Abstract
Natalizumab (Tysabri®) is highly efficacious in controlling disease activity in relapsing multiple sclerosis (MS) patients. As it is one of the more recent therapies for MS, there remains a need for long-term safety and efficacy data of natalizumab in a clinical practice setting. The Tysabri observational program (TOP) is an open-label, multicenter, multinational, prospective observational study, aiming to recruit up to 6,000 patients with relapsing-remitting MS from Europe, Canada and Australia. The objectives of this study are to collect long-term safety and efficacy data on disease activity and disability progression. We report here the interim results of the 563 patients included in TOP between December 2007 and 2012 from Belgium. This patient cohort was older at baseline, had longer disease duration, higher neurological impairment, and a higher baseline annualized relapse rate, when compared to patients included in the pivotal phase III AFFIRM trial. Nevertheless, the efficacy of natalizumab was comparable. The annualized relapse rate on treatment was reduced by 90.70 % (p < 0.0001) with a cumulative probability of relapse of 26.87 % at 24 months. The cumulative probabilities of sustained disability improvement and progression at 24 months were 25.68 and 9.01 %, respectively. There were no new safety concerns over the follow-up period. Two cases of progressive multifocal leukoencephalopathy were diagnosed. Our results are consistent with other observational studies in the post-marketing setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natalizumab (Tysabri®) is the first humanized monoclonal antibody directed against α4β1-integrin authorized June 27th 2006 by the European Medicines Agency (EMA) for the treatment of multiple sclerosis (MS). It is indicated as monotherapy in patients suffering from breakthrough disease despite treatment with interferon-beta (IFN-β) for at least 1 year or in patients with at least two relapses in a single year. In Belgium, natalizumab has been available since December 2007 with current reimbursement criteria aligned on EMA recommendations.
In the pivotal trial AFFIRM, in which 627 patients were randomized to receive 30 natalizumab infusions, both primary endpoints were met: the annualized relapse rate (ARR) was reduced by 68 % at 1 year (p < 0.001) and the 3-month sustained disability progression rate [as assessed by the Expanded Disability Status Scale (EDSS)], was reduced by 42 % (p < 0.001) over 2 years compared with placebo-treated patients [1]. However, during the extension phase of the pivotal natalizumab trial, concerns were raised about the occurrence of a rare but severe infection Progressive Multifocal Leukoencephalopathy (PML), an opportunistic infection caused by the JC virus (JCV) [1–3]. In the post-marketing setting, as of March 6th 2014, there have been 448 cases of PML recorded worldwide, with an overall risk estimated at 3.55 per 1,000 treated patients (95 % confidence interval of 3.23–3.89 per 1,000). Of note, this risk is calculated on the total number of patients having been exposed to at least one dose of natalizumab (Biogen Idec, medical information service, March 20th 2014).
In view of the crucial need for long-term safety and efficacy data of natalizumab in the post-marketing setting, the Tysabri observational program (TOP) was started. TOP is an open-label, multicenter, multinational, prospective observational study, aiming to recruit up to 6,000 patients with relapsing-remitting MS from Europe, Canada and Australia. Fifteen countries are currently participating with recruitment until December 31st 2013.
We report here the interim results of the 563 Belgian patients included in TOP between December 2007 and December 2012.
Methods
Patients
All patients gave written informed consent. The study protocol was approved by the central and local ethics committees. Relapsing-remitting MS patients, fulfilling reimbursement criteria and naïve to natalizumab were eligible. Enrolment was possible up to the 4th natalizumab infusion. Patient characteristics and contraindications to natalizumab treatment were verified, in accordance with prescribing information and Belgian prescription requirements (Supplemental Table 1).
Study medication administration
Medication was administered once every 4 weeks, per IV infusion in hospital setting.
Study procedure
Data collection
All data were recorded electronically at study site into a centralized database, using a Web-based electronic Case Report Form (CRF; for an example of the baseline CRF, see Supplemental Table 2). Source data are kept at the prescribing physician’s site. Data from the registry was extracted on December 1st 2012 for analysis.
Enrolment visit
Data concerning basic demographics, medical history, most recent brain MRI, history of disease-modifying treatments (DMTs), last EDSS score before enrolment and EDSS score at enrolment and relapse history were recorded. In addition, pregnancy status for female patient was also recorded.
Follow-up visits
Information from regular clinical visits were recorded in the database and repeated every 6 months as per protocol. Information was collected regarding treatment status, EDSS score, relapse occurrence since the last visit, modification in DMTs and serious adverse event (SAE) occurrence (for an example of the Follow-up Visit Case Report Form, see Supplemental Table 3). Female patients were asked about pregnancy and spontaneous abortions since the last visit. Relapses were not considered as an SAE for the purpose of this study.
Study endpoints
The primary endpoint of this study is the assessment of long-term safety in patients receiving natalizumab, through analysis of the incidence and pattern of SAEs. Secondary endpoints of the study include: analysis of relapse rate, analysis of disability progression, evaluation of baseline disease characteristics as prognostic indicators for disease activity and disability progression over time, and finally, evaluation of short-term disease outcomes as prognostic indicators for disease activity and progression over time.
Statistical analysis
Continuous data changes such as EDSS and ARR values were compared using Wilcoxon’s signed-rank test. Baseline EDSS used for analysis was the first non-missing value of: (1) physician-reported EDSS at enrollment; (2) calculated EDSS at enrollment or (3) last EDSS reported before enrollment. If baseline EDSS was calculated EDSS at enrollment, calculated EDSS was used for follow-up; if baseline EDSS was reported EDSS at enrollment or last reported EDSS before enrollment, reported EDSS was used for follow-up. Time to first relapse, time to sustained disability progression and time to sustained disability improvement were estimated using the Kaplan–Meier method. Sustained disability progression was defined as an increase of one point or more in the EDSS score from enrolment, sustained for 24 weeks. Sustained disability improvement was defined as a decrease in one point or more in the EDSS score from baseline, sustained at 24 weeks. The same definition of baseline EDSS was used as indicated above for the disability progression or improvement analysis.
Results
Baseline demographics
As of December 1st 2012, 563 patients were enrolled in the study. Of note 54 patients (9.6 %) were recruited in the study, with a baseline visit but no follow-up visits recorded. Of the 563 patients included, 70.2 % (n = 395) were females, with a median age of 37.0 years (range 17–70 years) (Fig. 1a; Table 1). There was no significant difference in the age distribution across genders (data not shown). As a whole, the female-to-male ratio was 2.35–1. Median disease duration was 6.8 years (range 0.0–40.2) (Fig. 1b; Table 1). Disease duration was <1 year in 57 patients (10.1 %). Median baseline EDSS was 3.0 (range 0.0–8.5) (Table 1). EDSS distribution at enrolment is shown in Fig. 1c. Median relapse rate in the year prior to natalizumab was 2.0 (range 1–8) and 2.0 (range 1–10) in the 2 years prior to natalizumab initiation (Fig. 1d; Table 1). In the year before starting natalizumab treatment, 204 patients (36.2 %) had 1 relapse, 238 patients (42.3 %) had 2 relapses and 121 (21.5 %) had 3 or more relapses. Relapses were treated by steroids in 501 patients (89 %). Two-hundred sixty patients (46.2 %) had one relapse and 234 (41.6 %) had two or more relapses resulting in steroid treatment in the year before the start of natalizumab (data not shown).
Baseline characteristics of Belgian patients included in the TOP study. a Age distribution of patients at first dose is depicted. b Distribution of disease duration in patients at first dose. Data are missing for 2 patients (0.4 %). c EDSS distribution at enrollment. Data are missing for 2 patients at baseline (0.4 %). d Distribution of relapses 1 or 2 years prior to natalizumab in patients at enrollment
Prior treatment
Five-hundred and one patients (89 %) had been previously treated with one or more DMTs and/or immunosuppressive drug (IS). Among them, 47 (8.3 %) had been previously exposed to one or more IS medications. Sixty-two patients (11 %) were treatment-naïve, 296 patients (52.6 %) had one previous DMT and 205 patients (36.4 %) had 2 or more previous DMTs (Fig. 2a). Median DMT-use duration was 3 years (range 0–15.8) (Fig. 2b; Table 1). Medications that were ever used, used in the past 24 months and the last medication used prior to natalizumab are shown, respectively, in Fig. 3. Four-hundred and fifty-one patients (80.1 %) had used IFN-β products in the past. In 366 of these patients (65 %), IFN-β was the last treatment before natalizumab. In 106 patients (18.8 %), glatiramer acetate (GA) was the last treatment before the start of natalizumab. Thirty-eight patients (6.7 %) had previously received mitoxantrone, among which 11 (2 %) received it during the 2 years preceding natalizumab treatment.
Baseline MRI
Baseline MRI characteristics were recorded for 495 patients (87.9 %). Eighty-four percent of patients had 9 or more T2 hyperintense lesions and, 67.5 % had one or more gadolinium-enhancing lesions. A few patients (11.1 %) had <9 T2 lesions on brain MRI (Table 1).
Treatment discontinuation
One-hundred and thirty patients (23.1 %) did not receive natalizumab every month. Among them, 96 patients (17.1 %) discontinued natalizumab. Reasons recorded for treatment discontinuation are listed in Supplemental Table 4. Medication change (n = 23) and insufficient efficacy (n = 17) were listed as the main reasons. Twelve patients (2.1 %) discontinued the treatment due to pregnancy desire, 6 (1.1 %) due to the occurrence of a SAE, 13 (2.3 %) due to JCV seropositivity and two (0.4 %) due to anti-natalizumab antibodies. Twenty patients (20.8 %) discontinued treatment due to safety concerns, treatment duration, JCV seropositivity and previous exposure to immunosuppressants. Finally, 44/96 patients (45.8 %) remained in the study, despite having stopped treatment.
Study drop outs
Eighty-seven patients (15.5 %) withdrew from the study during follow-up. Among those, 28 were lost to follow-up or moved away. A subgroup of these patients (n = 52) discontinued the treatment, while 35 patients remained on natalizumab. Reasons for study withdrawal, listed in Supplemental Table 5, are globally similar to the ones for treatment discontinuation.
Safety
In total, 78 SAEs were recorded in 65 patients (11.5 %), among which two cases of PML (Table 2). The first case occurred in a 45-year-old female patient, with no previous IS. At the 27th natalizumab infusion, she reported to be more absent-minded. The 6-month brain MRI showed a new suspicious right subcortical frontal lesion. Diagnosis was confirmed by JCV DNA polymerase chain reaction (PCR) on the cerebrospinal fluid, showing 186 viral copies per ml. The patient recovered favorably after five plasmapheresis sessions and a 3-day course of intravenous methylprednisolone followed by an oral taper for 2 months. The second case was diagnosed upon routine brain MRI follow-up in an asymptomatic 27-year-old male patient with no previous IS, after 38 natalizumab infusions [4]. Diagnosis was confirmed by JCV DNA PCR showing 712 viral copies per ml. The patient recovered without further symptoms after five plasmapheresis sessions and intravenous corticosteroids followed by a two-month oral taper. During treatment with natalizumab, severe infections were rare and isolated events (pneumonia, colitis, cystitis, streptococcal meningitis, lumbar discitis, influenza virus infection, cytomegalovirus infection). Malignant tumors were diagnosed in two patients (one with cervix cancer and one with glioblastoma).
Efficacy
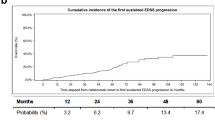
At the time of data extraction, 303 patients (53.8 %) had completed more than 25 natalizumab infusions. A total of 1,134.05 person-years of exposure to the drug was reached. In the subgroup of patients with data up to year 2 (n = 208), the median baseline EDSS score was 3.5. The median EDSS score slightly decreased to 3.0 at 6 months and remained stable thereafter at 12, 18 and 24 months of natalizumab treatment (p ≤ 0.0005) (Fig. 4; Table 3). The subsequent variations of the EDSS score are unrepresentative due to the low proportion of patients having reached more than 24 months of treatments (Table 3). At 24 months, survival curve analysis shows a 25.68 % cumulative risk of sustained disability improvement and a 9.01 % cumulative risk of disability progression for those who reached the 24-month follow-up (Fig. 5). The ARR was significantly reduced from a mean of 1.94 to 0.18 (90.7 % reduction, p < 0.0001) for a total of 1,170.8 subject-years followed (Table 4). Relapses requiring steroids or hospitalization were both reduced following natalizumab (Table 3). The significant reduction in ARR was independent of relapse history or treatment history (p < 0.0001, in all cases) (Table 4; Fig. 6a). Similarly, treatment-naïve patients showed a reduction in the mean ARR from 2.26 to 0.19 (91.6 % reduction, p < 0.0001) (Table 4). ARR in the subgroup of patients who were only on IFN-β, GA or switched from one treatment to another prior to natalizumab were similarly reduced following therapy escalation (data not shown). In contrary to the global data analysis, there was no significant difference in the magnitude of the reduction of the ARR post-natalizumab according to baseline EDSS score (< or >3) (data not shown) [5]. ARR in the subgroups according to treatment duration remained low throughout the study period (data not shown). During the course of the study, 133 patients experienced a relapse (23.6 %). The cumulative relapse risk was 26.87 % at 24 months (Fig. 6b).
Median EDSS score during the first two years of follow-up in Belgian TOP patients. Results are shown for the subgroup of patients for which baseline and year 2 data are available (number of patients for which data are available is indicated in corresponding bars). EDSS values were compared using Wilcoxon's signed-rank test
Cumulative probability of sustained disability improvement or progression up to 5 years. Time to sustained disability progression and time to sustained disability improvement were estimated using the Kaplan Meier method. a Sustained disability progression was defined as an increase of one point or more in the EDSS score from enrollment, sustained for 24 weeks. b Sustained disability improvement is defined as a decrease in one point or more in the EDSS score from baseline, sustained at 24 weeks
Comparison of relapse rates pre-and post-treatment by natalizumab and cumulative probability of relapse up to 5 years. a Annualized relapse rates (ARR) pre- and post-natalizumab treatment in different subgroups. ARR values were compared using Wilcoxon’s signed-rank test. b Time to first relapse was estimated using the Kaplan Meier method
Discussion
Natalizumab has been licensed in the EU since June 27th 2006 for second-line monotherapy in relapsing-remitting MS patients with clinically active disease despite IFN-β treatment or in patients with rapidly evolving relapsing disease. Concerns about long-term safety and efficacy have prompted several national and international initiatives to monitor patients starting treatment with natalizumab.
We report the interim results of the first 563 patients included in the TOP study in Belgium, up to December 1st 2012. At that time, about 1,100 patients were receiving natalizumab in Belgium. December 2012 was chosen as cut-off to present this interim analysis, because 56.8 % patients had reached a follow-up period of 24 months.
Baseline demographics of the patients included in TOP show that compared to the patients in the pivotal study AFFIRM, patients initiating natalizumab in Belgium were older, had a longer median disease duration, higher disease activity and disability, as assessed by the EDSS score [1]. These results are in line with those from other published national registries [6–14]. Another indicator of disease activity, apart from the ARR prior to natalizumab, is the baseline MRI, showing gadolinium enhancement in 67.5 % of cases. This percentage is high in comparison to the global TOP data (46.7 %), and this is due to specific Belgian reimbursement criteria in the first 2 years where gadolinium enhancement was required to start natalizumab (from December 2007 to January 2010) (Biogen Idec, data on file).
In the Belgian TOP cohort, disease duration was 1 year or less in 10.1 % of patients, corresponding to most of the treatment-naïve subgroup (11 %). However, most patients included in TOP have failed one or more DMT(s) before starting natalizumab (89 %), as described by others [6–13].
In our cohort, 368 patients were enrolled up to December 2010 prior to the use of the JCV stratify essay and prior to general knowledge about the risk factors established for PML [15]. Previous IS use was reported in 47 patients (8.3 %). In contrast, an Italian cohort and a Danish one report even higher proportions of patients with previous treatment by IS drugs (33 and 19.2 %, respectively) [7, 11].
Clinical disease activity dropped significantly following the start of natalizumab treatment, with a 90.7 % reduction in the ARR. The effect was also present in patients with highly active disease prior to natalizumab (i.e., with more than one relapse in the preceding year). The drop in the ARR was comparable, regardless of the treatment history (treatment-naïve, IFN-β or GA as last treatment before natalizumab; one or more than one treatment before natalizumab). Annualized relapse rate dropped also by 89.5 % in patients with prior IS treatment. Interestingly, the magnitude of the effect is in line with the drop in ARR shown in the AFFIRM study and also in subsequently published national registries or observational studies [6–13]. Results from the global TOP study indicate that post-baseline ARR after 4 years for patients receiving natalizumab therapy decreased from 1.99 at baseline to 0.28 [5, 16]. The proportion of patients remaining relapse-free during the study was 76.4 %. In this present subgroup of TOP patients from Belgium, relapse risk was 15.95 and 10.92 % during the first and second year of natalizumab treatment, respectively.
Median EDSS remained stable up to 24 months following the start of natalizumab, an effect also reported by other groups and also demonstrated in the global TOP cohort [5, 6, 8–10, 12, 13, 16]. EDSS data at 24 months were only available in 36.9 % of the included patients. It will be interesting to see whether these effects are sustained on the long term, as an insufficient number of patients have attained a longer follow-up period. The proportion of progression-free patients was 92.2 %, whereas the proportion of patients experiencing sustained disability improvement was 21.9 %. Considering disability progression, it will be interesting to obtain long-term data on a higher number of patients, as it is still unknown whether natalizumab delays secondary progression of disease.
Globally, SAE occurrence was infrequent (11.5 %) and consistent with natalizumab’s known safety profile. Two cases of PML out of 563 patients enrolled have been reported until December 2012 (0.4 %). Malignant tumors were reported in two patients. No increased signals for these cancers have been raised, either in post-marketing observational studies or in Adverse Event Reporting’s to national pharmacovigilance instances. Isolated cases of serious infections were reported. It is noteworthy that no other opportunistic infections were reported besides PML. One case of fulminant hepatitis of unspecified origin was reported. Anaphylactic shock and allergic reaction were reported as SAEs in six patients (1.1 %). The numbers are relatively low in comparison to what is reported in the AFFIRM trial (4 % of hypersensitivity reactions) and in other post-marketing studies, maybe suggesting underreporting by treating physicians [6–14]. This may also be due to the design of the study, authorizing up to three natalizumab injections before enrolment. Since allergic reactions most often occur during the second and third injections, some patients may have experienced these reactions, without them having been reported following inclusion in the study.
Treatment with natalizumab was discontinued in 18 patients out of 96 (18.75 %) due to adverse events (serious or non-serious) or lack of tolerability. The discontinuation rate reported is in line with other observational cohorts [6–14]. Safety concern, natalizumab treatment duration concern, prior immunosuppression or JCV seropositivity were cited in 20 cases (20.8 %) as the reasons for treatment suspension. This may be because only half of the patients have reached 2 years of treatment. It also could be because anti-JCV antibody status has been incorporated only since the second half of 2011 in treatment decision algorithms. Due to availability of other treatment options and implementation of risk stratifications strategies, the proportion of treatment discontinuations might rise in the future. It must be noted that a protocol amendment has recently been introduced to enable collection of patient anti-JCV antibody status within the TOP registry.
Although it provides strong and consistent data about safety and efficacy, the TOP study presents certain weaknesses, inherent to its open-label and observational design. Selection bias might influence certain outcomes. In the absence of a control group, one might argue that the drop in the relapse rate might, at least in part, reflect regression to the mean. For this reason, the clinical endpoints of TOP patients will be compared to those of an external, prospectively determined control cohort derived from the international MSBase Registry, by propensity score analysis [17]. Another caveat in the study is the fact that follow-up data are missing in 54 patients (9.6 %) initially enrolled. Treatment discontinuation rate (17.1 %) and subject withdrawal from the study (15.5 %) may be considered high, depriving the study of valuable datasets. For example, patients discontinuing natalizumab due to perceived lack of efficacy or medication change might be secondary progressive patients. This may, therefore, bias the results on the proportion of patients experiencing disability progression within the study. At the end of 2012, the occurrence of one suspected and six confirmed cases of PML has been reported in Belgium, of which only two were included in the TOP registry (Biogen Idec, Safety and Benefit-Risk Management). This study does not therefore accurately estimate the true incidence of PML within the Belgian patient population treated with natalizumab.
In conclusion, efficacy and safety data collected in this interim analysis of 514 patients from Belgium, in which natalizumab is largely administered as a second-line treatment, are consistent with the outcomes of the AFFIRM pivotal study and the interim results of the global TOP cohort [5, 16]. As in other observational studies, patients starting natalizumab in Belgium are older, have longer disease duration, higher disease activity and disease burden than in the phase III pivotal trial [6–14]. The results obtained from the various post-marketing studies are in line with the post hoc analysis of the subgroups from the AFFIRM study with highly active disease [18]. Hence we can conclude that in the clinical practice setting, for patients with active disease, regardless of treatment history, EDSS and disease duration, a marked decrease in ARR and stabilization of the neurological impairment was observed with natalizumab after 2 years of treatment. Results after more than 2 years of treatment are still waiting to be confirmed in a greater number of patients. The planned 10-year follow-up period will provide crucial data on these long-term efficacy and safety outcomes.
References
Polman CH et al (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354(9):899–910
Rudick RA et al (2006) Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 354(9):911–923
Sandborn WJ et al (2005) Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med 353(18):1912–1925
Phan-Ba R et al (2012) MRI preclinical detection and asymptomatic course of a progressive multifocal leucoencephalopathy (PML) under natalizumab therapy. J Neurol Neurosurg Psychiatry 83(2):224–226
Butzkueven H et al (2014) Efficacy and safety of natalizumab in multiple sclerosis: interim observational programme results. J Neurol Neurosurg Psychiatry. doi:10.1136/jnnp-2013-306936
Piehl F et al (2011) Swedish natalizumab (Tysabri) multiple sclerosis surveillance study. Neurol Sci 31(Suppl 3):289–293
Oturai AB et al (2009) Efficacy of natalizumab in multiple sclerosis patients with high disease activity: a Danish nationwide study. Eur J Neurol 16(3):420–423
Melin A et al (2012) Effect of natalizumab on clinical and radiological disease activity in a French cohort of patients with relapsing-remitting multiple sclerosis. J Neurol 259(6):1215–1221
Outteryck O et al (2010) Demographic and clinic characteristics of French patients treated with natalizumab in clinical practice. J Neurol 257(2):207–211
Fernandez O et al (2012) Natalizumab treatment of multiple sclerosis in Spain: results of an extensive observational study. J Neurol 259(9):1814–1823
Sangalli F et al (2010) Efficacy and tolerability of natalizumab in relapsing-remitting multiple sclerosis patients: a post-marketing observational study. Neurol Sci 31(3):299–302
Prosperini L et al (2010) Natalizumab treatment in multiple sclerosis: the experience of S. Andrea MS Centre in Rome. Neurol Sci 31(3):303–307
Putzki N et al (2010) Efficacy of natalizumab in second line therapy of relapsing-remitting multiple sclerosis: results from a multi-center study in German speaking countries. Eur J Neurol 17(1):31–37
Holmen C et al (2011) A Swedish national post-marketing surveillance study of natalizumab treatment in multiple sclerosis. Mult Scler 17(6):708–719
Bloomgren G et al (2012) Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 366(20):1870–1880
Kappos L et al (2012) Long-term safety and efficacy and association between baseline treatment history and postbaseline relapses in multiple sclerosis patients treated with natalizumab in the TYSABRI(R) observational program (TOP) (P04.134). Neurology 78:P04.134
Spelman (2012) Comparison of natalizumab and interferon-beta/glatiramer acetate efficacy using a propensity-matched registry data (S133). Mult Scler J 18(4 suppl):9–53
Hutchinson M et al (2009) The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol 256(3):405–415
Conflict of interest
The natalizumab Observational Program is funded by Biogen Idec. Although the authors had full editorial control, Biogen Idec reviewed the manuscript prior to submission and provided feedback to the authors.
V. van Pesch: has received travel grants, honoraria for consultancy and lectures from Bayer-Schering, Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis-Genzyme and Teva.
E. Bartholomé: EB is Chief Medical Officer of Medical Device Works. EB has received scientific and travel grants from Abbvie, Bayer Schering, Biogen Idec, Novartis, Sanofi-Aventis-Genzyme, Merck Serono and TEVA. EB has had consultancy contracts with Biogen Idec, Novartis, Sanofi-Aventis-Genzyme, and Merck Serono. EB is or has been member of Advisory Boards for Biogen Idec, Novartis, Sanofi-Aventis-Genzyme, and Serono.
V. Bissay: has been a member of an Advisory Board for Biogen Idec and has received travel grants from Bayer Schering, Biogen Idec, Novartis, Merck Serono and TEVA.
O. Bouquiaux: has received scientific grants from Biogen Idec and Novartis.
M. Bureau: has no disclosures to report.
Jo Caekebeke: scientific and/or travel grants from Abboth, GSK, Bayer Schering, Biogen Idec, Novartis, Merck Serono and TEVA.
J. Debruyne: has received grants from Novartis, Biogen Idec, Bayer Schering, Merck Serono and Teva.
I. Declercq: no disclosures reported.
D. Decoo: has received scientific and travel grants from Abbvie, Bayer Schering, Biogen Idec,
Novartis, Sanofi-Aventis/Genzyme, Merck Serono and Teva. DD has consultancy contracts with Biogen Idec, Novartis, Sanofi-Aventis/Genzyme and GLG. DD is or has been member of Advisory Boards for Biogen Idec, Novartis, Sanofi-Aventis-Genzyme.
P. Denayer: no disclosures to report.
E. Desmet: has received grants from Biogen Idec and Teva.
B. D’Hooghe: has been a consultant for Biogen Idec, Bayer Schering, Merck Serono, Allergan, Novartis and Genzyme Sanofi.
B. Dubois: is a Clinical Investigator of the Fund for Scientific Research (FWO-Vlaanderen), and is supported by the Merck Chair Multiple Sclerosis. She also received an educational grant from Novartis.
M. Dupuis: no disclosures reported.
S. El Sankari: has received travel grants from Biogen Idec, Bayer Schering, Merck Serono, Teva, Novartis and Sanofi Genzyme. SE is a member of an advisory board for Biogen Idec.
K. Geens: has received scientific and/or travel grants from Abboth, Glaxo-Smith-Kline, Bayer Schering, Biogen Idec, Novartis, Sanofi-Aventis-Genzyme, Merck Serono and TEVA.
D. Guillaume: DG has received scientific and travel grants from Bayer Schering, Biogen Idec, Novartis, Sanofi-Aventis and Sanofi-Genzyme, Merck Serono and TEVA. DG has been member of Advisory Boards for Biogen Idec, Novartis, Sanofi-Genzyme.
W. van Landeghem: has received scientific and/or travel grants from Abboth, Allergan, Bayer, Biogen Idec, Boehringer, Cyberonics, Glaxo-Smith-Kline, Ipsen, Medelec, Merck Serono, Novartis, Sanofi-Aventis-Genzyme, UCB and TEVA.
A. Lyssandropoulos: has received scientific and travel grants from Bayer, Biogen Idec,
Sanofi-Aventis-Genzyme, Serono and TEVA. AL has had consultancy contracts with Bayer and Novartis. AL is or has been the member of Advisory Boards for Biogen Idec and Sanofi-Aventis-Genzyme.
A. Maertens: has received travel grants from Biogen Idec, Bayer Schering, Merck Serono, Teva, Novartis and Sanofi Genzyme.
R. Medaer: no disclosures reported.
A. Melin: no disclosures reported.
K. Peeters: no disclosures reported.
R. Phan–Ba: has served at Scientific Advisory Boards for Genzyme Sanofi Aventis and has received funding for travel from Genyzme–Sanofi Aventis, Bayer Schering Pharma and Biogen Idec.
C. Retif: no disclosures reported.
P. Seeldrayers: is a member of Advisory Boards for Biogen Idec, Novartis, Merck Serono, Bayer Schering, Teva and Sanofi Genzyme. PS has received travel grant and research support from Biogen Idec.
A. Symons: has been a member of advisory boards of Biogen Idec, Genzyme and Merck Serono. AS is currently an employee of Biogen Idec.
E. Urbain: has received scientific and travel grants from Biogen Idec, Bayer Schering, Sanofi-Aventis, Novartis and Merck Serono.
P. Vanderdonckt: has received scientific and/or travel grants from Abboth, GSK, Bayer Schering, Biogen Idec, Novartis, Merck Serono, TEVA and Cyberonics.
E. Van Ingelghem: no disclosures reported.
L Vanopdenbosch: has received honoraria for consultancy and lectures, educational and occasional institutional grants by Novartis, Merck Serono, Teva, Biogen Idec, Bayer Schering and UCB. LV serves on the editorial board of Tijdschrift van Neurologie en Neurochirurgie.
E. Vanroose: has received travel grants from Biogen Idec, Bayer Schering, Merck Serono, Teva, Novartis and Sanofi Genzyme.
B. Van Wijmersch: has received scientific and travel grants from Biogen Idec, Novartis, Sanofi-Aventis-Genzyme, TEVA, Merck Serono and Bayer Schering.
B. Willekens: has served as a member of advisory boards for Biogen Idec, Genzyme, Bayer Schering, Merck Serono, Novartis; has received honoraria for lectures from Biogen Idec, Novartis and Bayer Schering; has received travel grants from Biogen Idec, Merck Serono, Bayer schering, Novartis and TEVA.
C. Willems: has no disclosures to report.
C. Sindic: has received honoraria for consultancy and lectures, educational and research grants from Bayer-Schering, Biogen Idec, GSK Biological Vaccines, Merck Serono, Novartis, Sanofi-Aventis and Teva.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the Belgian Study Group for Multiple Sclerosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Pesch, V., Bartholomé, E., Bissay, V. et al. Safety and efficacy of natalizumab in Belgian multiple sclerosis patients: subgroup analysis of the natalizumab observational program. Acta Neurol Belg 114, 167–178 (2014). https://doi.org/10.1007/s13760-014-0308-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-014-0308-9