Abstract
Summary
The association between Parkinson’s disease and fracture was not completely understood. This nationwide study investigated increased risk of fracture in patients with Parkinson’s disease. In the nested cohort study, Parkinson’s disease was associated with pneumonia, septicemia, stroke, urinary tract infection, and mortality after fracture admission.
Introduction
Falls are a common complication in people with Parkinson’s disease (PD). This study evaluated fracture risk and post-fracture outcomes in patients with PD.
Methods
We identified 1,423 adults aged 40 years and older newly diagnosed with PD using the Taiwan National Health Insurance Research Database from 2000 to 2003. Comparison cohort consisted of 5,692 adults without PD randomly selected from the same dataset, frequency matched in age and sex. Followed-up events of fracture from January 1, 2000, until December 31, 2008, were ascertained from medical claims. Adjusted hazard ratios (HR) and 95 % confidence interval (CI) of fracture associated with PD were evaluated. Another nested cohort study of 397,766 hospitalized fracture patients analyzed for adjusted odds ratios (ORs) and 95 % CIs of adverse events after fracture among patients with and without PD between 2004 and 2010.
Results
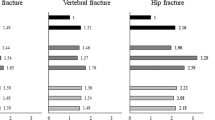
The incidences of fracture for people with and without PD were 39.5 and 23.9 per 1,000 person-years, respectively (p < 0.0001). Compared with control, the adjusted HR of fracture was 2.25 (95 % CI 1.97–2.58) for PD patients. Previous PD was associated with risks of pneumonia (OR 1.44, 95 % CI 1.36–1.52), septicemia (OR 1.41, 95 % CI 1.33–1.49), stroke (OR 1.40, 95 % CI 1.32–1.50), urinary tract infection (OR 1.53, 95 % CI 1.46–1.61), and mortality (OR 1.25, 95 % CI 1.15–1.35) after fracture.
Conclusions
PD was associated with higher risk of fracture. Patients with PD had more complications and mortality after fracture. Fracture prevention and attention to post-fracture adverse events are needed for this susceptible population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the most common serious movement disorder and is characterized by its cardinal features of tremor, bradykinesia, and rigidity [1]. Patients with PD also commonly show postural instability after a few years [1]. With prevalence of 315 per 100,000 persons in people aged 40 years and older worldwide [2], this progressive neurodegenerative disorder affects approximately 1 % of people aged 60 years and older [3–5]. It has been estimated that annual direct medical expenditures for people with PD were more than twice those of people without PD, with projected annual direct health care expenditures of US$23 billion for individuals with PD in the USA alone [6]. Severe consequences of PD such as increased hospitalization and mortality also represent major health and policy concerns [7].
Falls were identified as a common complication in people with PD [1, 8], and these incidents can lead to various fractures, disabilities, and mortality [9]. More than two million fracture incidents cost an estimated US$17 billion in the USA in 2005 [10]. Fractures are also an important public health burden in the UK, with estimated overall costs of £5.1 billion in 2010 [11].
Previous studies reported an association between PD and fracture risk [12–20]. However, these were limited by small sample sizes [12, 14, 17–19] and by focusing solely on male or female patients [14, 20] or on specific types of fractures [15, 16, 19], inadequate diagnosis of PD [14], or inadequate adjustment for potential confounders [12, 14, 16–19]. The association of PD and post-fracture mortality remains unknown.
We conducted two nationwide population-based retrospective cohort studies using Taiwan’s National Health Insurance Research Database to evaluate the risk of fractures and to investigate the impact of PD on adverse events and in-hospital mortality after fractures.
Methods
Source of data
Taiwan’s National Health Insurance Program has recorded all medical reimbursement claims since 1996, and the Taiwan National Health Insurance Research Database containing these claims is available to researchers with identification of beneficiaries scrambled to protect patient privacy. Sets of information available for this study include sex, date of birth, diagnoses at outpatient visits, admissions and discharges, health care provided, medications prescribed, medical institutions, and physicians providing services. For research and administrative purposes, Taiwan’s National Health Research Institute also provided a data subset of one million beneficiaries randomly selected from insurance enrollees aged 0–113 years, this subset represents nearly 5 % of Taiwan’s insured population. Information about medical care for these persons was collected from 1996 to 2008. The validity of studies based on the Taiwan National Health Insurance Research Database has been well accepted by peer reviewers for prominent scientific journals worldwide [21–24].
Ethical approval
Insurance reimbursement claims used in this study were from Taiwan’s National Health Insurance Research Database, which is available for public access. This study was conducted in accordance with the Helsinki Declaration. To protect personal privacy, the electronic database was decoded with patient identifications scrambled for further public access for research. According to National Health Research Institutes regulations, informed consent is not required because of the use of decoded and scrambled patient identifications. However, this study was evaluated and approved by Taiwan’s National Health Research Institutes (NHIRD-103-121).
Study design
In this longitudinal cohort of one million insured individuals, we identified as the exposed cohort 1,423 patients age 40 years or older newly diagnosed with PD (without any previous record of diagnosis or treatment for PD from the database established in 1996) between 2000 and 2003 who had no history of fracture before the index date of diagnosis. To identify patients with PD more strictly, at least three outpatient visits or inpatient medical services with principal diagnosis of PD were required. During the same index period, we identified 5,692 people age 40 years or older matched by age and sex who had no diagnosis of PD (fourfold non-PD controls) without any history of fracture as the nonexposure cohort. Patients with any diagnosis of fracture between January 1, 1996, and December 31, 2003, were excluded to ensure that all study participants were free of fracture at the start of both cohorts. In this study, participants with previously known osteoporosis as well as bisphosphonate treatment before the index date were excluded. Follow-up started from January 1, 2000 and lasted until December 31, 2008 or censoring due to death, loss to follow-up, or other causes. We sought to validate whether individuals with PD faced an increased risk of fracture.
To investigate the impact of PD on post-fracture outcomes, a nested retrospective cohort study was conducted. We identified 397,766 hospitalized patients with first fracture event between 2004 and 2010, including 7,479 patients with pre-fracture PD and 390,287 without PD. We compared post-fracture septicemia, pneumonia, urinary tract infection, stroke, and mortality for a 30-day period postoperatively among fracture patients with or without pre-fracture PD.
Measures and definitions
We identified income status by defining low-income patients as those qualifying for waived medical copayment as verified by the Bureau of National Health Insurance. Population density was calculated by dividing the population (persons) by the area (square kilometers) for each administrative unit of Taiwan and then sorting these areas into quartiles of low, moderate, high, and very high urbanization. These categories were used as surrogates for residential urbanization. Use of osteoporosis medications such as alendronate, pamidronate disodium, risedronate sodium, zoledronic acid, and hormone replacement therapy was also analyzed.
We used the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) to define coexisting medical conditions and post-fracture complications. Pre-fracture PD (ICD-9-CM 332) was defined as primary exposure. Coexisting medical conditions determined from medical claims for the follow-up period included mental disorders (ICD-9-CM 290-319), hypertension (ICD-9-CM 401-405), chronic obstructive pulmonary disease (ICD-9-CM 490-496), ischemic heart disease (ICD-9-CM 410-414), diabetes (ICD-9-CM 250), stroke (ICD-9-CM 430-438), hyperlipidemia (ICD-9-CM 272.0, 272.1, and 272.2), osteoporosis (ICD-9-CM 733.0), asthma, congestive heart failure, liver cirrhosis (ICD-9-CM 571), and inflammatory bowel disease (ICD-9-CM 555, 556). We defined alcohol-related illnesses as alcoholic psychoses (ICD-9-CM code 291), alcohol dependence syndrome (ICD-9- CM code 303), alcohol abuse (ICD-9-CM code 305), alcoholic fatty liver (ICD-9-CM code 571.0), acute alcoholic hepatitis (ICD-9-CM code 571.1), alcoholic cirrhosis of the liver (ICD-9-CM code 571.2), and alcoholic liver damage (ICD-9-CM code 571.3). Renal dialysis was identified by administration code (D8, D9). Fracture is the main outcome of this retrospective cohort study, including skull bone fracture (ICD-9-CM 800-804) and fractures of neck and trunk (ICD-9-CM 805-809), upper limb (ICD-9-CM 810-819), lower limb (ICD-9-CM 820-829), and hip (ICD-9-CM 820). The association of PD with various fractures (including skull bone, neck, trunk, upper limb, lower limb, hip fracture, and at least two types of fracture) and post-fracture mortality was also analyzed. Complications after fracture were analyzed as secondary outcomes, including septicemia (ICD-9-CM 038 and 998.5), pneumonia (ICD-9-CM 480-486), urinary tract infection (ICD-9-CM 599.0), and stroke (ICD-9-CM 430-438). In-hospital post-fracture mortality was also considered as outcome in the nested cohort study.
Statistical analysis
In the retrospective cohort study including all participants, we used chi-square tests to compare sociodemographic characteristics and coexisting medical conditions between people with and without PD. We calculated the hazard ratios (HRs) with 95 % confidence intervals (CIs) for risk of fracture after PD, adjusting for age, sex, low income, urbanization, stay in teaching hospital, mental disorders, hypertension, chronic obstructive pulmonary disease, ischemic heart disease, diabetes, stroke, hyperlipidemia, osteoporosis, asthma, congestive heart failure, liver cirrhosis, alcohol-related illness, inflammatory bowel disease, renal dialysis, anxiolytics, antiepileptics, antipsychotics, antidepressants, and oral steroids in multivariate Cox proportional hazards regression models. The adjusted HRs of fracture associated with diabetes in the sex- and age-stratified analyses were also calculated.
In the nested cohort study, the sociodemographics and coexisting medical conditions between fracture patients with and without PD were compared using chi-square tests. The adjusted odds ratios (ORs) and 95 % CIs of post-fracture septicemia, pneumonia, urinary tract infection, and stroke as well as mortality associated with pre-fracture PD were calculated in the multivariate logistic regressions with adjustment for age, sex, low income, urbanization, mental disorders, hypertension, chronic obstructive pulmonary disease, ischemic heart disease, diabetes, stroke, hyperlipidemia, osteoporosis, asthma, congestive heart failure, liver cirrhosis, alcohol-related illness, inflammatory bowel disease, renal dialysis, anxiolytics, antiepileptics, antipsychotics, antidepressants, oral steroids, and fracture-repair surgery. Risks and mortality of subtypes of fracture for patients with PD were also calculated in the multivariate Cox models and logistic regression models, respectively. SAS version 9.1 (SAS Institute, Inc., Cary, NC) statistical software was used for data analyses; two-sided p < 0.05 indicated significant differences between groups.
Results
After the frequency matching in age and sex between cohorts with and without PD, there were no significant differences in age and sex (Table 1). Compared with the cohort without PD, patients with PD had higher proportions with mental disorders (p < 0.0001), chronic obstructive pulmonary disease (p < 0.0001), ischemic heart disease (p < 0.0001), diabetes (p = 0.0022), stroke (p < 0.0001), congestive heart failure (p = 0.0127), and renal dialysis (p = 0.0440). Proportionally, more patients with PD used selected medications than did those without PD; these included antidepressants (p < 0.0001), antiepileptics (p < 0.0001), antipsychotics (p < 0.0001), and anxiolytics (p < 0.0001).
Table 2 shows higher incidence of fracture was found in patients with PD than in those without PD (39.5 vs. 23.9 per 1,000 person-years, p < 0.0001) during the follow-up period; the corresponding HR of fracture associated with PD was 2.25 (95 % CI 1.97–2.58). The increased fracture rates were consistently found in all age groups and either gender. The association between PD and fracture risk was significant both in females (HR 2.11, 95 % CI 1.76–2.52) and in males (HR 2.46, 95 % CI 1.99–3.03). Patients with PD were at greater risk of fracture in groups aged 40–49 (HR 2.80, 95 % CI 1.11–7.07), 50–59 (HR 3.19, 95 % CI 1.80–5.65), 60–69 (HR 2.91, 95 % CI 2.17–3.89), 70–79 (HR 2.13, 95 % CI 1.76–2.57), and ≥80 (HR 1.95, 95 % CI 1.38–2.76).
In the nested cohort study including 397,766 patients with fracture (Table 3), the increased fracture rates were consistently found in all age groups and either gender. Higher proportions of patients with PD than patients without PD were noted among females (p < 0.0001), older people (p < 0.0001), those living in the most highly urbanized areas (p = 0.0005), and those with low incomes (p = 0.0004), stay in teaching hospital (p < 0.0001), hypertension (p < 0.0001), mental disorders (p < 0.0001), diabetes (p < 0.0001), chronic obstructive pulmonary disease (p < 0.0001), ischemic heart disease (p < 0.0001), cancer (p < 0.0001), osteoporosis (p < 0.0001), stroke (p < 0.0001), asthma (p < 0.0001), congestive heart failure (p < 0.0001), inflammatory bowel disease (p = 0.0008), and alcohol-related illness (p < 0.0001). Proportionately, more fracture patients with PD used antidepressants (p < 0.0001), antiepileptics (p < 0.0001), antipsychotics (p < 0.0001), anxiolytics (p < 0.0001), and oral steroids (p < 0.0001) than did fracture patients without PD. Among fracture patients with previous PD, men had higher mean age than women (77.3 ± 8.5 vs. 76.8 ± 8.0, p = 0.0078); among fracture patients without previous PD, women had higher mean of age than men (66.7 ± 13.7 vs. 61.3 ± 14.6, p < 0.0001) (this data is not shown in the tables).
In the nested retrospective cohort study (Table 4), patients with PD had higher risk of mortality (OR 1.25, 95 % CI 1.15–1.35), pneumonia (OR 1.44, 95 % CI 1.36–1.52), septicemia (OR 1.41, 95 % CI 1.33–1.49), stroke (OR 1.40, 95 % CI 1.32–1.50), and urinary tract infection (OR 1.53, 95 % CI 1.46–1.61) after fracture compared with those without PD.
Higher risks of neck or trunk fracture (HR 2.33, 95 % CI 1.84–2.96), upper limb fracture (HR 1.87, 95 % CI 1.49–2.44), lower limb fracture (HR 1.91, 95 % CI 1.54–2.36), and hip fracture (HR 2.56, 95 % CI 2.02–3.26) were found in patients with PD than in those without PD (Table 5). Pre-fracture PD was associated with mortality after upper limb fracture (OR 1.74, 95 % CI 1.40–2.15), lower limb fracture (OR 1.21, 95 % CI 1.10–1.33), and hip fracture (OR 1.13, 95% CI 1.03–1.24).
Discussion
Using claims data from Taiwan’s National Health Insurance, this nationwide retrospective cohort study showed approximately twofold increased risk of all types of fracture in patients with PD. A further nested cohort study showed that patients with PD were associated with increased post-fracture pneumonia, septicemia, stroke, urinary tract infection, and mortality rate when compared with the non-PD population.
Older age [25], female gender [25], low income [26], and urbanization [26] were considered as sociodemographic risk factors for fracture among patients with PD [4, 5, 17]. These were the potential confounding factors when analyzing the associations between PD and fracture. To avoid bias when investigating risk and outcomes of fracture in patients with PD, we used multivariate regression models to adjust these sociodemographic characteristics. In the subgroup analysis, female gender was associated with higher risk of fracture, a finding compatible with results of previous studies [13, 16, 17]. Women have a higher incidence of osteoporosis because they lose bone mass faster than men due to menopause. Osteoporosis was considered the most important risk factor for fracture, and this association has been well studied in both general populations and PD patients [10, 11, 14, 20]. Hypertension [27], diabetes [22], mental disorders [28], asthma or chronic obstructive pulmonary disease [29. 30], ischemic heart disease [29, 30], hyperlipidemia [31], stroke [29], liver cirrhosis [30], and renal failure [30] have been shown to be independently associated with risk of fracture. These were also comorbidities for patients with PD [32, 33]. However, previous studies were limited by inadequate control for coexisting medical conditions when investigating the association between PD and fracture risk [12, 14, 16, 17]. The current study found that fracture risk increased in patients with PD after adjustment for these potential confounding factors.
Among all subtypes of fracture, hip fracture is the most closely linked to falls [34]. Falls are identified as a frequent complication for people with PD [1, 8, 15, 16, 19]. Consistent with previous studies [1, 8, 15, 16, 19], this study found that the highest risk of subtype fracture is hip fracture for patients with PD. Patients with PD also had increased risk of neck or trunk, upper limb, and lower limb fracture in the present investigation. The novel finding of this study is that patients with PD also had twofold risk of at least two types of fracture compared with those without PD. For post-fracture mortality, we investigated whether PD had impact on mortality after upper or lower limb or hip fracture and at least two types of fracture. Therefore, it is important to prevent falls and various types of fractures caused by daily activities in patients with PD, and specific health care protocols are needed to address this issue.
To the best of our knowledge, the present study is the first investigation reporting increased post-fracture pneumonia, septicemia, stroke, urinary tract infection, and mortality in patients with PD. Fracture and consequent need for hospitalization may cause deterioration of PD patients after surgery [35]. Difficulties with self-care during inpatient treatment may be the main reason more adverse events were found after fracture in patients with PD. During hospitalization after fractures, PD patients are often cared for by non-neurologists who might not be familiar with PD, and complex PD medication regimens might be interrupted resulting in reduced efficacy. Other clinical problems associated with PD patients’ reduced mobility such as infection, pressure ulcer, and confusion might also be involved [36]. The importance of guidelines and education for interdisciplinary health care teams and of timely neurological consultation for management of PD patients during hospitalization should not be underestimated [35–37].
Several reasons may explain why patients with PD had increased risk of fracture. First, it is reasonable to consider that postural instability may contribute to the correlation between PD and fracture because frequent falls are an important and disabling feature of PD [8]. The second possible cause is PD patients’ low bone mineral density that may lead to subsequent fractures [14]. Sunlight deprivation, decreased dietary intake of vitamin D, and prolonged periods of immobility in advanced PD may all contribute to reduced bone mass [38, 39]. Third, sarcopenia by malnutrition in patients with PD and possible direct negative effects of PD medication on the osseous system are also possible explanations for the increased risk of fracture in patients with PD [40–42]. The side effects of PD-related medication include postural hypotension and confusion causing loss of protective reflexes during falls [43]. In addition, although we adjusted for dementia and other mental illness in the regression models, the treatments for these comorbidities are also risk factors for fracture [28, 44].
The strengths of the present study included large sample size, cohort study design, multivariate adjustment, and analyzing all types of fracture. It was also not restricted by specific patient groups because the National Health Insurance Research Database is a nationwide, population-based and highly representative database. Although previous studies have reported that PD patients had increased fracture risk [12–14, 16, 17, 45], the current study is the first investigation finding that patients with PD have increased risks of post-fracture adverse outcomes such as pneumonia, septicemia, stroke, and urinary tract infection after multivariate adjustment for sociodemographics and coexisting medical conditions.
This study has limitations. First, we used insurance claims data that lacks information on sociodemographics and lifestyle correlating with the risks of fracture or PD. Second, a study from this reimbursement claims database possibly under-reports patients with minor fractures who neglected to seek treatment or did not know that they had experienced fractures. However, these data should be distributed equally between both groups without causing bias in the results. Third, though the accuracy of major diagnosis codes from the research database in studies based on these has been accepted by peer reviewers for prominent scientific journals worldwide [21–23], validity of PD, fracture, and other comorbidity and complication codes might still be a limitation of this study. Future research is needed that includes prospective design and lifestyle information as covariates in multivariate analyses. Fourth, although the risk of fracture among patients with PD in our study is similar with previous investigations [12–20], part of our findings (such as subtypes of fracture) could not be generalized to Caucasian directly because the results of this study are based on the data from Taiwan National Health Insurance Research Database that represents the population of Taiwan. In addition, to define patients with PD by physician diagnoses may be not totally correct because misdiagnosis may occur although the possibility of misdiagnosis is relatively very low under the inclusive criteria of physician’s primary diagnosis of PD for three visits. Finally, the complications of PD may not evolve linearly with longer disease duration but increase exponentially after 10 or more years of disease duration. Thus, underestimation of the influence of PD on risk of fracture and post-fracture adverse events may exist in this study.
In conclusion, PD is an important independent risk factor for fracture and post-fracture adverse outcomes. This study provided comprehensive assessment of fracture risk and post-fracture outcomes in patients with PD. Further studies are needed to develop specific strategies to decrease fracture risks and post-fracture adverse outcomes for this fragile patient population.
References
Samii A, Nutt JG, Ransom BR (2004) Parkinson’s disease. Lancet 363:1783–1793
Pringsheim T, Jette N, Frolkis A, Steeves TD (2014) The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 29:1583–1590
Nussbaum RL, Ellis CE (2003) Alzheimer’s disease and Parkinson’s disease. N Engl J Med 348:1356–1364
De Rijk MC, Launer LJ, Berger K et al (2000) Prevalence of Parkinson’s disease in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54:S21–S23
De Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5:525–535
Huse DM, Schulman K, Orsini L, Castelli-Haley J, Kennedy S, Lenhart G (2005) Burden of illness in Parkinson’s disease. Mov Disord 20:1449–1454
Berger K, Breteler MM, Helmer C et al (2000) Prognosis with Parkinson’s disease in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54:S24–S27
Pickering RM, Grimbergen YAM, Rigney U et al (2007) A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov Disord 22:1892–1900
Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR (2009) Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 301:513–521
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22:465–475
Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, Jönsson B (2011) Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 6:59–155
Genever RW, Downes TW, Medcalf P (2005) Fracture rates in Parkinson’s disease compared with age- and gender-matched controls: a retrospective cohort study. Age Ageing 34:21–24
Pouwels S, Bazelier MT, de Boer A, Weber WE, Neef C, Cooper C, de Vries F (2013) Risk of fracture in patients with Parkinson’s disease. Osteoporos Int 24:2283–2290
Fink HA, Kuskowski MA, Taylor BC, Schousboe JT, Orwoll ES, Ensrud KE (2008) Association of Parkinson’s disease with accelerated bone loss, fractures and mortality in older men: the Osteoporotic Fractures in Men (MrOS) study. Osteoporos Int 19:1277–1282
Harris-Hayes M, Willis AW, Klein SE, Czuppon S, Crowner B, Racette BA (2014) Relative mortality in U.S. Medicare beneficiaries with Parkinson disease and hip and pelvic fractures. J Bone Joint Surg Am 96:e27
Chen YY, Cheng PY, Wu SL, Lai CH (2012) Parkinson’s disease and risk of hip fracture: an 8-year follow-up study in Taiwan. Parkinsonism Relat Disord 18:506–509
Melton LJ 3rd, Leibson CL, Achenbach SJ, Bower JH, Maraganore DM, Oberg AL, Rocca WA (2006) Fracture risk after the diagnosis of Parkinson’s disease: influence of concomitant dementia. Mov Disord 21:1361–1367
Johnell O, Melton LJ 3rd, Atkinson EJ, O'Fallon WM, Kurland LT (1992) Fracture risk in patients with parkinsonism: a population-based study in Olmsted County, Minnesota. Age Ageing 21:32–38
Sato Y, Kaji M, Tsuru T, Oizumi K (2001) Risk factors for hip fracture among elderly patients with Parkinson’s disease. J Neurol Sci 182:89–93
Schneider JL, Fink HA, Ewing SK, Ensrud KE, Cummings SR (2008) The association of Parkinson’s disease with bone mineral density and fracture in older women. Osteoporos Int 19:1093–1097
Liao CC, Shen WW, Chang CC, Chang H, Chen TL (2013) Surgical adverse outcomes in patients with schizophrenia: a population-based study. Ann Surg 257:433–438
Liao CC, Lin CS, Shih CC, Yeh CC, Chang YC, Lee YW, Chen TL (2014) Increased risk of fracture and postfracture adverse events in patients with diabetes: two nationwide population-based retrospective cohort studies. Diabetes Care 37:2246–2252
Liao CC, Chou YC, Yeh CC, Hu CJ, Chiu WT, Chen TL (2014) Stroke risk and outcomes in patients with traumatic brain injury: 2 nationwide studies. Mayo Clin Proc 89:163–172
Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML (2011) Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 20:236–242
Court-Brown CM, Caesar B (2006) Epidemiology of adult fractures: a review. Injury 37:691–697
Guilley E, Herrmann F, Rapin CH, Hoffmeyer P, Rizzoli R, Chevalley T (2011) Socioeconomic and living conditions are determinants of hip fracture incidence and age occurrence among community-dwelling elderly. Osteoporos Int 22:647–653
Yang S, Nguyen ND, Center JR, Eisman JA, Nguyen TV (2014) Association between hypertension and fragility fracture: a longitudinal study. Osteoporos Int 25:97–103
Baker NL, Cook MN, Arrighi HM, Bullock R (2011) Hip fracture risk and subsequent mortality among Alzheimer’s disease patients in the United Kingdom, 1988–2007. Age Ageing 40:49–54
Dennison EM, Compston JE, Flahive J et al (2012) Effect of co-morbidities on fracture risk: findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 50:1288–1293
Hippisley-Cox J, Coupland C (2012) Derivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: prospective open cohort study. BMJ 344:e3427
Trimpou P, Odén A, Simonsson T, Wilhelmsen L, Landin-Wilhelmsen K (2011) High serum total cholesterol is a long-term cause of osteoporotic fracture. Osteoporos Int 22:1615–1620
Bhattacharjee S, Sambamoorthi U (2013) Co-occurring chronic conditions and healthcare expenditures associated with Parkinson’s disease: a propensity score matched analysis. Parkinsonism Relat Disord 19:746–750
Leibson CL, Maraganore DM, Bower JH, Ransom JE, O'brien PC, Rocca WA (2006) Comorbid conditions associated with Parkinson’s disease: a population-based study. Mov Disord 21:446–455
Kauppi M, Stenholm S, Impivaara O, Mäki J, Heliövaara M, Jula A (2014) Fall-related risk factors and heel quantitative ultrasound in the assessment of hip fracture risk: a 10-year follow-up of a nationally representative adult population sample. Osteoporos Int 25:1685–1695
Gerlach OH, Broen MP, van Domburg PH, Vermeij AJ, Weber WE (2012) Deterioration of Parkinson’s disease during hospitalization: survey of 684 patients. BMC Neurol 12:13
Gerlach OH, Winogrodzka A, Weber WE (2011) Clinical problems in the hospitalized Parkinson’s disease patient: systematic review. Mov Disord 26:197–208
Chou KL, Zamudio J, Schmidt P et al (2011) Hospitalization in Parkinson disease: a survey of National Parkinson Foundation Centers. Parkinsonism Relat Disord 17:440–445
Sato Y, Kikuyama M, Oizumi K (1997) High prevalence of vitamin D deficiency and reduced bone mass in Parkinson’s disease. Neurology 49:1273–1278
Sato Y, Iwamoto J, Honda Y (2011) Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in Parkinson’s disease. Parkinsonism Relat Disord 17:22–26
Invernizzi M, Carda S, Viscontini GS, Cisari C (2009) Osteoporosis in Parkinson’s disease. Parkinsonism Relat Disord 15:339–346
Gnädinger M, Mellinghoff HU, Kaelin-Lang A (2011) Parkinson’s disease and the bones. Swiss Med Wkly 141:w13154
Dobson R, Yarnall A, Noyce AJ, Giovannoni G (2013) Bone health in chronic neurological diseases: a focus on multiple sclerosis and parkinsonian syndromes. Pract Neurol 13:70–79
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373:2055–2066
Pouwels S, van Staa TP, Egberts AC, Leufkens HG, Cooper C, de Vries F (2009) Antipsychotic use and the risk of hip/femur fracture: a population-based case-control study. Osteoporos Int 20:1499–1506
Vestergaard P, Rejnmark L, Mosekilde L (2007) Fracture risk associated with parkinsonism and anti-Parkinson drugs. Calcif Tissue Int 81:153–161
Acknowledgments
This study is based in part on data obtained from the National Health Insurance Research Database, which is provided by Taiwan’s Bureau of National Health Insurance of the Ministry of Health and Welfare, and managed by the National Health Research Institutes (NHIRD-103-121). The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, the Ministry of Health and Welfare, or the National Health Research Institutes.
Funding
This study was supported by a grant (NSC102-2314-B-038-021-MY3) from Taiwan’s Ministry of Science and Technology.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
T.-L. Chen and C.-C. Liao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Huang, YF., Cherng, YG., Hsu, S.P.C. et al. Risk and adverse outcomes of fractures in patients with Parkinson’s disease: two nationwide studies. Osteoporos Int 26, 1723–1732 (2015). https://doi.org/10.1007/s00198-015-3052-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3052-y