Abstract
I present a general theory for the initial domestication of plants and animals that is based on niche construction theory and incorporates several behavioral ecological concepts, including central-place provisioning, resource catchment, resource ownership and defensibility, and traditional ecological knowledge. This theory provides an alternative to, and replacement for, current explanations, including diet breadth models of optimal foraging theory, that are based on an outmoded concept of asymmetrical adaptation and that attempt to explain domestication as an adaptive response to resource imbalance resulting from either environmental decline or human population growth. The small-scale human societies that first domesticated plants and animals share a number of basic interrelated attributes that when considered as an integrated and coherent set of behaviors provide the context for explaining initial domestication not as an adaptive response to an adverse environmental shift or to human population growth or packing but rather as the result of deliberate human enhancement of resource-rich environments in situations where evidence of resource imbalance is absent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The initial domestication of plants and animals and the subsequent development of agricultural economies mark a major evolutionary transition in earth’s history. Small-scale human societies in perhaps as many as a dozen separate world areas independently brought a wide variety of different species under domestication between about 11,000 and 5,000 years ago, and these domesticates provided the lever with which we have transformed much of the earth into agricultural landscapes that feed an ever increasing global population. Over the past several decades, biologists and archaeologists working with different data sets at different scales of analysis, and from a number of complementary perspectives, have employed a range of new techniques that have substantially improved our understanding of many different aspects of the initial domestication of plants and animals (e.g., Doebley et al. 2006; Zeder et al. 2006; Bar-Yosef and Price 2011; Gepts and Famula 2012).
Although there is considerable variation in the quality and quantity of information that is currently available from the different independent centers of domestication, these regions provide an excellent opportunity for comparative analysis in terms of the sequence, timing, and rate of domestication of different species as well as the environmental and cultural contexts within which domestication occurred. It is also at the regional scale of analysis that the most interesting and challenging developmental questions can be most successfully addressed, and where most researchers focus their efforts (Zeder and Smith 2009).
Along with rapid, if variable, advances in our understanding of the domestication process in different world areas at a regional scale of analysis, there is also a continuing strong interest in formulating and refining overarching models and theories that can help to illuminate the underlying similarities of the domestication process on a global scale. The vast majority of the universal explanations for initial domestication and agricultural origins that have been proposed over the past half century, however, including, most recently, the diet breadth models of optimal foraging theory (e.g., Kennett and Winterhalder 2006; Piperno 2006, 2011), are based on a “unidirectional” definition of adaptation that was the consensus within evolutionary theory up through the 1970s and still dominates today. According to this traditional definition, adaptation is a one-way street in which environments change and species adapt: “Adaptation is always asymmetrical; organisms adapt to their environment, never vice versa” (Williams 1992, p. 484). In these unidirectional “environments change, humans adapt” explanations, the initial domestication of plants and animals worldwide during the early Holocene is seen as an adaptive response by small-scale human societies to an imbalance between supply (available resources, particularly larger, high-value prey), and demand (human food requirements) that followed the well-documented climatic and environmental changes associated with the Pleistocene–Holocene transition. Humans are viewed as adjusting to the adverse imbalance between caloric supply and demand that developed at the close of the Pleistocene by first expanding their diets to include more lower-value plant and animal species, and as their diet breadth increased, eventually turning to domestication of lower-ranked dietary additions as an adaptive solution.
Two different general causes for this early Holocene resource imbalance are employed in these unidirectional adaptation explanations for initial domestication. On the demand side of the equation, human population growth in the early Holocene is often identified as the cause of resource depression and subsequent initial domestication. Increasing human population is sometimes considered as an inexorable universal trend, or alternatively, at the regional scale of analysis, as either forcing groups into marginal environments or confining them within increasingly inadequate resource territories (e.g., Binford 1968; Cohen 1977; Richerson et al. 2001).
In contrast to these Malthusian explanations, other unidirectional adaptation theories, including several recent optimal-foraging-theory diet breadth models, identify the resource imbalance or resource depression as originating on the supply side of the equation rather than on the demand side. In these explanations, climatic change in the late Pleistocene led to the loss of high-value resources, either through rising sea level (Binford 1968) or as a result of megafaunal extinctions (Hawkes et al. 1982, p. 395; Piperno 2006, 2011), resulting in humans having to expand their diet (and eventually domesticate) in order to survive in substantially downgraded biotic communities.
Here I outline a general theory for initial domestication that is opposed to the prevailing approaches that are based on the concept of asymmetrical adaptation. This alternative explanation is in response to the basic paradigm shift that has been underway in the biological disciplines over the past decade as the perception of adaptation as an asymmetrical process is being replaced by the recognition that many species, including humans, play an active role in modifying their environments, and that such niche-construction activities constitute, along with natural selection, a second major participant in evolution (Odling-Smee et al. 2003). Ironically, by insisting that researchers interested in understanding initial domestication must abandon outmoded biological theories in favor of newly established, more viable ones, Piperno effectively argues against employment of the diet breadth models she advocates: “to avoid a serious paradigm lag with modern biological principles and ensure that our theories can accommodate complex and learned human actions, archaeologists must incorporate these now-standard approaches in biology” (Piperno 2006, p. 137).
The theory outlined below proposes that small-scale societies all share a number of basic interrelated attributes that, when considered as an integrated and coherent general pattern of behavior, provide a simple and compelling foundation for explaining initial domestication not as an adaptive response to an adverse environmental shift but rather as reflecting deliberate human enhancement of resource-rich environments in situations where evidence of resource imbalance is absent (Smith 2007).
Five General Attributes of Small-Scale Human Societies
The present-day and historically described small-scale societies from which the following five general attributes are drawn constitute an appropriate reference class for considering the transition to food-production economies in that they span the transition from having no reliance to having a limited reliance on domesticates (Smith 2001, 2011a). This characterization of five general attributes of small-scale human societies can also be considered a form of meta-analysis or second-level pattern recognition in that each attribute is viewed through the lens of synthetic studies carried out by other scholars having considerable knowledge of the specific topics under consideration.
Small-Scale Societies Have Well-Defined Resource Areas
Small-scale human societies, along with populations of many other species, typically use resources by ranging out from and returning to a central place. For some species, such central locations may serve primarily to provide protection from predation (refuging), or for sleep (central place foraging), but for small-scale human societies as well as a variety of other species of birds, carnivores, rodents, and insects, such central places serve as a place to consume acquired food and often to share it with others (central-place provisioning) (Marlowe 2006).
The daily and annual range and patterns of movement for resource acquisition out from such central locations or places have been studied for many species over the past half century, with a primary focus being the size and shape of the surrounding resource area: “Individuals disperse radially from C [a fixed point, a core, or central place] to some well-defined limit. Between C and this dispersal limit lies the arena … where resource acquisition takes place” (Hamilton and Watt 1970, p. 263). This geographical area from which a group draws its food and raw materials is often termed its resource catchment, which is comparable to the concept of drainage catchment—the area from which a stream draws its water. A basic tenet of resource-catchment analysis is that the farther one moves out and away from a central place, the greater the amount of energy that must be expended for the procurement of resources: “The additional energy and the energy equivalent of time required to reach these more distant and less heavily exploited ranges act to limit dispersal distances” (Hamilton and Watt 1970, p. 263). As a result, the size and shape of the resource-catchment zones of small-scale societies will depend upon the distribution, spacing, and seasonal availability of different resources around their settlements (e.g., Bettinger et al. 1997; Bird and Bliege Bird 1997; Kelly 2007). Catchment zones around settlements in resource-rich environments are smaller in general than those where resources are less abundant and less predictable. In theory, the intensity of exploitation of resources should decrease as one moves farther away from a settlement, eventually reaching a point where energy expenditure is greater than the value of the energy captured and exploitation is unprofitable. This conceptual boundary line defines the outer edge of the resource catchment of a settlement.
General estimates of the size of resource-catchment zones for settlements of small-scale societies have been expressed in terms of a circle with the settlement as the center point and the diameter of the circle expressed in either distance or actual travel time. A distance of about six miles and a walking time of 2 hours, for example, have been suggested as the maximum distance that hunter-gatherers will normally or habitually travel from their camp to procure resources (Higgs et al. 1967; Lee 1969; Vita-Finzi and Higgs 1970). Low-level food-producing societies can be expected to have even smaller resource catchments (Chisholm 1968), and a walking time of 1 hour has been suggested for full agriculturalists (Vita-Finzi and Higgs 1970).
Such static-circle resource-catchment projections, along with more detailed time-contour catchment projections produced by compiling actual 2-hour-walk radii, have been employed extensively by archaeologists in an effort to estimate resource-catchment areas of long-abandoned settlements (Roper 1979). Historical records and analysis of present-day small-scale societies, in contrast, provide much more accurate and reliable delineation of resource-catchment zones of small-scale societies across different environmental zones (e.g., Steward 1933, 1938; Gottesfeld and Johnson 1994; Russell-Smith et al. 1997).
Resource-catchment analysis of small-scale societies continues to be an active area of inquiry, as a variety of new approaches are being employed (e.g., Bliege-Bird et al. 2008). Although the size and shape of human resource-catchment areas are variable across environmental zones, they represent a universal and long-term aspect of how small-scale human societies have successfully adapted to their local ecosystems.
Small-Scale Societies Maintain and Consistently Update a Comprehensive Knowledge of Local Ecosystems
Small-scale human societies develop and maintain, in the form of shared oral traditions, beliefs, myths, and stories, huge amounts of information about their environment, and this information is passed down from generation to generation: “Detailed observation and experimentation with the natural environment over many generations led to a profound native knowledge of how natural systems work” (Anderson 1999, p. 88). A society’s continuing knowledge of its local landscape is of obvious critical importance to its well-being. The better a society’s traditional and sustained understanding of how their local ecosystem works, the better their chances for survival over many generations: “Traditions are the products of generations of intelligent reflection tested in the rigorous laboratory of survival. That they have endured is proof to their power” (Hunn 1993, p. 13).
The term “traditional ecological knowledge” (TEK) is often used to refer to these environmental information sets: “a cumulative body of knowledge, practice, and belief, evolving by adaptive processes and handed down through generations by cultural transmission, about the relationship of living beings (including humans) with one another and with their environment” (Berkes 2008, p. 7). Over the past half century, many aspects of TEK have been documented for small-scale societies occupying different ecosystems worldwide, from the arctic tundra to tropical forests. Considerable attention has been focused, for example, on the often comprehensive and detailed taxonomies of plants and animals developed by small-scale societies (e.g., Berlin 1992) and the associated knowledge of the life cycles, seasonal availability, and patterns of behavior of the many species that are relied on for survival (e.g., Anderson 2005; Deur and Turner 2005; Berkes 2008; Bliege-Bird et al. 2008).
The ability to accurately identify and locate resources in both time and space is an important and obvious element of TEK, and over their life spans members of small-scale societies will construct and refine high-resolution cognitive maps of the seasonal habitat preferences and spatial distribution of a wide variety of high-value target species of plants and animals. These maps of resource distribution are both abstract and specific. They are abstract in that a variety of general indicators (e.g., time of year, weather, topography, habitat) of any location will provide a context of reference and expectation for encountering target species. Any upland stand of mature oaks and hickories in the eastern woodlands of North America, for example, could be expected during October and November to hold the promise of encountering not only oak mast and hickory nuts but also turkey and white-tailed deer. Similarly, fall-line waterfalls and rapids of coastal rivers and streams in many world areas could be expected to be filled with anadromous species of fish during seasonal spawning runs. This abstract ability to assess the potential occurrence of different target resources based on the general characteristics of any newly encountered location is based on the corporate memory of innumerable past encounters of similar environmental settings and associated resources.
Cognitive maps of resource distribution are at the same time specific in that small-scale societies characteristically have a long history of sustained resource use within a well-defined area of land—their resource catchment. As a result, the multigenerational accumulation of knowledge regarding the environmental context of target species often does not result from random exploration of unknown landscapes but rather from repeated return visits to specific and well-known locales within their established resource catchment. TEK, like politics, is always local: “Traditions are enduring adaptations to specific places” (Hunn 1993, p. 13), and “The practice of indigenous knowledge is, above all, the story of how social/cultural systems adapt to specific ecosystems” (Berkes 2008, p. 71).
A number of scattered stands of oaks and hickories of known location within a society’s resource catchment, for example, would be identified on the basis of past experience as being highly promising in terms of future fall nut and mast collecting and encountering deer, turkey, and other species that include nuts in their diet. Such similar but perhaps widely dispersed nut/deer/turkey resource locales, like other resource locations (e.g., shallow-water saltwater or freshwater bivalve beds, backwater fish-spawning pools, and piñon stands) would be routinely monitored and repeatedly visited on a periodic basis over many generations. As a result, most decisions by small-scale societies regarding resource-acquisition efforts are made long before the actual moment of encountering the resources in question and within a much larger context of overall knowledge and planning.
Such periodic monitoring and assessment of the status of resources by small-scale societies is situated within an overarching framework of expectation embodied in systems of TEK. Rather than being written down, these expectations regarding resources, both in the abstract and specific, are retained in stories and legends as well as in the overall worldviews and belief systems of the societies. Resource-catchment areas thus are more than the physical landscape. They include the living environment, and the living environment in turn plays a central role in shaping the system of values and meaning developed by small-scale societies: “Ecological knowledge and activities (are) symbolically and instrumentally embedded in the places and life worlds out of which they developed and which they help constitute” (Butz 1996, p. 52). Similarly, “Stories and legends are part of culture and indigenous knowledge because they signify meaning. Such meaning and values are rooted in the land and closely related to a ‘sense of place’” (Berkes 2008, p. 6). Along with serving as a basic vehicle for the multigenerational cultural transmission of information regarding the identity, characteristics, spatiotemporal occurrence, and status of a wide range of different resources, TEK also organizes this information in a coherent overall framework of understanding of how the world works and the place of human societies within the local ecosystem.
Small-Scale Societies Establish Various Forms of “Ownership” of Wild Resources
Resource-catchment areas around the settlements of small-scale societies can vary greatly in terms of the relative abundance, distribution, and predictability of different food sources, resulting in a corresponding diversity of different forms and combinations of possible resource ownership and defensibility by small-scale societies (Dyson-Hudson and Smith 1978; Cashdan 1983a; Kelly 2007).
At one end of the spectrum of variation, in environments generally characterized by relatively low resource availability, small-scale societies will have relatively large resource catchments, but overall ownership (control of outsider access) to land areas and resources will be relatively weak. Against this general background of low resource availability and weak development of ownership, however, specific locations within the resource-catchment area that offer high resource abundance and predictability will be the subject of clear and strongly enforced control of access: “Resources that are predictable in their spatiotemporal distribution have greater economic defendability than unpredictable resources” (Dyson-Hudson and Smith 1978, p. 24). Ownership of a wide variety of different types of such high-value resource locales or patches within the resource catchments of small-scale societies has been documented, including settings as diverse as particular stretches of beach associated with clam beds, seasonal fish-run river bottlenecks, root “gardens,” and piñon groves or even individual piñon trees (Gottesfeld and Johnson 1994; Deur and Turner 2005; Kelly 2007; Fowler and Rhode 2011).
At the other end of the spectrum, in environments with high abundance and predictability of subsistence resources, ownership is not limited just to small and scattered individual resource locales such as piñon groves but rather is extended to encompass a society’s entire resource-catchment area. The resource-catchment area becomes, in effect, a defended territory: “We define a territory as an area occupied more or less exclusively by an individual or group by means of repulsion through overt defense or some form of communication” (Dyson-Hudson and Smith 1978, p. 22). The boundaries of a small-scale society’s resource-catchment area can be defended by various combinations of both active physical efforts and social negotiation with potential outside visitors (Dyson-Hudson and Smith 1978; Cashdan 1983a; Kelly 2007). The Gitksan and Wet’suwet’en in inland British Columbia, for example, maintained strongly defended territories, usually coincident with watersheds, with boundaries running along drainage divides and encompassing the full range of altitudinal zones from valley bottom to alpine zones. The traditional penalty for trespass was death (Gottesfeld and Johnson 1994).
The theoretical tipping point for the shifting of ownership and defense (either socially negotiated or physical) from specific locales within a resource catchment to a society’s entire catchment zone has been defined in cost-benefit terms and comes when the cost of maintaining such borders in terms of the time, energy, and potential risk involved is outweighed by the benefits gained in terms of exclusive use of the catchment’s resources (Dyson-Hudson and Smith 1978): “The territorial strategy evolved is the one that maximizes the increment of fitness due to extraction of energy from the defended area, as compared with the loss of fitness due to the effort and perils of defense” (Wilson 1975, p. 269).
Patterns of ownership and differential access to resources by small-scale societies, often referred to under the general heading of “land tenure” (Kelly 2007), can be extremely complex, variable, and mutable, and issues surrounding the concepts of resource catchment and territory continue to be a focus of research and analysis. The important point in terms of the present discussion is that small-scale human societies characteristically establish control over specific resource locations of high value and will shift ownership to entire resource-catchment areas when the return from perimeter defense of entire resource zones outweighs the costs.
Small-Scale Societies Engineer Ecosystems During Multiple Generations Through TEK Transfer
Along with maintaining a detailed and sophisticated multigenerational knowledge of the biotic community within their resource-catchment areas, small-scale societies also exhibit an inherent capacity and proclivity for modifying or “engineering” their local ecosystems. They are not passive participants in local environments, confined to simply using what the ecosystem offers in the way of natural resources—adapting to what’s available. To the contrary, small-scale societies deliberately modify both their local environments and their relationship with their environments in a variety of ways, large and small (Odling-Smee et al. 2003; Laland et al. 2007; Laland and O’Brien 2010).
It is not just humans, however, who have the capacity to alter their local environments and construct their own niches. In the 1980s, Richard Lewontin proposed that organisms do not simply respond to the environment but in fact interact with and modify their surroundings. He argued for a significant revision of the general concept of adaptation that was then prevalent in evolutionary theory, which he described as follows: “The environment ‘poses’ the ‘problem’; the organisms ‘posit solutions,’ of which the best is finally ‘chosen’” (Lewontin 1983, p. 276). Taking issue with this “one-way” view of adaptation, Lewontin proposed that “organisms do not adapt to their environments; they construct them out of the bits and pieces of the external world” (Lewontin 1983, p. 280). Odling-Smee et al. (2003) argue that niche construction is universal and should be regarded, alongside natural selection, as a second major participant in evolution: “There are in fact two logically distinct routes to the evolving match between organisms and their environments: either the organism changes to suit the environment, or the environment is changed to suit the organism” (p. 18).
At the same time that evolutionary biologists and ecologists have been looking more closely at niche construction across a broad range of species, niche construction by human populations has also drawn increasing interest (Laland et al. 2007; Laland and O’Brien 2010; Kendal et al. 2011). For more than 50 years, scholars have been describing how societies situated in a variety of different ecosystem settings have deliberately changed their environments to suit their preferences, modifying “natural” landscapes and managing “wild” species of plants and animals. Given the range of different societies and environments that have been considered by researchers from different generations and different disciplinary perspectives, and the rich variety of different human “ecosystem-improvement” strategies that have been encountered, it is not surprising that a somewhat confusing array of different terms and phrases have been coined to characterize human manipulation of environments (Smith 2011a).
Although reflective of broad geographical, temporal, and behavioral coverage, this profusion of different descriptive terms also reflects a scattered and often species-specific scale of documentation and analysis. This in turn has emphasized the seemingly distinctively different and unique character of each example of resource management. As a result, attention has been drawn away from broader and more inclusive consideration of what all of these human behavior patterns have in common: they all represent human strategies of niche construction.
The concept of niche construction solves this problem of too many terms and the associated lack of a global perspective. It not only provides a general label for all forms of human management of wild (and domesticated) species but also offers a single unifying approach for integrating consideration of human and nonhuman modification of ecosystems (Odling-Smee et al. 2003; Laland et al. 2007). With the adoption of niche construction as a unifying concept, all of these different strategies for environmental manipulation, and all of the various descriptive terms that have been employed, come into broader focus as comprising a large and coherent category of human behavior.
By situating human efforts at environmental modification within a much larger context of niche-construction activities by a wide range of nonhuman species, niche construction theory (NCT) also substantially expands the foundation of empirical comparative evidence in support of human niche construction. Odling-Smee et al. (2003) provide a broad overview of the ways in which a wide range of different plant and animal species carry out “ecosystem engineering” or “niche construction.”
NCT also offers an explanation for why human and nonhuman species alike modify ecosystems: because such efforts have the potential to provide individuals and populations with an evolutionary advantage. Niche construction occurs when an organism modifies the relationship between itself and its environment. By modifying their surrounding environments, and associated selective pressures, populations can increase the chances of survival of subsequent generations of their species: “Niche construction by organisms significantly modifies the selection pressures acting on them, on their descendants, and on unrelated populations,” and as a result, “niche constructing organisms frequently influence their own evolution by modifying their own selective environments” (Odling-Smee et al. 2003, p. 2).
Although the occurrence of niche construction across a wide range of species is recognized, along with its potential ecological consequences, discussion regarding its relative importance in evolution continues. There is little argument, however, with the capacity of humans to modify environments, and the evolutionary importance of such efforts: “Humans are not just passive vehicles for genes, they actively modify sources of natural selection in environments. They are the ultimate niche constructors” (Odling-Smee et al. 2003, p. 28; emphasis added).
In order to provide an evolutionary advantage to small-scale human societies, human niche-construction efforts targeting wild species of plants and animals must persist over multiple generations. Niche construction typically does not consist of short-term, one-time efforts but rather requires sustained, consistent, and repetitive activities on the part of the human ecosystem engineers. In nonhuman species, this repetitive niche construction is primarily transmitted genetically: “Each individual inherits genes that express the behavior in multiple generations” (Odling-Smee et al. 2003, p. 9)
In addition, modifications made to the local physical environment by the members of one generation can be passed on to the next generation through the process of ecological inheritance, which comprises
… whatever legacies of modified natural selection pressures that are bequeathed by niche constructing ancestral organisms to their descendants. Thus, ecological inheritance more closely resembles the inheritance of territory or property than it does the inheritance of genes. (Odling-Smee et al. 2003, p. 13; see also Odling-Smee and Laland 2012, this issue)
Small-scale human societies bequeath to the next generation landscapes that have already been modified and shaped in a variety of ways, and over many generations.
In addition to being passed down from one generation to the next through genetic and ecological inheritance, humans also, of course, communicate niche-construction strategies, the sum total of accumulated “copying” knowledge, through cultural inheritance (Laland and Brown 2002; Laland et al. 2007) and the transfer of TEK. In stories, myths, legends, and general worldview, and through the continuous intergenerational narrative of real-life lessons regarding the attributes and value of different components of the local environment, and how to enhance them, TEK carries a strong aspect of continuity and stability. Of obvious importance in evolutionary terms, however, TEK is not static and unchanging:
“TEK is rooted in, and informed by, a traditional or customary lifestyle, but it adapts to change and incorporates contemporary information and technology. New information is continually added and old information deleted, as the environment is transformed, as weather patterns shift, or as species are wiped out or introduced” (Menzies and Butler 2006, p. 7).
Embedded within TEK transfer, cultural niche-construction strategies also build upon themselves over time:
[C]ultural niche construction, guided by culturally transmitted information, is a particularly potent modifier of environments, with major evolutionary and genetic consequences both for humans, and other species in shared ecosystems… cultural processes can amplify the evolutionary feedback loop that is generated by niche construction…. Trans-generational cultural niche construction modifies environments in ways that favour ever-more culture, causing cultural niche construction to become ever-more powerful. (Laland et al. 2007, p. 59)
Niche Construction Increases the Abundance and Accessibility of Targeted Wild Species
Niche construction by small-scale societies is in large measure designed to increase the relative abundance and reliability of preferred wild species of plants and animals within resource-catchment areas, and to reduce the amount of time and energy required to harvest them. In effect, these societies restructure their local ecosystems so that more of the solar radiation entering them each year is transformed into new organic matter in the plant species (and subsequently in the animals that feed on them) upon which they depend for food and raw material. Through niche construction, they redirect a higher percentage of an ecosystem’s net primary production to species of economic value to humans. These linked goals of increasing resource abundance, predictability, and availability are accomplished through a few simple strategies that focus directly on plant species, with animal consumer species influenced more indirectly (Smith 2011a).
The relative abundance of targeted plants is increased on the one hand by employing fire and other methods in the selective removal of lower-value species that would compete with them for sunlight, soil moisture, and nutrients. Lower-value species would include, for example, many species of non-fruit- or nut-bearing trees that sequester large amounts of organic matter (standing crop biomass) in woody tissue that is of no nutritional value to either humans or to the animals that humans consume. Plant species high on the list as targets for niche-construction efforts by small-scale societies, in contrast, are ones in which some substantial percentage of their organic matter is contained in tissue of nutritional value to humans, either reproductive propagules (e.g., seeds, nuts, mast, fruits, berries) or underground energy-storage organs (e.g., roots, rhizomes, bulbs, tubers).
At the same time, niche-construction efforts are also directed toward actively increasing the abundance of economically valuable target species. This is accomplished by expanding existing stands or patches of target species as well as by creating new stands by planting seeds or transplanting young plants of the species in question into areas that meet, or are modified to meet, their soil, moisture, and nutrient requirements.
By increasing the overall abundance of target species within the resource-catchment area through expansion of existing stands and the creation of new ones, small-scale human societies also reduce the amount of time and energy required to both monitor and harvest the species that are the subject of human niche construction. Monitoring the status of economically valuable plants through the growing season to assess their relative growth and health, as well as predation threats, becomes less time consuming as their distribution across the landscape becomes more consolidated into larger stands. Similarly, harvesting becomes easier both logistically and in terms of time as stands become larger in size. In addition, monitoring, harvesting, and protection from predation (both human and non-human) is particularly improved for those newly created stands that are situated close to human settlements and near frequently used routes and destinations.
By shifting plant-community composition toward earlier successional-stage plant species, small-scale human societies can also indirectly increase, through trophic cascade, the relative abundance of a wide range of browsing herbivores that are highly valued as food resources (e.g., Bliege-Bird et al. 2008). At the same time, direct efforts to reduce acquisition effort and increase the predictability of high-value animal species are largely limited to channeling and constraining their movement to allow for easier harvesting. This is accomplished by creating and maintaining, often over long periods of time, structural modifications to the landscape such as fish weirs designed to direct fish into enclosures for capture and similar terrestrial fences placed to facilitate the driving of large herbivores into corrals for killing (Bar-Oz et al. 2011; Smith 2011a).
The reliability and predictability of economically valuable plant and animal species is also enhanced in another important respect as a result of the investment of time and energy by small-scale societies in reducing low-value plant species within their resource-catchment areas, increasing the size and number of stands of favored plant species, and constructing structures to facilitate harvesting of animal prey. As the economic value of expanded and newly created resource locales increases, there is an associated strengthening of bonds of ownership over them by the kin groups that maintain them and “keep them living” (Deur and Turner 2005). Strengthened ownership, in turn, carries with it the dual benefits of recognition on the part of outside groups that access is restricted, providing additional protection to the resource locales, while also giving the “owners” exclusive control over the long-term management and maintenance of the resource patches. The resource locales are effectively removed from the “tragedy of the commons” and the associated threats to their continued existence. In addition, as the overall relative abundance of high-value resources within catchment areas is increased through niche construction, and resources are consolidated into larger patches, land-tenure strategies shift away from focusing on specific resource locales and toward the cost-benefit tipping point where ownership and defense (either socially negotiated or physical) encompass a society’s entire catchment zone.
A Cultural Niche Construction Theory of Initial Domestication
Based on the five general characteristics of small-scale human societies outlined above, a theory of initial domestication can be proposed that involves the following sequence of developments.
-
(1)
The onset of warmer and wetter climates at the end of the Pleistocene, accompanied by an increase in CO2 levels and the establishment of more stable and quiescent weather patterns (Richerson et al. 2001), resulted in the emergence in many world regions of new and more productive ecosystems, particularly in the temperate latitude zones that have witnessed the development of the earth’s highest levels of ecologically relevant terrestrial net productivity (Huston and Wolverton 2009).
-
(2)
Against this backdrop of global climate change and increasing net primary production, the world’s small-scale human societies, including those situated in the regions identified as being independent centers of domestication, occupied a broad spectrum of newly emerging early Holocene ecosystems, some of which can be recognized as being particularly attractive in terms of high human-carrying capacity.
-
(3)
Within such resource-rich “hotspot” settings associated with river floodplain corridors and lake and marsh/estuary margins, which are among the most productive natural habitats in the world, small-scale human societies encountered an unusually rich density and diversity of potential plant and animal food sources (Smith 2007).
-
(4)
Ranging outward from small central-place settlements (Marlowe 2006) consisting of at most a dozen or so household units, small-scale human societies occupying these resource-rich environments established resource-catchment zones, and detailed TEK systems (Berkes 2008) were developed, refined, and passed down from generation to generation through cultural inheritance.
-
(5)
Broad-based human utilization of plant and animal species in these early Holocene “hot spot” environments, often recognized in the archaeological record as the “broad-spectrum revolution” (Zeder 2012), represents an extended phase of wide-ranging exploration, experimentation, and social learning (Laland and Brown 2002) by small-scale human societies as they comprehensively “auditioned” component species of these unusually rich biotic communities to assess their potential both for sustained economic utilization and as targets of niche construction (Smith 2007).
Emerging out of the initial somewhat chaotic and selective sorting out of various potential economic solutions that took place during the broad-spectrum revolution, ownership and enhancement of high-value resource locales within resource catchments developed, with a subsequent eventual shift by small-scale societies to territorial defense of entire resource-catchment zones (Dyson-Hudson and Smith 1978).
With the establishment of defended resource locales and resource-catchment territories, the associated formation of systems of TEK, and multiple generations of trial and error, of social learning or “copying” behavior (Laland et al. 2007) focused on enhancement of local ecosystems through niche construction, a wide range of species of plants and animals were subject to varying degrees and forms of manipulation and life-cycle intervention.
Among the species that were auditioned to establish their economic value and potential for niche construction efforts by small-scale human societies across diverse ecosystems in the Early Holocene, many were identified as low-value candidates and targeted for active discouragement, while a smaller group of species with high economic utility responded in ways that encouraged and rewarded further experimentation and investment of human capital. The positive feedback loops that developed between small-scale societies and some members of this latter species group resulted in important and sustained traditions of management of essentially “wild” populations (Smith 2011a), whereas others evolved into relationships of domestication (Smith 2007), many of which continued to expand and intensify up through the millennia to the present day.
Discussion
The cultural niche construction theory (CNC) of initial domestication presented here provides an alternative to, and replacement for, prior explanations that have been proposed to account for the initial domestication of plants and animals (including diet breadth models) that are based on an outmoded concept of asymmetrical adaptation (Smith 2009). By offering a direct and diametrically opposed alternative explanation, the CNC theory will also, I hope, serve to broaden the focus of future discussion and debate beyond the artificial constraints that are imposed by considering diet breadth and related human behavioral ecology models in isolation. T.C. Chamberlin recognized the danger of researchers becoming too attached to favored approach or theory more than a century ago when he proposed the method of multiple working hypotheses: “With this method the dangers of parental affection for a favorite theory can be circumvented (Chamberlin 1897).
For more than a quarter century, scholars have remarked on the unfortunate tendency of proponents of optimal foraging theory (OFT) to consider optimal foraging or diet breadth models in isolation and to insist on their exclusive application both in biology and in the study of human societies (e.g., Piperno 2006, 2011). A year after Winterhalder (1986) judged OFT to be a “paragon of robustness” in biology, for example, Gray reached a very different conclusion in his comprehensive critique of OFT’s basic assumptions, predictions, precision, testability, insight, generality, and degree of empirical support: “Despite its current popularity OFT faces a long list of serious problems” (Gray 1987, p. 93). In a passage that applies as well to anthropology today as it did to biology in 1987, Gray noted that
“while it is true that many pages of many journals are full of OFT, this fact alone is hardly a strong argument for OFT. Popularity is a poor measure of content and quantity is no estimate of quality. The history of science is littered with research programs which at the time stimulated lots of intellectual effort, but with the benefit of hindsight, appear sadly misguided” (Gray 1987, p. 75).
One of the many concerns raised by Gray regarding the application of optimal foraging models in biology is the issue of “parental affection” for favorite intellectual offspring (Chamberlin 1897) and the resultant reluctance on the part of OFT proponents to consider any alternative perspectives or explanations. When faced with discrepancies between an OFT model and its observations and the question of “whether the approach is fundamentally flawed or whether a more specific assumption is inaccurate,” the all-too-frequent response has been to propose an “endless number of highly plausible ad hoc modifications” (Gray 1987, p. 81), thus avoiding consideration of the possibility of core conceptual flaws in OFT models. Comparing this response to the manner in which medieval astronomers added more and more epicycles to their models of the solar system in order to preserve their faith in the circular orbit of planets, Gray cites the vivid statement of Croizat-Chaley (1978, p. 119): “My viewpoint is that when somebody sets foot into a trap, he should do his utmost to break fully loose from it, not hobble about claiming that the trap is but a rare kind of shoe.”
Anthropologists have raised similar concerns over the past 30 years regarding the reluctance of OFT proponents to look beyond their own models for possible explanations: “Our research strategies must permit us to compare the relative merits of alternative explanations of observed behavior” (Blundell 1983, p. 642). Or, “If the models don’t fit, make new ones” (Cashdan 1983b, p. 642). Or, “I urge ecological anthropologists to test existing models and formulate new ones” (Smith 1983, p. 648). Presented here in general outline, the CNC theory represents just such a newly formulated explanation and may encourage OFT proponents to follow my earlier suggestion (Smith 2006a) that they consider alternatives to their own explanations rather than viewing it as someone else’s responsibility. As Bettinger (2006, p. 321) put it, “My more fundamental problem with the method of multiple working hypotheses is its suggestion that I should spend time developing plausible alternatives. In my view the responsibility for that falls squarely on those who doubt the hypothesis I’m working on; it keeps me busy enough as it is.”
The dozen or so independent centers of initial domestication that have been identified worldwide offer excellent case-study opportunities to consider the degree to which the CNC theory and other alternative explanations are supported by available empirical evidence. A number of specific test implications or predictions can be derived from each competing explanatory framework and compared with available archaeological information, allowing their relative strength to be directly compared. Test implications for the CNC theory, for example, include the following: (1) initial evidence of domestication should occur in resource-rich ecosystem settings rather than marginal environments; (2) settlements should be small but reflect long-term occupation or reoccupation; (3) evidence of utilization of a broad spectrum of resources should be present; (4) evidence of population packing and over-exploitation of resources should be absent; (5) evidence of burning and other markers of niche construction, such as vegetation clearing and the presence of water-management features, may be observable in the archaeological record (Smith 2011a); and (6) evidence of multigenerational corporate ownership of established resource-catchment territories, including group burial features and other ceremonial structures, may be observable in the archaeological record.
All of the test implications for the CNC theory listed above have been confirmed in eastern North America, one of the best-documented independent centers of domestication (Smith 2006b, 2011b; Smith and Yarnell 2009), and the CNC theory also appears to be supported by currently available evidence in a number of other world regions (Smith 2007; Bar-Yosef and Price 2011; Zeder 2012). It will be interesting to see, over the next several decades, to what extent continuing research in other independent centers of initial domestication worldwide will, like eastern North America, provide increasing support for, and additional refinement of, the CNC theory, while also allowing for the comparative testing of alternative explanations.
Another advantage in comparing the relative strength of alternative universal explanations for initial domestication, rather than restricting consideration to a single model in isolation, is that it highlights test implications that may have been considered to be “critical” [those that support one explanation and undercut another (Smith 1977)], but which in fact appear to provide support for more than one competing theory or model. Evidence of the broad-spectrum revolution (test implication 3, above), for example, has been assumed in recent years to reflect resource depression and to be a critical test implication providing exclusive support for diet breadth explanations. The broad-scale revolution however, has also been long identified as evidence for small-scale societies experimenting with rich resource abundance and could reflect the sorting out of potential subsistence strategies and the establishment of TEK systems rather than resource depression (Zeder 2012). For example, Caldwell (1958) coined the term “primary forest efficiency” to describe the Late Archaic societies of eastern North America that were on the cusp of domesticating a number of species of local seed plants:
According to the concept of primary forest efficiency, Archaic peoples learned over the millennia how to ever more efficiently and skillfully exploit a wider and wider spectrum of natural food resources in their generous woodland environment. The result was the establishment of cyclical seasonal procurement schedules that focused on the harvesting of foods that were abundant, nutritious, and relatively easy to gather or capture. (Gibbon and Ames 1998, p. 686)
In this regard CNC theory clearly calls into question a core assumption of the diet breadth model. The deliberate and sustained enhancement of a specific set of species of plants and animals by Early and Mid-Holocene small-scale societies through niche construction would result in the newly enhanced species moving up in any optimization rank list of species based on net caloric return per unit of energy expended. Given this, changes in the relative abundance of different species in archaeobiological assemblages that have been frequently identified as evidence of resource depression requiring small-scale societies to turn to lower-ranking species (e.g., a reduction in occurrence of larger prey and an increase in smaller prey and seed plants) could just as reasonably represent the “upward mobility” of species whose relative abundance and ease of acquisition were enhanced through deliberate human design. If the relative explanatory value of the diet breadth model is seriously compromised in situations where human niche construction must be factored in, a key challenge will be identifying when in the archaeological record humans began to modify ecosystems in a substantial and sustained manner.
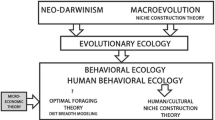
Although I have cast CNC theory in opposition to diet breadth models in order to encourage debate and further comparison of alternative explanations in the independent centers of domestication worldwide, CNC theory clearly falls comfortably under the general heading of “behavioral ecology.” Derived from niche construction theory, CNC theory also incorporates several behavioral ecology concepts and perspectives, including central-place provisioning, resource catchment, resource ownership and defensibility, and traditional ecological knowledge. Perhaps most importantly, the niche-construction paradigm and the concept of cultural niche construction, as embodied in CNC theory, appears to hold great promise in serving as a framework of explanation that can accommodate and successfully integrate both biologically and culturally informed approaches to understanding the evolution of human behavior (Laland and O’Brien 2010, 2012 this issue).
References
Anderson MK (1999) The fire, pruning, and coppice management of temperate ecosystems for basketry material by California Indian tribes. Hum Ecol 27:79–113
Anderson MK (2005) Tending the wild. University of California Press, Berkeley
Bar-Oz G, Zeder M, Hole F (2011) Role of mass-kill hunting strategies in the extirpation of Persian gazelle (Gazella subgutturosa) in the northern Levant. Proc Nat Acad Sci 108:7345–7350
Bar-Yosef O, Price TD (eds) (2011) The origins of agriculture: new data, new ideas. Curr Anthropol 52 (Special Supplement 4)
Berkes F (2008) Sacred ecology, 2nd edn. Routledge, New York
Berlin B (1992) Ethnobiological classification: principles of categorization of plants and animals in traditional societies. Princeton University Press, Princeton
Bettinger R (2006) Agriculture, archaeology, and human behavioral ecology. In: Kennett D, Winterhalder B (eds) Behavioral ecology and the transition to agriculture. University of California Press, Berkeley, pp 304–322
Bettinger RL, Malhi R, McCarthy H (1997) Central place models of acorn and mussel processing. J Archaeol Sci 24:887–899
Binford LR (1968) Post-Pleistocene adaptations. In: Binford SR, Binford LR (eds) New perspectives in archeology. Aldine, Chicago, pp 313–341
Bird D, Bliege Bird R (1997) Contemporary shellfish gathering strategies among the Meriam of the Torres Strait Islands, Australia: testing predictions of a central place foraging model. J Archaeol Sci 24:39–63
Bliege-Bird R, Bird DW, Codding BF, Parker CH, Jones JH (2008) The “fire stick farming” hypothesis: Australian Aboriginal foraging strategies, biodiversity, and anthropogenic fire mosaics. Proc Nat Acad Sci 105:14796–14801
Blundell V (1983) Comment on “Anthropological applications of optimal foraging theory: a critical review” by E. A. Smith. Curr Anthropol 24:625–651
Butz D (1996) Sustaining indigenous communities: symbolic and instrumental dimensions of pastoral resource use in Shimshal, Northern Pakistan. Can Geogr 40:36–53
Caldwell J (1958) Trend and tradition in the prehistory of the Eastern United States. Illinois State Museum, Springfield
Cashdan E (1983a) Territoriality among human foragers: ecological models and an application to four bushman groups. Curr Anthropol 24:47–66
Cashdan E (1983b) Comment on “Anthropological applications of optimal foraging theory: a critical review” by E. A. Smith. Curr Anthropol 24:625–651
Chamberlin TC (1897) The method of multiple working hypotheses. Science 15:92–96
Chisholm MC (1968) Rural settlement and land use: an essay in location. Aldine, Chicago
Cohen MN (1977) The food crisis in prehistory: overpopulation and the origins of agriculture. Yale University Press, New Haven
Croizat-Chaley L (1978) Hennig (1966) entre rosa (1918) y Lovtrup (1977): medio siglo de “sistematica pilogentica”. B Acad Cien Fis Matemat Nat Caracas 38:59–147
Deur D, Turner N (eds) (2005) Keeping it living: traditions of plant use and cultivation on the Northwest Coast of North America. University of Washington Press, Seattle
Doebley J, Gaut B, Smith BD (2006) The molecular genetics of crop domestication. Cell 127:1309–1321
Dyson-Hudson R, Smith EA (1978) Human territoriality: an ecological reassessment. Amer Anthropol 80:21–41
Fowler K, Rhode D (2011) Plant foods and foodways among the Great Basin’s indigenous peoples. In: Smith BD (ed) The subsistence economies of indigenous North American societies. Smithsonian Institution Scholarly Publications, Washington, pp 233–270
Gepts P, Famula T (eds) (2012) Biodiversity in agriculture: domestication, evolution, and sustainability. Cambridge University Press, Cambridge
Gibbon G, Ames K (1998) Archaeology of prehistoric North America. Routledge, London
Gottesfeld L, Johnson M (1994) Conservation, territory, and traditional beliefs: an analysis of Gitksan and Wet’suwet’en subsistence, northwest British Columbia, Canada. Hum Ecol 22:443–465
Gray RD (1987) Faith and foraging: a critique of the “paradigm argument from design”. In: Kamil AC, Krebs JR, Pulliam HR (eds) Foraging behavior. Plenum, New York, pp 69–140
Hamilton WJIII, Watt KEF (1970) Refuging. Ann Rev Ecol Evol Syst 1:263–286
Hawkes K, Hill K, O’Connell JF (1982) Why hunters gather: optimal foraging and the Ache of eastern Paraguay. Amer Ethnol 9:379–398
Higgs ES, Vita-Finzi C, Harris DR, Fagg FA (1967) The climate, environment, and industries of stone age Greece: part III. Proc Prehist Soc 33:1–29
Hunn E (1993) What is traditional ecological knowledge? In: Williams NM, Baines G (eds) Traditional ecological knowledge: wisdom for sustainable development. Centre for Resource and Environmental Studies, Australian National University, Canberra, pp 13–15
Huston MA, Wolverton S (2009) The global distribution of net primary production: resolving the paradox. Ecol Monogr 79:343–377
Kelly RL (2007) The foraging spectrum: diversity in hunter-gatherer lifeways (2nd ed). Percheron, New York
Kendal J, Tehrani J, Odling-Smee J (eds) (2011) Human niche construction. Philos Trans R Soc B 366:784–934
Kennett D, Winterhalder B (eds) (2006) Behavioral ecology and the transition to agriculture. University of California Press, Berkeley
Laland K, Brown GR (2002) Sense and nonsense: evolutionary perspectives on human behavior. Oxford University Press, Oxford
Laland KN, O’Brien MJ (2010) Niche construction theory and archaeology. J Archaeol Method Theory 17:303–322
Laland KN, O’Brien MJ (2012) Cultural niche construction: an introduction. Biol Theory 6. doi:10.1007/s13752-012-0026-6
Laland KN, Kendal JR, Brown GR (2007) The niche construction perspective: implications for evolution and human behavior. J Evol Psych 5:51–66
Lee RB (1969) !Kung bushman subsistence: an input-output analysis. In: Vayda AP (ed) Environment and cultural behavior. Natural History Press, Garden City, pp 47–79
Lewontin RC (1983) Gene, organism, and environment. In: Bendall DS (ed) Evolution from molecules to men. Cambridge University Press, Cambridge, pp 273–285
Marlowe FW (2006) Central place provisioning: the Hadza as an example. In: Hohmann G, Robbins MM, Boesch C (eds) Feeding ecology in apes and other primates: ecological physical and behavioral aspects. Cambridge University Press, Cambridge, pp 359–377
Menzies C, Butler C (2006) Introduction: understanding ecological knowledge. In: Menzies C (ed) Traditional ecological knowledge and natural resource management. University of Nebraska Press, Lincoln, pp 1–17
Odling-Smee J, Laland KN, Feldman MW (2003) Niche construction: the neglected process in evolution. Princeton University Press, Princeton
Odling-Smee J, Laland KN (2012) Ecological inheritance and cultural inheritance: what are they and how do they differ? Biol Theory 6. doi:10.1007/s13752-012-0030-x
Piperno D (2006) The origins of plant cultivation and domestication in the Neotropics: a behavioral ecological approach. In: Kennett D, Winterhalder B (eds) Behavioral ecology and the transition to agriculture. University of California Press, Berkeley, pp 137–166
Piperno D (2011) The origins of plant cultivation and domestication in the New World tropics. In: Bar-Yosef O, Price TD (eds) The origins of agriculture: new data, new ideas. Curr Anthropol 52 (Special Suppl 4):453–470
Richerson PJ, Boyd R, Bettinger RL (2001) Was agriculture impossible during the Pleistocene but mandatory during the Holocene? Am Antiquity 66:387–411
Roper DC (1979) The method and theory of site catchment analysis: a review. Advances Archaeol Method Theory 2:119–140
Russell-Smith J, Lucas D, Gapindi M et al (1997) Aboriginal resource utilization and fire management practice in western Arnhem Land, monsoonal northern Australia: notes for prehistory, lessons for the future. Hum Ecol 25:159–195
Smith BD (1977) Archaeological inference and inductive confirmation. Am Anthropol 79:589–617
Smith EA (1983) Anthropological applications of optimal foraging theory: a critical review. Curr Anthropol 24:625–651
Smith BD (2001) Low level food production. J Archaeol Res 9:1–43
Smith BD (2006a) Human behavioral ecology and the transition to food production. In: Kennett D, Winterhalder B (eds) Behavioral ecology and the transition to agriculture. University of California Press, Berkeley, pp 289–303
Smith BD (2006b) Eastern North America as an independent center of plant domestication. Proc Nat Acad Sci 103:12223–12228
Smith BD (2007) Niche construction and the behavioral context of plant and animal domestication. Evol Anthropol 16:188–199
Smith BD (2009) Core conceptual flaws in human behavioral ecology. Commun Integr Biol 2:533–534
Smith BD (2011a) General patterns of niche construction and the management of “wild” plant and animal resources by small-scale pre-industrial societies. Philos Trans R Soc B 366:836–848
Smith BD (2011b) The cultural context of plant domestication in eastern North America. In: Bar-Yosef O, Price TD (eds) The origins of agriculture: new data, new ideas. Curr Anthropol 52 (Special Suppl 4):471–484
Smith BD, Yarnell RA (2009) Initial formation of an indigenous crop complex in eastern North America at 3800 B.P. Proc Nat Acad Sci 106:6561–6566
Steward J (1933) Ethnography of the Owens Valley Paiute. U Cal Pub Am Archaeol Ethnol 33:233–350
Steward J (1938) Basin-Plateau aboriginal sociopolitical groups. Bur Am Ethnol Bull 120:346
Vita-Finzi C, Higgs ES (1970) Prehistoric economy in the Mt. Carmel area of Palestine. Proc Prehist Soc 36:1–37
Williams GC (1992) Gaia, nature worship, and biocentric fallacies. Q Rev Biol 67:479–486
Wilson EO (1975) Sociobiology: the new synthesis. Harvard University Press, Cambridge
Winterhalder B (1986) Diet choice, risk, and food sharing in a stochastic environment. J Anthropol Archaeol 5:369–392
Zeder MA (2012) The broad spectrum revolution at 40: resource diversity, intensification, and an alternative to optimal foraging explanations. J Anthropol Archaeol 31:241–264
Zeder MA, Smith BD (2009) A conversation on agricultural origins: talking past each other in a crowded room. Curr Anthropol 50:681–691
Zeder MA, Emshwiller E, Bradley D, Smith BD (eds) (2006) Documenting domestication: new genetic and archaeological paradigms. University of California Press, Berkeley
Acknowledgments
I thank Kevin Leland and Mike O’Brien for including me in the KLI workshop and the other participants for stimulating and enjoyable discussions. I also thank Russell Gray, Kevin Laland, Mike O’Brien, Thomas Payne, Joyce Marcus, and Melinda Zeder for their suggestions for improving this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, B.D. A Cultural Niche Construction Theory of Initial Domestication. Biol Theory 6, 260–271 (2011). https://doi.org/10.1007/s13752-012-0028-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13752-012-0028-4