wootton R, Brereton PJ, Clark MB, Hesp R, Hodkinson HM, Klenerman L et al (1979) Fractured neck of femur in the elderly: an attempt to identify patients at risk. Clin Sci 57(1):93–101
Van Den Bos F, Speelman AD, Samson M, Munneke M, Bloem BR, Verhaar HJJ (2012) Parkinson’s disease and osteoporosis. Age Ageing 42(2):156–162
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281
Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K (2005) Abnormal bone and calcium metabolism in immobilized Parkinson’s disease patients. Mov Disord 20(12):1598–1603
Evatt ML, DeLong MR, Khazai N, Rosen A, Triche S, Tangpricha V (2008) Prevalence of vitamin D insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol 65(10):1348–1352
Snow-Harter C, Bouxsein M, Lewis B, Charette S, Weinstein P, Marcus R (1990) Muscle strength as a predictor of bone mineral density in young women. J Bone Miner Res 5(6):589–595
Blain H, Vuillemin A, Teissier A, Hanesse B, Guillemin F, Jeandel C (2001) Influence of muscle strength and body weight and composition on regional bone mineral density in healthy women aged 60 years and over. Gerontology 47(4):207–212
Pang MYC, Eng JJ, McKay HA, Dawson AS (2005) Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporos Int 16(12):1769–1779
Cano-de-la-Cuerda R, Pérez-de-Heredia M, Miangolarra-Page JC, Munoz-Hellín E, Fernández-de-las-Penas C (2010) Is there muscular weakness in Parkinson’s disease? Am J Phys Med Rehabil 89(1):70–76
Lorefält B, Toss G, Granérus A (2007) Bone mass in elderly patients with Parkinson’s disease. Acta Neurol Scand 116(4):248–254
Johnell O, Gullberg BO, Kanis JA, Allander E, Elffors L, Dequeker J et al (1995) Risk factors for hip fracture in European women: the MEDOS study. J Bone Miner Res 10(11):1802–1815
van Nimwegen M, Speelman AD, Smulders K, Overeem S, Borm GF, Backx FJG et al (2010) Design and baseline characteristics of the ParkFit study, a randomized controlled trial evaluating the effectiveness of a multifaceted behavioral program to increase physical activity in Parkinson patients. BMC Neurol 10(1):70
Sato Y, Kaji M, Tsuru T, Oizumi K (2001) Risk factors for hip fracture among elderly patients with Parkinson’s disease. J Neurol Sci 182(2):89–93
Bikle DD (2008) Integrins, insulin like growth factors, and the skeletal response to load. Osteoporos Int 19(9):1237–1246
Santos A, Bakker AD, Klein-Nulend J (2009) The role of osteocytes in bone mechanotransduction. Osteoporos Int 20(6):1027–1031
Minaire P (1989) Immobilization osteoporosis: a review. Clin Rheumatol 8(2):95–103
Wyshak G (1981) Hip fracture in elderly women and reproductive history. J Gerontol 36(4):424–427
Smith JR (1980) Dietary and hormonal factors in bone loss. Henry Ford Hosp Med J 28(2–3):171–181
Vestergaard P, Rejnmark L, Mosekilde L (2007) Fracture risk associated with parkinsonism and anti-Parkinson drugs. Calcif Tissue Int 81(3):153–161
Rogers JD, Sanchez-Saffon A, Frol AB, Diaz-Arrastia R (2003) Elevated plasma homocysteine levels in patients treated with levodopa: association with vascular disease. Arch Neurol 60(1):59–64
Melton LJ (2000) Who has osteoporosis? A conflict between clinical and public health perspectives. J Bone Miner Res 15(12):2309–2314
Garriguet D (2011) Bone health: osteoporosis, calcium and vitamin D. Health Rep 22(3):7
Dhanwal DK, Dennison EM, Harvey NC, Cooper C (2011) Epidemiology of hip fracture: worldwide geographic variation. Indian J Orthop 45(1):15
Dhanwal D, Cooper C, Dennison E (2010)Geographic variation in osteoporotic hip fracture incidence: the growing importance of Asian influences in coming decades.J Osteoporos 2010:757102
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ali Hosseinzadeh, Malahat Khalili, Behnaz Sedighi, Sohrab Iranpour, and Ali Akbar Haghdoost declare that they have no conflict of interest. The authors alone are responsible for the content and writing of this paper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Hosseinzadeh, A., Khalili, M., Sedighi, B. et al. Parkinson’s disease and risk of hip fracture: systematic review and meta-analysis. Acta Neurol Belg 118, 201–210 (2018). https://doi.org/10.1007/s13760-018-0932-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-018-0932-x