Abstract
New sets of thiochromenes hybridized with five-membered rings as pyrazole and oxazole and/or six-membered ring as pyrimidine and thiopyrimidine were constructed. The novel constructed compounds were evaluated for their antimicrobial potential against B. subtilis and S. aureus as examples of gram-positive-bacteria, E. coli and P. aeruginosa as examples of gram-negative bacteria in addition to A. flavus and C. albicans as fungal strains. The results recorded promising antimicrobial potential with inhibition zone diameter range from 8 to 25 mm against the tested bacteria. Regarding antifungal activity, all the screened compounds revealed antifungal effect against A. flavus except thiochromene derivative 4 with zone of inhibition ranged from 9 to 16 mm. Moreover, all compounds recorded moderate to high antifungal potential towards C. albicans (ZI range = 8–19 mm) except thioxopyrazolothiochromene derivative 6 which did not exhibit any effect. To suggest mode of action of these candidates as antimicrobials, in silico docking study was carried out inside dihydropteroate synthase enzyme. Compound 8c recorded the best binding energy score (−5.47 kcal/mol) forming good fitting within DHPS active site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most real risks to global health is multidrug resistance emergence by pathogenic microbes [1, 2]. A wide variety of biochemical mechanisms account for bacterial resistance including mutational modification of target protein, drug’s enzymatic inactivation, prevention of drug access to the targeted enzyme [3, 4]. Furthermore, misuse of antibiotics speeds up the emergence of multidrug resistant bacterial species [5]. So there is still a great demand for development of new antimicrobials to overcome the emergence and development of novel chemotherapeutic agents to combat the emergence and increasing spread of resistant pathogens. Dihydropteroate synthase enzyme (DHPS) is an essential enzyme for microbes which catalyzes the formation of 7,8-dihydropteroate—a precursor of tetrahydrofolate—formed through reacting p-aminobenzoic acid and 7,8-dihydropterin pyrophosphate [6,7,8]. Folate analogues are essential cofactors for nucleic acid and protein biosynthesis in all cells [9]. Whereas humans get folates from diet, almost all microbes biosynthesize folate de novo [10]. So, targeting DHPS is considered as a rational for design selective antimicrobial agents. Thiochromene-based heterocycles recorded versatile biological importance as HIF hydroxylase inhibitor [11], dopamine D3 receptor agonist [12, 13], anticancer [14], herbicide [15], antimicrobial [16, 17] and HIV protease inhibitor [18]. Prompted by all the previous facts and our researches concerned with designing and preparing bioactive agents [19,20,21,22,23,24,25,26,27,28,29,30], we are encouraged to synthesize some novel thiochromene derivatives and evaluating these novel compounds for their antibacterial potential against B. subtilis, S. aureus, E. coli and P. aeruginosa and antifungal potential towards A. flavus and C. albicans. In addition, in silico docking study was carried out for the synthesized compounds to display the probability of these compounds to suppress DHPS enzyme.
Results and discussion
Chemistry
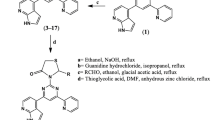
In the present work, the target compounds were constructed as illustrated in Schemes 1, 2 and 3. The starting compound 4 was prepared via reacting mercaptobenzaldehyde (1), acetamide (2) and ethyl chloroacetate (3) in the presence of piperidine in a catalyst (Scheme 1).
Reacting compound 4 with carbon disulphide and potassium hydroxide in ethanol afforded the propanedithioic acid derivative 5 (Scheme 2). 1HNMR spectrum of this compound 5 displayed the disappearance of methyl protons at 1.91 ppm in addition to the appearance of two singlet signals at 2.71 and 13.25 ppm referred to CH2 and SH groups. Moreover, in 13CNMR the presence of a peak at 229.78 ppm referred to C = S confirmed the structure of compound 5. Cyclizing the propanedithioic acid 5 with hydrazine hydrate in ethanol afforded thioxopyrazole derivative 6 in 68% yield. 1HNMR of the product 6 revealed the presence of single signal exchanged with D2O at 9.99 ppm attributed to NH proton and disappearance of SH proton at 13.25 ppm of the precursor 5. The chalcone derivatives 7a-c were prepared from reacting the acetamide derivative 4 with the appropriate aldehydes in presence of piperidine as a catalyst. The 1HNMR spectra of these chalcones 7a-c recorded the presence of CH = CH and additional aromatic protons in range of 6.93–8.45 ppm.
Cyclizing chalcones 7a-c with hydrazine hydrate in acetic acid afforded acetylpyrazole derivatives 8a-c in good yield (Scheme 3). Chemical structure of these pyrazoles was elucidated by spectral and elemental analysis. 1HNMR showed the presence of a singlet signal at 2.12 ppm referred to CH3 protons, in addition to doublet and triplet peaks at 3.47 and 5.03 ppm attributed to CH2 and CH protons.
On the other hand, the phenylpyrazole derivatives 9a-c were constructed from treating the chalcones with phenylhydrazine in DMF using piperidine as a catalyst. 1HNMR chart of compound 9a recorded the appearance of doublet and triplet peaks at 3.48 and 5.24 ppm referred to CH2 and CH protons in addition to the presence of 15 aromatic protons at 6.92–8.35 ppm. Reacting chalcones 7a-c with hydroxylamine afforded isoxazole derivatives 10a-c in good yields. Pyrimidine derivatives 11a-c and thiopyrimidine derivatives 12a-c were constructed from reacting chalcones 7a-c with urea and/or thiourea. These target compounds 10a-c, 11a-c and 12a-c were confirmed by spectral and elemental analyses.
Antimicrobial potential
All the twenty novel prepared compounds 4-12a-c were screened in vitro for their antibacterial activity against two gram-positive bacterial strains as B. subtilis and S. aureus and gram-negative bacterial strains as E. coli and P. aeruginosa using ampicillin as a standard at concentration 100 ppm adapting disc-diffusion technique. The results are listed in Table 1 which revealed that all the tested compounds exhibited antimicrobial potential with inhibition zone diameter ranged from 8 to 25 mm towards all tested bacterial strains. Concerning antimicrobial potential against B. subtilis, the pyrazolothiochromene derivative 9c (ZI = 25 mm) and pyrimidiothiochromene derivative 11c (ZI = 25 mm) recorded comparable antimicrobial potential to that recorded by ampicillin (ZI = 26 mm). Moreover, the phenylpyrazole derivatives 9a-c (ZI = 16–25 mm) displayed higher antimicrobial potential towards B. subtilis than acetylpyrazole analogues 8a-c (ZI = 13–18 mm). Moreover, pyrimidiothiochromene candidates 11a-c (ZI = 14–25 mm) recorded higher antibacterial effect than thiopyrimidine analogues 12a-c (12–15 mm). Furthermore, the pyrazolothiochromene 8c (ZI = 19 mm) was the most active antibacterial agent towards S. aureus followed by thiochromene derivative 7b (ZI = 17 mm) and then thiochromenes 4, 7c and pyrimidothiochromenes 11a and 11c (ZI = 14 mm). Moreover, the acetylpyrazole derivative 8c (ZI = 24 mm), phenylpyrazole 9b (ZI = 23 mm) and oxazole derivative 10b (ZI = 24 mm) recorded similar antibacterial potential towards E. coli as displayed by ampicillin (ZI = 25 mm). The thioxopyrazole derivative 6 was found to be the least active antibacterial agent towards all the tested bacteria with zone of inhibition range from 8 to 9 mm. The antifungal potential of the target compounds 4-12a-c towards A. flavus and C. albicans was screened using amphotericin B as a standard. From the obtained results, all the screened compounds revealed antifungal effect against A. flavus except thiochromene derivative 4 with zone of inhibition ranged from 9 to 16 mm. Besides, thiochromene candidate 7c (ZI = 16 mm), acetylpyrazole 8b (ZI = 15 mm) and oxazole derivatives 9c and 10b (ZI = 14 mm) assigned similar antifungal potential to A. flavus as shown by amphotericin B (ZI = 15 mm). While the antifungal potential against C. albicans revealed that all compounds recorded moderate to high antifungal potential (ZI range = 8–19 mm) except thioxopyrazolothiochromene derivative 6 which did not exhibit any effect. The highest antifungal activity to C. albicans was recorded by phenylpyrazole derivatives 9a (ZI = 18 mm) and 9b (ZI = 19 mm) followed by 9c (ZI = 16 mm) and then compounds 8b, 10a and 10b (ZI = 14 mm).
In Silico docking study
To predict the mode of action of the newly constructed compounds 6-12a-c as antimicrobial agents, molecular docking study was conducted on these compounds inside DHPS enzyme using MOE 2005.06. Crystal structure of DHPS with the cocrystallized ligand (pyrimido [4,5-c]pyridazine derivative) was downloaded from protein data bank (PDB: 4DAI). Outcomes from docking the target compounds are explained in Table 2.
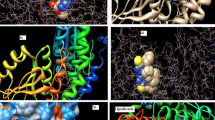
Compound 8c showed good fitting within DHPS revealing three hydrogen bonding interactions with Asp101, Arg254 and Ser218 through binding with NH, thiochromene C = O and OH moieties. Moreover, compound 8c formed arene cation interactions with Lys220 and Arg254 through binding with phenyl moiety (Fig. 1).
The oxazolothiochromene derivative 10c recorded binding energy score = -5.09 kcal/mol forming four hydrogen bonds as follows: (1) Asp101 with NH, (2) Arg254 with thiochromene C = O, (3) Asn120 with thiochromene C = O and (4) Arg219 with OH via H2O molecule (Fig. 2).
One the other hand, compound 11c showed binding with Glu79, Lys104 and Asp101 though hydrogen bonding with hydroxyl and amino groups, besides arene cation interaction with Arg254 and Lys220 aminoacids (Fig. 3).
Experimental
Chemistry
Reagents were purchased from Sigma Aldrich (Bayouni Trading Co. Ltd., Al-Khobar, Saudi Arabia) and used without further purification. Reaction progress was monitored by TLC on silica gel pre-coated F254Merck plates. Spots were visualized by ultraviolet irradiation. Melting points were determined on a Gallenkamp electrothermal melting point apparatus and are uncorrected. IR spectra were recorded as potassium bromide disc using Bruker-Vector 22 FTIR Spectrophotometer. The NMR spectra were recorded with a Varian Mercury VXR-300 NMR spectrometer at 300 and 75 MHz for 1H and 13C NMR spectra, respectively, using DMSO-d6 as solvents. Mass spectra were recorded on a Hewlett Packard MS-5988 spectrometer at 70 eV. Elemental analyses were carried out at the Micro-analytical Center of Cairo University, Giza, Egypt.
N-(2-Oxo-2H-thiochromen-3-yl)acetamide (4)
A mixture of acetamide (0.59 g, 0.01 mol) and ethyl chloroacetate (1.22 g, 0.01 mol) in ethanol (30 mL), piperidine (0.5 ml) as catalyst and mercaptobenzaldehyde (1.38 g, 0.01 mol) was heated under reflux for 7–8 h, and its progress was checked by TLC. The formed precipitate was filtered, dried and crystallized from ethanol/DMF (3:1) to give the expected product (1) (65% yield) as green crystals. mp 200 − 202 °C.; IR (KBr): ν (cm−1) 1660, 1718 (2C = O), 3400 (N–H); 1H NMR (DMSO − d6): δ 1.91 (s, 3H, CH3), 7.32–8.19 (m, 5H, aromatic protons), 12.3 (br. s, 1H, N − H); 13C NMR (DMSO − d6): δ 24.5, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.0 (C = C), 170.5 and 187.8 (2C = O): ms (m/z, %): 219.0 (M+, 55%). Anal. Calcd for C11H9NO2S (219.26): C, 60.26; H, 4.14; N, 6.39; S, 14.62%. Found: C, 60.21; H, 4.13; N, 6.02; S, 14.57%.
3-Oxo-3-((2-oxo-2H-thiochromen-3-yl)amino)propanedithioic acid (5)
A mixture of 4 (2.19 g, 0.01 mol) and carbon disulfide (15 mL) in ethanol (30 mL) and KOH was heated in reflux for 7–8 h. The solid product formed on hot was filtered, washed with MeOH, dried and crystallized from MeOH-DMF (3:1) to afford 5. (60% yield) as brown crystals. mp 183–185 °C; IR (KBr): ν (cm−1) 1660, 1718 (2C = O), 3100–3400 (NH); 1H NMR (DMSO − d6): δ 2.71 (s, 2H, CH2), 7.28–8.19 (m, 5H, aromatic protons), 12.25 (br. s, 1H, NH), 13.25 (s, 1H, SH); 13C NMR (DMSO − d6): δ 62.7, 117.9, 121.3, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 138.0, 138.3, 148.2, 168.3, 187.5 and 229.7: ms (m/z, %): 295.0 (M+, 40%). Anal. Calcd for: C12H9NO2S3 (295.40): C, 48.79; H, 3.07; N, 4.74; S, 32.56%. Found: C, 48.74; H, 3.02; N, 4.68; S, 32.53%.
3-((5-Thioxo-4,5-dihydro-1H-pyrazol-3-yl)amino)-2H-thiochromen-2-one (6)
To solution of 5 (0.300 g, 1 mmol) in EtOH, hydrazine hydrate (0.5 mL) was added. The mixture was refluxed for 6 h. The solid product so obtained after cooling was collected by filtration and crystallized from EtOH to afford 12 in (68% yield) as orange crystals. mp 270–273 °C; IR (KBr): ν (cm−1) 1660, 1718 (2C = O), 3100–3400 (2NH); 1H NMR (DMSO − d6): δ 1.56 (s, 2H, CH2), 7.38–8.19 (m, 5H, aromatic protons), 9.99 (s, 1H, NH), 12.25 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 50.4, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.1, 106.6, 138.3, 118.4, 150.3, 157.5, 187.5 and 201.7: ms (m/z, %): 275.0 (M+, 40%). Anal. Calcd for: C12H9N3OS2 (275.35): C, 52.34; H, 3.29; N, 15.26; S, 23.29%. Found: C, 52.36; H, 3.34; N, 15.32; S, 23.35%.
General synthesis of compounds 7a-c
A solution of 4 (0.01 mol) in absolute ethanol (30 mL) was treated with different aromatic aldehydes (0.01 mol) in the presence of piperidine as catalyst. The reaction mixture was heated under reflux for 4–6 h. (monitored by TLC). The solvent was then evaporated under reduced pressure and the residue treated with ice/water acidified by HCl. The solid product was collected and crystallized from the proper solvent to afford 7a-c.
N-(2-Oxo-2H-thiochromen-3-yl)cinnamamide (7a)
(64% yield) as white crystals. mp 115–117 °C; IR (KBr): ν (cm−1) 1660, 1718 (2C = O), 3100–3400 (NH); 1H NMR (DMSO − d6): δ 6.95 (s, 1H, CH), 7.23–8.45 (m, 11H, aromatic protons & O = C–CH), 12.23 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138, 106.6, 138.3, 128.5, 135.4, 109.5, 141.7, 165.5 and 187.5: ms (m/z, %): 307.0 (M+, 25%). Anal. Calcd for: C18H13NO2S (307.37): C, 70.34; H, 4.26; N, 4.56; S, 10.43%. Found: C, 70.30; H, 4.21; N, 4.54; S, 10.38%.
3-(4-Chlorophenyl)-N-(2-oxo-2H-thiochromen-3-yl)acrylamide (7b)
(55% yield) as grey crystals. mp 135–137 °C; IR (KBr): ν (cm−1) 1660, 1718 (2C = O), 3100–3400 (NH); 1H NMR (DMSO − d6): δ 6.98 (s,1H, CH), 7.28–8.10 (m, 10H, aromatic protons & O = C–CH), 12.23 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.1, 106.6, 138.3, 128.5, 135.4, 109.5, 141.7, 165.5 and 187.5: ms (m/z, %): 341.0 (M+, 25%). Anal. Calcd for: C18H12ClNO2S (341.81): C, 63.25; H, 3.54; Cl, 10.37; N, 4.10; S, 9.38%. Found: C, 63.28; H, 3.59; Cl, 10.42; N, 4.15; S, 9.41%.
3-(4-Hydroxyphenyl)-N-(2-oxo-2H-thiochromen-3-yl)acrylamide (7c)
(58% yield) as yellow crystals. mp 167–169 °C; IR (KBr): ν (cm−1) 1660, 1718 (2C = O), 3100–3400 (NH,OH); 1H NMR (DMSO − d6): δ 6.93 (s,1H, CH), 7.03–8.18 (m, 10H, aromatic protons & O = C–CH), 10.26 (s, 1H, OH), 12.23 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.3, 106.6, 138.3, 128.5, 135.4, 158.1, 109.5, 141.7, 165.5 and 187.5 (2C = O): ms (m/z, %): 323.0 (M+, 30%). Anal. Calcd for: C18H13NO3S (323.37): C, 66.86; H, 4.05; N, 4.33; S, 9.92%. Found: C, 66.89; H, 4.07; N, 4.37; S, 9.96%.
Preparation of compounds 8a-c
A solution of compound 7a-c (0.01 mol) and hydrazine hydrate (0.01 mol) in hot ethanol (30 ml) containing few drop of catalytic amount of glacial acetic acid (3 drops) was refluxed for 8–10 h and estimated by (TLC) till completion, and then the reaction was filtered while hot and the solvent was then evaporated under reduced pressure and the residue was heated with petroleum ether and then treated with ice water and crystalized from methanol to provide 8a-c.
3-((1-Acetyl-5-phenyl-4,5-dihydro-1H-pyrazol-3-yl)amino)-2H-thiochromen-2-one (8a)
(58% yield) as yellow crystals. mp 167–169 °C; IR (KBr): ν (cm−1) 1660, 1718 (2C = O), 3100–3400 (NH); 1H NMR (DMSO − d6): δ 2.12 (s, 3H, CH3), 3.47 (d, 2H, CH2), 5.03 (t, 1H, CH), 7.48–8.29 (m, 10H, aromatic protons), 12.23 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 23.6, 40.0, 63.9, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.7, 106.6, 138.3, 128.5, 135.4, 118.3, 150.8 (C = C), 168.8 and 187.5 (2C = O): ms (m/z, %): 363.0 (M+, 30%). Anal. Calcd for: C20H17N3O2S (363.43): C, 66.10; H, 4.71; N, 11.56; S, 8.82%. Found: C, 66.13; H, 4.76; N, 11.60; S, 8.87%.
3-((1-Acetyl-5-(4-chlorophenyl)-4,5-dihydro-1H-pyrazol-3-yl)amino)-2H-thiochromen-2-one (8b)
(63% yield) as reddish brown crystals. mp 215–217 °C; IR (KBr): ν (cm−1) 1660, 1718 (2C = O), 3100–3400 (NH); 1H NMR (DMSO − d6): δ 2.12 (s, 3H, CH3), 3.47 (d, 2H, CH2), 5.03 (t, 1H, CH), 7.3–8.1 (m, 9H, aromatic protons), 12.25 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 23.6, 40.0, 63.9, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.6, 106.6, 138.3, 128.5, 135.4, 118.3, 150.8, 168.8 and 187.5: ms (m/z, %): 397.0 (M+, 35%). Anal. Calcd for: C20H16ClN3O2S (397.88): C, 60.37; H, 4.05; Cl, 8.91; N, 10.56; S, 8.06%. Found: C, 60.39; H, 4.09; Cl, 8.95; N, 10.62; S, 8.12%.
3-((1-Acetyl-5-(4-hydroxyphenyl)-4,5-dihydro-1H-pyrazol-3-yl)amino)-2H-thiochromen-2-one (8c)
69% yield) as greenish crystals. mp 245–247 °C; IR (KBr): ν (cm−1) 1660, 1718 (2C = O), 3100–3400 (NH,OH); 1H NMR (DMSO − d6): δ 2.12 (s, 3H, CH3), 3.47 (d, 2H, CH2), 5.03 (t, 1H, CH), 7.3–8.1 (m, 9H, aromatic protons), 10.25 (s, 1H, OH), 12.25 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 23.6, 40.03, 63.9, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.6, 106.6, 138.34, 128.5, 135.4, 118.3, 150.8, 168.8 and 187.5: ms (m/z, %): 379.0 (M+, 35%). Anal. Calcd for: C20H17N3O3S (379.43): C, 63.31; H, 4.52; N, 11.07; S, 8.45%. Found: C, 63.34; H, 4.57; N, 11.11; S, 8.48%.
Synthesis of compounds 9a-c
A solution of (7a-c) (0.01 mol) and phenylhydrazine (0.01 mol) in dimethylformamide (30 mL) was prepared, and piperidine was used as catalyst. The mixture was refluxed and heated for 10-11 h (controlled by TLC). The resulting product was subject to reduced pressure to remove solvent and then poured into cold water and crystallized from methanol to provide (9a-c).
3-((1,5-Diphenyl-4,5-dihydro-1H-pyrazol-3-yl)amino)-2H-thiochromen-2-one (9a)
(66% yield) as red crystals. mp 188–190 °C; IR (KBr): ν (cm−1) 1660 (C = O), 3100–3400 (NH); 1H NMR (DMSO − d6): δ 3.48 (d, 2H, CH2), 5.24 (t, 1H, CH), 6.92–8.35 (m, 15H, aromatic protons), 12.23 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 23.6, 40.0, 63.9, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.8, 106.6, 138.3, 128.5, 135.4, 118.3, 150.8, 187.5: ms (m/z, %): 397.0 (M+, 26%). Anal. Calcd for: C24H19N3OS (397.49): C, 72.52; H, 4.82; N, 10.57; S, 8.07%. Found: C, 72.53; H, 4.85; N, 10.62; S, 8.13%.
3-((5-(4-Chlorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl)amino)-2H-thiochromen-2-one (9b)
(72% yield) as reddish brown crystals. mp 210–212 °C; IR (KBr): ν (cm−1) 1660 (C = O), 3100–3400 (NH); 1H NMR (DMSO − d6): δ 3.48 (d, 2H, CH2),5.24(t,1H,CH), 6.99–8.41 (m, 14H, aromatic protons), 12.23 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 40.0, 63.9, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.9, 106.6, 138.3, 128.5, 135.4, 118.3, 150.8, 187.5: ms (m/z, %): 431.0 (M+, 26%). Anal. Calcd for: C24H18ClN3OS (431.94): C, 66.74; H, 4.20; Cl, 8.21; N, 9.73; S, 7.42%. Found: C, 66.72; H, 4.16; Cl, 8.17; N, 9.69; S, 7.39%.
3-((5-(4-Hydroxyphenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl)amino)-2H-thiochromen-2-one (9c)
(76% yield) as reddish brown crystals. mp 220–222 °C; IR (KBr): ν (cm−1) 1660 (C = O), 3100–3400 (NH, OH); 1H NMR (DMSO − d6): δ 3.48 (d, 2H, CH2), 5.24 (t, 1H, CH), 7.15–8.25 (m, 14H, aromatic protons), 10.22 (s, 1H, OH), 12.23 (br. s, 1H, NH); 13CNMR (DMSO − d6): δ 40.0, 63.9, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.9, 106.6, 138.3, 128.5, 135.4, 118.3, 150.8, 187.5 (C = O): ms (m/z, %): 413.0 (M + , 35%). Anal. Calcd for: C24H19N3O2S (413.49): C, 69.71; H, 4.63; N, 10.16; S, 7.75%. Found: C, 69.72; H, 4.66; N, 10.20; S, 7.78%.
Preparation of compounds 10a-c
A solution of (7a-c) (0.01 mol) and hydroxylamine hydrochloride in ethanol (30 ml) containing catalytic amount of sodium hydroxide was prepared. The mixture was refluxed and heated for 10-12 h and then filtered hot; the solvent was evaporated and the remaining boiled with petroleum ether (60–80). The residue was poured with ice water and the solid collected and crystalized from ethanol to give (10a-c).
3-((5-Phenyl-4, 5-dihydroisoxazol-3-yl) amino)-2H-thiochromen-2-one (10a)
(54% yield) as orange crystals. mp 166–168 °C; IR (KBr): ν (cm−1) 1660 (C = O), 3100–3400 (NH); 1H NMR (DMSO − d6): δ 3.41 (d, 2H, CH2), 5.96 (t, 1H, CH), 7.25–8.64 (m, 10H, aromatic protons), 12.25 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 30.1, 80.8, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.9, 106.6, 138.3, 128.5, 135.4, 118.3, 150.8, 166.0, 187.5: ms (m/z, %): 322.0 (M+, 26%). Anal. Calcd for: C18H14N2O2S (322.38): C, 67.06; H, 4.38; N, 8.69; S, 9.95%. Found: C, 67.07; H, 4.42; N, 8.74; S, 9.98%.
3-((5-(4-Chlorophenyl)-4,5-dihydroisoxazol-3-yl)amino)-2H-thiochromen-2-one (10b)
(54% yield) as orange crystals. mp 166–168 °C; IR (KBr): ν (cm−1) 1660 (C = O), 3100–3400 (NH); 1H NMR (DMSO − d6): δ 3.41(d, 2H, CH2), 5.96 (t, 1H, CH), 6.98–8.76 (m, 9H, aromatic protons), 12.25 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 30.1, 80.8, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.8, 106.6, 138.34, 128.55, 135.4, 118.3, 150.8, 166.0, 187.5: ms (m/z, %): 356.0 (M+, 16%). Anal. Calcd for: C18H13ClN2O2S (356.83): C, 60.59; H, 3.67; Cl, 9.94; N, 7.85; S, 8.99%. Found: C, 60.57; H, 3.60; Cl, 9.90; N, 7.80; S, 8.93%.
3-((5-(4-Hydroxyphenyl)-4, 5-dihydroisoxazol-3-yl)amino)-2H-thiochromen-2-one (10c)
(67% yield) as red crystals. mp 146–148 °C; IR (KBr): ν (cm−1) 1660 (C = O), 3100–3400 (NH,OH); 1H NMR (DMSO − d6): δ 3.41 (d, 2H, CH2), 5.96 (t, 1H, CH), 6.88–8.67 (m, 9H, aromatic protons), 10.23 (s, 1H, OH), 12.25 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 30.1, 80.8, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.9, 106.6, 138.3, 128.5, 135.4, 118.3, 150.8, 166.0, 187.5: ms (m/z, %): 338.0 (M+, 40%). Anal. Calcd for: C18H14N2O3S (338.38): C, 63.89; H, 4.17; N, 8.28; S, 9.48%. Found: C, 63.92; H, 4.21; N, 8.33; S, 9.53%.
4-((2-Oxo-2H-thiochromen-3-yl)amino)-6-phenyl-5,6-dihydropyrimidin-2(1H)-one (17a)
(50% yield) as deep brown crystals. mp 216–218 °C; IR (KBr): ν (cm−1) 1718, 1660 (2C = O), 3100–3400 (2NH); 1H NMR (DMSO − d6): δ 2.21 (d, 2H, CH2), 5.95 (t, 1H, CH),7. 3–8.1 (m, 10H, aromatic protons), 11.24 (s, 1H, NH), 12.25 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 40.2, 45.6, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.6, 106.6, 138.3, 128.5, 135.4, 118.3, 150.8, 156.9, 160.0, 187.5: ms (m/z, %): 349.0 (M+, 25%). Anal. Calcd for: C19H15N3O2S (349.41): C, 65.31; H, 4.33; N, 12.03; S, 9.18%. Found: C, 65.38; H, 4.39; N, 12.07; S, 9.22%.
6-(4-Chlorophenyl)-4-((2-oxo-2H-thiochromen-3-yl)amino)-5,6-dihydropyrimidin-2(1H)-one(17b)
(50% yield) as deep brown crystals. mp 216–218 °C; IR (KBr): ν (cm−1) 1718,1660 (2C = O), 3100–3400 (2NH); 1H NMR (DMSO − d6): δ 2.21(d, 2H, CH2), 5.95 (t, 1H, CH),7. 3–8.1 (m, 10H, aromatic protons), 11.24 (s, 1H, NH), 12.25 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 40.2, 45.8, 124.0, 125.6, 137.0, 128.6, 132.5, 137.4, 121.3, 138.8, 106.6, 138.3, 128.5, 135.4, 118.3, 150.8, 156.9, 160.0, 187.5: ms (m/z, %): 383.0 (M+, 20%). Anal. Calcd for: C19H14ClN3O2S (383.85): C, 59.45; H, 3.68; Cl, 9.24; N, 10.95; S, 8.35%. Found: C, 59.47; H, 3.72; Cl, 9.29; N, 10.99; S, 8.38%.
6-(4-Hydroxyphenyl)-4-((2-oxo-2H-thiochromen-3-yl)amino)-5,6-dihydropyrimidin-2(1H)-one (17c)
(50% yield) as deep brown crystals. mp 234–236 °C; IR (KBr): ν (cm−1) 1718, 1660 (2C = O), 3100–3400 (2NH, OH); 1H NMR (DMSO − d6): δ 2.21 (d, 2H, CH2), 5.95 (t, 1H, CH), 7.3–8.1 (m, 10H, aromatic protons), 10.22(s, 1H, OH), 11.24 (s, 1H, NH), 12.25 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 40.2, 45.8, 124.0, 125.6, 137.0, 128.6, 132.5, 137.4, 121.3, 138.7, 106.6, 138.3, 128.5, 135.4, 118.3, 150.8, 156.8, 160.0, 187.5: ms (m/z, %): 365.0 (M+, 28%). Anal. Calcd for: C19H15N3O3S (365.41): C, 62.45; H, 4.14; N, 11.50; S, 8.78%. Found: C, 62.46; H, 4.19; N, 11.56; S, 8.80%.
3-((6-Phenyl-2-thioxo-1,2,5,6-tetrahydropyrimidin-4-yl)amino)-2H-thiochromen-2-one(18a)
(68% yield) as yellowish crystals. mp 233–235 °C; IR (KBr): ν (cm−1) 1660 (2C = O), 3100–3400 (2NH); 1H NMR (DMSO − d6): δ 2.11(d,2H,CH2),4.23(t,1H,CH),7. 3–8.1 (m, 10H, aromatic protons), 11.24(s, 1H, NH), 12.25 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 40.2, 54.1, 124.0, 125.6, 137.0, 128.6, 130.5, 137.4, 121.3, 138.9, 106.6, 138.3, 128.5, 135.4, 118.3, 150.8, 156.8, 160.0, 187.5: ms (m/z, %): 365.0 (M+, 38%). Anal. Calcd for: C19H15N3OS2 (365.47): C, 62.44; H, 4.14; N, 11.50; O, 4.38; S, 17.55%. Found: C, 62.47; H, 4.15; N, 11.56; O, 4.42; S, 17.58%.
3-((6-(4-chlorophenyl)-2-thioxo-1,2,5,6-tetrahydropyrimidin-4-yl)amino)-2H-thiochromen-2-one(18b)
(50% yield) as deep brown crystals. mp 216–218 °C; IR (KBr): ν (cm−1) 1718, 1660 (2C = O), 3100–3400 (2NH); 1H NMR (DMSO − d6): δ 2.21 (d, 2H, CH2), 5.95 (t, 1H, CH), 7.3–8.1 (m, 10H, aromatic protons), 11.24 (s, 1H, NH), 12.25 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 40.2, 45.8, 124.0, 125.6, 137.0, 128.6, 132.5, 137.4, 121.3, 138.8, 106.6, 138.3, 128.5, 135.4, 118.3, 150.8, 156.9, 160.0, 187.5: ms (m/z, %): 383.0 (M+, 20%). Anal. Calcd for: C19H14ClN3O2S (383.85): C, 59.45; H, 3.68; Cl, 9.24; N, 10.95; S, 8.35%. Found: C, 59.47; H, 3.72; Cl, 9.29; N, 10.99; S, 8.38%.
3-((6-(4-Hydroxyphenyl)-2-thioxo-1,2,5,6-tetrahydropyrimidin-4-yl)amino)-2H-thiochromen-2-one (18c)
(60% yield) as Reddish brown crystals. mp 225–227 °C; IR (KBr): ν (cm−1) 1660 (C = O), 3100–3400 (2NH, OH); 1H NMR (DMSO − d6): δ 2.22 (d, 2H, CH2), 5.97 (t, 1H, CH), 7.3–8.1 (m, 10H, aromatic protons), 10.23 (s, 1H, OH), 11.24 (s, 1H, NH), 12.25 (br. s, 1H, NH); 13C NMR (DMSO − d6): δ 40.2, 45.8, 124.0, 125.6, 137.0, 128.6, 132.5, 137.4, 121.3, 138.9, 106.6, 138.3, 128.5, 135.4, 118.3, 150.8, 156.9, 160.0: ms (m/z, %): 383.0 (M+, 20%). Anal. Calcd for: C19H15N3O2S2 (381.47): C, 59.82; H, 3.96; N, 11.02; S, 16.81%. Found: C, 59.85; H, 3.98; N, 11.06; S, 16.84%.
Antimicrobial potential
Zone of inhibition technique is adapted for estimating antimicrobial potential for the target compounds against P. aeruginosa, B. subtilis, S. aureus and E. coli as examples of bacterial species besides, C. albicans and A. flavus as used fungal strains [31].
Docking study
In silico docking study had been carried out using molecular operating environment (MOE, version 2005.6, Canada). The isolated crystal structures of DHPS and protease enzyme active sites were obtained from protein data bank (4DAI). Chemical structures of the novel candidates were built by MOE builder and minimized by force field MMFF94x. Docking of the cocrystallized ligand was performed to get its root mean standard deviation (RMSD), energy score and interactions with the amino acids. Preparing of the target candidates for docking was done through their 3D structure built by MOE. Some procedures were done before docking including 3D protonation of the structure, running conformational analysis and choosing the conformer of least energy and adapting the same docking protocol used with the ligand. The outcomes were obtained from docking study such as binding score, hydrogen bond numbers, binding groups and distance from aminoacids (Table 2).
Conclusion
In conclusion, we constructed novel derivatives of thiochromenes mixed with other heterocycle such as pyrazole 6, 8a-c and 9a-c and oxazole 10a-c, pyrimidine 11a-c and/or thiopyrimidine 12a-c. All the thiochromene candidates were screened for their antibacterial potential towards B. subtilis, S. aureus, E. coli and P. aeruginosa and their antifungal activity against A. flavus and C. albicans. From the obtained results, thiochromene derivatives 8c (ZI = 24 mm), 9b (ZI = 23 mm) and 10b (ZI = 24 mm) exhibited antibacterial potential towards E. coli similar to that shown by ampicillin (ZI = 25 mm). Regarding the antimicrobial activity towards S. aureus, compound 8c (ZI = 19 mm) was the most active followed by 7b (ZI = 17 mm) and then thiochromenes 4, 7c, 11a and 11c (ZI = 14 mm). All the tested compounds showed antifungal activity against A. flavus (ZI = 9–16 mm) except compound 4, while the antifungal potential against C. albicans revealed that all compounds recorded moderate to high antifungal potential (ZI range = 8–19 mm) except thioxopyrazolothiochromene derivative 6 which did not exhibit any effect. In silico docking study had been performed within dihydropteroate synthase (DHPS) to predict the binding mode of the novel compounds. The outcomes of this study displayed the ability of these compounds to bind with DHPS; in particular, compound 8c recorded excellent fitting within the enzyme forming three hydrogen bonds with Asp101, Arg254 and Ser218 amino acids.
In silico docking study was carried out inside dihydropteroate synthase enzyme. Compound 8c recorded the best binding energy score (−5.47 kcal/mol) forming good fitting within DHPS active site.
References
S. Hernando-Amado, T.M. Coque, F. Baquero, J.L. Martínez, Defining and combating antibiotic resistance from one health and global health perspectives. Nat. Microbiol. 4, 1432–1442 (2019)
S. Sarma, S. Upadhyay, Current perspective on emergence, diagnosis and drug resistance in Candida auris. Infect. Drug Res. 10, 155 (2017)
R.P. Novick, R.C. Clowes, S.N. Cohen, R. Curtiss, N. Datta, S. Falkow, Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol. Rev. 40, 168 (1976)
S. Remy, S. Gabriel, B.W. Urban, D. Dietrich, T.N. Lehmann, C.E. Elger et al., A novel mechanism underlying drug resistance in chronic epilepsy. Ann. Neurol.: Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 53, 469–479 (2003)
A. Valsamatzi-Panagiotou, M. Traykovska, R. Penchovsky, Mechanisms of antibacterial drug resistance and approaches to overcome. Drug Discov Target. Drug-Resistant. Bact. 9–37 (2020)
C.W. Pemble, P.K. Mehta, S. Mehra, Z. Li, A. Nourse, R.E. Lee et al., Crystal structure of the 6-hydroxymethyl-7, 8-dihydropterin pyrophosphokinase• dihydropteroate synthase bifunctional enzyme from Francisella tularensis. PloS one 5, e14165 (2010)
S.H. Satuluri, S.K. Katari, C. Pasala, U. Amineni, Novel and potent inhibitors for dihydropteroate synthase of Helicobacter pylori. J. Recept. Signal Transduct. 40, 246–256 (2020)
M.S. Cheong, K.H. Seo, H. Chohra, Y.E. Yoon, H. Choe, V. Kantharaj et al., Influence of sulfonamide contamination derived from veterinary antibiotics on plant growth and development. Antibiotics 9, 456 (2020)
N. Hagner, M. Joerger, Cancer chemotherapy: targeting folic acid synthesis. Cancer Manage. Res. 2, 293 (2010)
J.G. LeBlanc, G.S. de Giori, E.J. Smid, J. Hugenholtz, F. Sesma, Folate production by lactic acid bacteria and other food-grade microorganisms. Commun. Current Res. Edu. Topics Trends Appl. Microbiol. 1, 329–339 (2007)
W.-b. Ho, L.A. Flippin, C. Mossman, E.D. Turtle, L.R. Wright. Thiochromene derivatives as HIF hydroxylase inhibitors. Google Patents; (2015)
P. Sokoloff, T. Imbert, L. Vergnes, F. Cuisiat. Novel chromene and thiochromene carboxamide derivatives, methods for preparing same and therapeutic applications of same. Google Patents; (2010)
A. Sipos, M. Tóth, F.K. Mueller, J. Lehmann, S. Berényi, Synthesis and dopamine receptor binding affinity of 4 H-thiochromenoapomorphines. Monatshefte für Chemie-Chem. Mon. 140, 473–478 (2009)
A.G. Alshammari, A.-R.B. El-Gazzar, H.N. Hafez, Efficient synthesis of a new class of N-Nucleosides of 4H-thiochromeno [2, 3-d] pyrimidine-10-Sulfone as potential anticancer and antibacterial agents. Int. J. Org Chem. 3, 15–27 (2013)
H. Kamano, I. Nasuno, H. Yamamoto, K. Koike. Cyclohexanedione derivatives and herbicides containing them. Google Patents; (1999)
R. Choubey, N. Choubey, G. Garg, Antimicrobial activity of newly synthesized pyrazolidine-3, 5-dione substituted Thiochromene derivatives. Res. J. Pharm. Technol. 8, 1250–1258 (2015)
D.-J. Wang, Z. Hou, H. Xu, R. An, X. Su, C. Guo, Design, synthesis, and biological evaluation of 4-chloro-2H-thiochromenes featuring nitrogen-containing side chains as potent antifungal agents. Bioorg. Med. Chem. Lett. 28, 3574–3578 (2018)
P.T. Kaye, M.A. Musa, A.T. Nchinda, X.W. Nocanda, Novel heterocyclic analogues of the HIV-1 protease inhibitor. Ritonavir. Synthet. Commun. 34, 2575–2589 (2004)
I.H. El Azab, H.S. El-Sheshtawy, R.B. Bakr, A.A. Elkanzi, New 1, 2, 3-triazole-containing hybrids as antitumor candidates: design, click reaction synthesis, DFT calculations, and molecular docking study. Molecules 26, 708 (2021)
N.A. Elkanzi, R.B. Bakr, Microwave assisted, antimicrobial activity and molecular modeling of some synthesized newly pyrimidine derivatives using 1 4-diazabicyclo [2.2. 2] octane as a catalyst. Lett. Drug Des. Discov. 17, 1538–1551 (2020)
N.A. Elkanzi, H. Hrichi, R.B. Bakr, O. Hendawy, M.M. Alruwaili, E.D. Alruwaili et al., Synthesis, in vitro evaluation and molecular docking of new pyrazole derivatives bearing 1, 5, 10, 10a-tetrahydrobenzo [g] quinoline-3-carbonitrile moiety as potent antibacterial agents. J. Iran. Chem. Soc. 18(4), 977 (2020)
R.B. Bakr, N.A. Elkanzi, Preparation of some novel thiazolidinones, imidazolinones, and azetidinone bearing pyridine and pyrimidine moieties with antimicrobial activity. J. Heterocycl. Chem. 57, 2977–2989 (2020)
M. Al-Sanea, D. Parambi, M. Shaker, H. Elsherif, H. Elshemy, R. Bakr et al., Design, synthesis, and in vitro cytotoxic activity of certain 2-[3-Phenyl-4-(pyrimidin-4-yl)-1 H-pyrazol1-yl] acetamide derivatives. Russ. J. Org. Chem. 56, 514–520 (2020)
H. Hrichi, E.N.A. Ahmed, B.R. Badawy, Novel β-lactams and thiazolidinone derivatives from 1, 4-dihydroquinoxaline schiff’s base: synthesis, antimicrobial activity and molecular docking studies. Chem. J. Moldova 15, 86–94 (2020)
M.M. Al-Sanea, A. Elkamhawy, S. Paik, K. Lee, A.M. El Kerdawy, B.S.N. Abbas et al., Sulfonamide-based 4-anilinoquinoline derivatives as novel dual Aurora kinase (AURKA/B) inhibitors: Synthesis, biological evaluation and in silico insights. Bioorg. Med. Chem. 28, 115525 (2020)
R.B. Bakr, A. Mehany, (3, 5-Dimethylpyrazol-1-yl)-[4-(1-phenyl-1H-pyrazolo [3, 4-d] pyrimidin-4-ylamino) phenyl] methanone. Molbank 2016, M915 (2016)
R.B. Bakr, A.A. Ghoneim, A.A. Azouz, Selective cyclooxygenase inhibition and ulcerogenic liability of some newly prepared anti-inflammatory agents having thiazolo [4, 5-d] pyrimidine scaffold. Bioorg. Chem. 88, 102964 (2019)
K.R. Abdellatif, R.B. Bakr, Pyrimidine and fused pyrimidine derivatives as promising protein kinase inhibitors for cancer treatment. Med. Chem. Res. 30, 31 (2020)
M.A. Abdelgawad, A. Musa, A.H. Almalki, S.I. Alzarea, E.M. Mostafa, M.M. Hegazy et al., Novel phenolic compounds as potential dual EGFR and COX-2 inhibitors: design, semisynthesis in vitro biological evaluation and in silico insights. Drug Des. Develop. Therapy 15, 2325 (2021)
I.H. El Azab, R.B. Bakr, N.A. Elkanzi, Facile one-pot multicomponent synthesis of pyrazolo-thiazole substituted pyridines with potential anti-proliferative activity: synthesis, in vitro and in silico studies. Molecules 26, 3103 (2021)
S.A. Komykhov, K.S. Ostras, A.R. Kostanyan, S.M. Desenko, V.D. Orlov, H. Meier, The reaction of amino-imidazoles,-pyrazoles and-triazoles with α, β-unsaturated nitriles. J. Heterocycl. Chem. 42, 1111–1116 (2005)
Acknowledgements
I.H.E. thanks Taif University Researchers Supporting Project number (TURSP-2020/27), Taif University, Taif, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakr, R.B., Azab, I.H.E. & Elkanzi, N.A.A. Thiochromene candidates: design, synthesis, antimicrobial potential and in silico docking study. J IRAN CHEM SOC 19, 1413–1423 (2022). https://doi.org/10.1007/s13738-021-02391-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02391-w