Abstract
The present study reports the green synthesis of starch–maleate (SM) at ambient temperature in solvent-free system using Rhizopus arrhizus lipase as a biocatalyst and maleic acid (MA) as an esterification agent. The synthetic scheme was found to be efficient, economical, and ecofriendly. The newly synthesized SM samples were characterized using Fourier transform infrared (FTIR) and proton nuclear magnetic resonance (1H NMR) spectroscopic techniques. The degree of substitution (DS) was found in the range of 0.53–0.62. Moreover, DS was found to be temperature and time-dependent. X-ray diffraction (XRD) exhibited that maleation did not change the crystalline nature of native starch. Scanning electron microscopy (SEM) revealed that size of SM granules was in the range of 4–18 µm. The activation energy (Ea) of SM formation was calculated to be 42.94 kcal mol−1 which clearly indicated the effective and rapid interaction of functional groups. Hence, the solvent-free solid-state synthetic methodology proved to be excellent for the synthesis of novel biomaterials with appreciable high DS for drug delivery and sorption of heavy metal ions from water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Starch is a biopolymer with a very complex structure, formed by glycosidic linkages between glucose units. It is widely used in paper industry as coating and sizing of paper, cosmetics, agriculture, delayed drug release, disposable diapers, and engineering [1,2,3]. Starch is a mixture of amylose and amylopectin. Amylose is a linear combination of d-glucose units linked by α-1 → 4 linkages, while amylopectin also contained branching of α-d-(1 → 6) linkages after every 24–30 glucose units [4,5,6].
Owing to disadvantages of natural unmodified starch such as low viscosity, shelf life, solubility and thickening power, it can be modified through physical, chemical, and biotechnological methods to acquire useful derivatives with requisite characteristics [7,8,9]. Modified starch can stabilize starch granules during processing which makes it more suitable for industrial applications. The chemical modification of starch can also enhance molecular stability against mechanical shearing, acidic, and high-temperature hydrolysis [10, 11].
A number of researchers have modified starch to investigate the potential of its derivatives for controlled/sustained release of various drugs [12, 13]. The biocompatible and biodegradable nature of these starch derivatives makes them promising candidates for enhanced bioavailability of therapeutic agents in biological fluids. Abundance of free hydroxyl groups present on starch chains offer modification/tailoring for desired applications [14].
Most common chemical modifications include oxidation, esterification, and etherification of hydroxyl groups on starch chains [15, 16]. Starch is esterified to convert hydrophilic hydroxyl groups into more hydrophobic ester groups. The hydrophobicity of thermoplastic-esterified starch depends upon the nature of ester group and the degree of substitution (DS) [17, 18]. Esterification takes place under the influence of organic and inorganic acids and their derivatives, and enzymes like lipases [19, 20].
In contrast to the various types of modifications, some researchers have focused on different starch sources as the antecedents for derivatives suitable for pharmaceutical applications. Colon-targeted and gastrointestinal tract drug delivery carriers were synthesized and investigated using high amylose corn starch (CS) [13, 21]. Similarly, transmucosal delivery devices were fabricated using high amylose Hylon VII starch [22].
Starch is normally dissolved in aprotic solvents like dimethylacetamide (DMA), dimethylformamide (DMF), and dimethylsulfoxide (DMSO) for modification [23]. These solvents were difficult to be removed from the end products and hence rendered them harmful for biomedical applications. Nonetheless, starch esterification with a reasonable DS had been reported in aqueous media using sodium hydroxide as catalyst instead of N,N-dimethylaminopyridine (DMAP) and methane sulphonic acid [15, 16, 23,24,25]. Starch esterification in alkaline medium has been carried out mostly to obtain hydrophobic end groups such as acetylated starch [26, 27]. Starch monoesters with hydrophilic end groups have been derived from dicarboxylic acids such as maleic acid, fumaric acid, and succinic acid [28]. Starch maleate monoesters with DS up to 0.25 have been synthesized using microwave-assisted heating in DMSO and pyridine [23]. A little is known about the use of biocatalysts (enzymes) for the synthesis of starch monoesters in a solvent-free method.
Keeping in view the importance of SM, we synthesized SM by a simple, inexpensive, and biocompatible method from CS and maleic acid (MA) using Rhizopus arrhizus lipase (RAL) as biocatalyst under micro-aqueous conditions at ambient temperature. We were also aimed to characterize SM using Fourier transform infrared (FTIR), 1H NMR, and X-ray diffraction (XRD) spectroscopies. The physico-chemical characteristics and properties like DS, moisture absorbency, particle size, and surface morphology of native and maleated starch were also investigated.
Materials and methods
Materials
Corn starch (Sigma-Aldrich; CAS 9005-25-8) was dried under vacuum at 45 °C for 4 h to remove moisture. RAL (CAS 9001-62-1), Maleic acid (MA; CAS 110-16-7), sodium hydroxide (NaOH; CAS 1310-73-2), acetone (CAS 67-64-1), ethanol (CAS 64-17-5), and other reagents (analytical grade) were also obtained from Sigma-Aldrich (USA). All chemicals were used without any further purification. Ultrapure water (Millipore; 18.2 MΩ) was used where needed.
Methods
Fourier transform infrared (FTIR) spectroscopy
FTIR spectra of native and modified starch samples were acquired on IR Prestige-21 instrument (Shimadzu, Japan) using KBr pellet technique. The spectra of dried pellets were recorded with 4 cm−1 resolution and 16 scans in the range 4000–400 cm−1.
Proton nuclear magnetic resonance (1H NMR) spectroscopy
1H NMR spectra were acquired on a Bruker Avance II 400 MHz instrument (Germany). Samples were prepared in DMSO-d6 using TMS as an internal standard.
Powder X-ray diffractometry
The X-ray diffractograms of the powder samples were obtained using PANalytical X’Pert Pro X-ray diffractometer (The Netherlands) at gonio scan axis with Cu as anode material. Generator settings were 40 Ma and 40 kV at 25 °C. Scan type was continuous and scan size was 0.02 2θ with scan step time 0.15 s. Scanning range was 3°–60° 2θ with Kα 1.5 Å and Kβ 1.3 Å.
Scanning electron microscopy (SEM)
Scanning electron micrographs were taken using Philips/FEI QUANTA 200 SEM (USA) with a secondary electron beam and accelerating voltage of 200 V–30 kV. All samples taken on stubs were coated with Au–Pd alloy in a Cressington 208 HR Sputter Coater to reduce charging effect during SEM imaging. Micrographs of various parts were captured at different magnifications.
Synthesis of SM monoesters
Esterification of starch was carried out with MA and RAL (2370 I U g−1) in a solvent-free system. In a typical synthesis of SM, pre-dried corn starch (8.1 g, 0.05 mol) was mixed with finely ground MA (5.8 g, 0.05 mol) and triturated evenly in mortar for 10 min. The mixture was incubated at 35 °C for half an hour followed by the addition of RAL (1.0 g) and water (0.1 mL). The mixture was triturated again at regular intervals and at 35 °C in a time span of 5 h. The reaction mixture was washed with chilled water to remove any unreacted MA and RAL. The newly synthesized starch–maleate was further washed with acetone and dried in a vacuum oven at 50 °C.
Free fatty acid titration
Samples (0.2 g each time) were drawn at regular intervals from the reaction mixture, extracted in water, and then titrated for residual fatty acids with 0.1 M ethanolic KOH using thymolphthalein as an indicator. Similarly, blank samples (with inactivated enzyme) were titrated for the comparison. The initial reaction rate (acylation activity) of the enzyme was calculated from the slope of the progress curve of the acid consumption [29].
Determination of DS
SM (1.0 g) was mixed with aqueous ethanol (10 mL, 75%) and NaOH (10 mL, 0.5 M), and stirred for 30 min at 30 °C in stoppered conical flask. The excess NaOH was titrated with standard HCl solution (0.5 M) using phenolphthalein indicator to the colorimetric end point. A blank titration was also carried out using native starch. The DS was calculated using Eqs. (1 and 2):
where nMA is the number of moles of maleic acid attached to starch, VNaOH is the volume (mL), MNaOH is the molarity of free NaOH, VHCl is the volume (mL), and MHCl is the molarity of HCl consumed by excess NaOH:
where mstarch-mal is the mass of modified starch and nMA is the number of moles of maleic acid attached to starch.
Moisture absorbency
The moisture absorbing capacity was assessed at room temperature by taking weighed amount of dried SM sample and equilibrating it in a relative humidity of 100% for 5 days. The moisture absorbance of the SM sample was calculated according to Eq. (3):
where Wf and W0 are the moist and dry weights of the samples, respectively.
Effect of temperature on acylation and reaction kinetics
Temperature not only affects the rate of acylation but also affects the activity of the enzyme [30]. Effect of temperature on the initial rates of enzymatic acylation was investigated between 20 and 40 °C. Specific rate constants at different temperatures were calculated from reaction rates using Eq. (4):
The activity of reactants in solid phase is taken unity, so values of rate constants were taken equal to the initial rates. The natural logs of specific rate constants at different temperatures (ln k) were plotted against 1/T. Apparent activation energy is calculated from the Arrhenius plot obtained using Eq. (5):
Results and discussion
Synthesis of SM
SM was prepared in a solvent-free system by reacting starch with maleic acid in the presence of RAL as biocatalyst. Reaction yield was monitored by varying mole ratios of MA. Figure 1 displays the synthetic scheme of SM. The reaction conditions and results of synthesis are summarized in Table 1.
FTIR spectroscopy
Structural characterization of unmodified CS and SM was executed using FTIR spectroscopy. Figure 2 shows the overlay spectra of both unmodified CS and SM. Basic composition unit of unmodified CS is anhydroglucose unit with characteristic groups of C2- and C3-linked secondary hydroxyl and C6-linked primary hydroxyl groups. The IR peak 3269 cm−1 in spectrum of unmodified CS corresponds to O–H stretching. C–H stretching was observed at 2932 cm−1, whereas the peak at 1640 cm−1 was presumably a feature of tightly bound water [31]. Other significant peaks were C–O–C asymmetrical stretching at 1152 cm−1, C–O stretching at 1078 cm−1, and glycosidic bond vibration found at 929 cm−1.
After modification, there appeared a characteristic C=O stretching at 1705 cm−1 confirming the successful synthesis of SM as an ester conjugate. Moreover, C=O vibration became more intense with higher value of DS. A C=C stretching of maleate moiety in spectrum of SM was observed overlapping with peak of bound water at 1638 cm−1. It could also be confirmed by C–H stretch of sp2 hybrid carbon at 3068 cm−1. These FTIR data verified the successful esterification of starch by MA as reported in the literature [32].
1H NMR spectroscopy
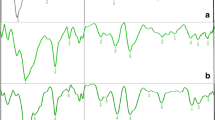
Structure of SM was also confirmed by 1H NMR spectroscopy and the acquired spectrum is given in Fig. 3. Proton signals of sp2 hybrid carbons of maleate moieties were observed at δ 6.22 and 6.27 ppm (H7 and H8, respectively). Both protons appear as single signal in the spectrum of maleic acid but here split into two signals due to different neighboring environment after esterification with starch, thus also confirming the successful synthesis of SM. The characteristic AGU protons of CS appeared at δ 3.02–4.01 ppm (H2–H7), whereas proton signal of anomeric carbon (H1) appeared at δ 5.07 ppm.
Powder X-ray diffractometry
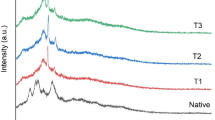
Both unmodified and modified starch samples were analyzed using powder X-ray diffractometry. Figure 4 displays the X-ray diffractograms of unmodified CS and SM. X-ray diffraction peaks of unmodified starch were found around 10, 11, 15, 17, 18, 20, 23, and 30 of 2θ indicating A type crystallization (diameter of granules is greater than 9.9 µm). Esterification occurred at amorphous areas of starch, so the crystallization type of native starch did not change after esterification with MA. Intensity of diffraction peaks of esterified starch was weaker than that of unmodified CS. It was reported that degree of crystallinity of innate starch was 27% as compared to the esterified starch (24%), indicating that esterification could disrupt crystalline structure to some extent [33]. This decrease in crystallinity may be attributed to the weakening of hydrogen bonding and increase thermoplasticity of SM. The structure of starch is hypocrystalline.
SEM analysis
Granules of unmodified CS appeared in the size regime of 4–18 µm. Figure 5a shows angular-to-polyhedral shape granules of unmodified CS, while maleation did not change the granular structure; however, the surface became rougher with esterification (see Fig. 5b).
Effect of temperature and time on DS
Reaction of MA with CS was investigated in the presence of RAL at different temperatures. It was explored that DS of SM 1 was 0.06 and 0.53 after 5 h at 25 and 35 °C, respectively. Same trend was observed for all other SM conjugates (2–5). It means that DS increases with increase in temperature up to 35 °C (Fig. 6a). Similar effect of temperature on enzymatic esterification has already been reported [34].
DS of SM was greatly affected by reaction time, as well. Figure 6b shows the effect of time on DS of SM 1. It can be observed that with increase in reaction time, DS increased and reached value of 0.53 after 5 h (see Table 1). Moreover, it was also found to be directly proportional to the MA consumption.
The initial rates of MA consumption at different temperatures were determined from values of residual acid in the reaction mixture (Table 2). It was observed that with increase in temperature, both consumption of MA and rate of reaction increased.
Furthermore, the plot between ln k and 1/T as described by Arrhenius equation was used to calculate activation energy of the lipase-mediated esterification of starch which was found to be 42.94 kcal. mol−1.
Moisture absorbency of SM
Moisture absorbency of starch changes by modification of its hydroxyl groups into hydrophilic carboxylic groups. The effect of carboxylate groups on water absorption was not significant in SM samples with low DS. However, water absorbency of SM samples was found to increase with higher DS up to a certain DS value and then started to decrease with higher DS. This trend could be attributed to the increasing hydrophobic unsaturated double bonds in MA moieties in SM samples with high DS values. Moreover, it was also observed that rates of water absorption by SM samples were faster initially and slowed down with passage of time.
Conclusions
Starch–maleate was successfully synthesized using RAL as biocatalyst. The reaction scheme being solvent-free and operating at ambient temperature proved efficient and provided good yield. It was observed that esterification with maleic acid did not affect the crystalline structure of starch. However, maleation made the surface of starch granules rough. Moreover, the DS of SM was found to increase with time and temperature (optimum for enzyme activity). The water absorbency and hydrophilicity of SM samples increased with increasing DS up to a certain level and then started decreasing due to increasing hydrophobicity exhibited by unsaturation present in MA moieties. Hence, this RAL-catalyzed synthetic scheme can be employed in modification of biocompatible and biodegradable polymers, and their potential can further be exploited in drug delivery and as composites.
References
M.R. Almeida, R.S. Alves, L.B. Nascimbem, R. Stephani, R.J. Poppi, L.F.C. de Oliveira, Anal. Bioanal. Chem. 397, 2693–2701 (2010)
H. Liu, F. Xie, L. Yu, L. Chen, L. Li, Prog. Polym. Sci. 34, 1348–1368 (2009)
G.E. Luckachan, C. Pillai, J. Polym. Environ. 19, 637–676 (2011)
H. Liu, L. Yu, F. Xie, L. Chen, Carbohydr. Polym. 65, 357–363 (2006)
J. Stagner, V.D. Alves, R. Narayan, A. Beleia, J. Polym. Environ. 19, 589 (2011)
R. Karmakar, D. Ban, U. Ghosh, Int. Food Res. J. 21, (2014)
J.I. Moran, V.P. Cyras, A. Vazquez, J. Polym. Environ. 21, 395–404 (2013)
D. Wei, H. Wang, H. Xiao, A. Zheng, Y. Yang, Carbohydr. Polym. 123, 275–282 (2015)
D. Wei, H. Wang, Z. Ziaee, F. Chibante, A. Zheg, H. Xiao, Mater. Sci. Eng. C. 58, 986–991 (2016)
T. Tsuruta, T. Koyama, M. Yasutake, K. Hatano, K. Matsuoka, Carbohydr. Res. 393, 15–22 (2014)
V.P. Cyras, L.B. Manfredi, M.-T. Ton-That, A. Vázquez, Carbohydr. Polym. 73, 55–63 (2008)
J. Alias, I. Silva, I. Goni, M. Gurruchaga, Carbohydr. Polym. 74, 31–40 (2008)
L. Chen, X. Li, L. Li, S. Guo, Curr. Appl. Phys. 7, e90-e93 (2007)
R. Sun, X. Sun, Carbohydr. Polym. 47, 323–330 (2002)
H. Chi, K. Xu, X. Wu, Q. Chen, D. Xue, C. Song, W. Zhang, P. Wang, Food Chem. 106, 923–928 (2008)
J. Fang, P. Fowler, C. Sayers, P. Williams, Carbohydr. Polym. 55, 283–289 (2004)
A. Alissandratos, N. Baudendistel, S.L. Flitsch, B. Hauer, P.J. Halling, BMC Biotechnol. 10, 82 (2010)
B. Kaur, F. Ariffin, R. Bhat, A.A. Karim, Food Hydrocol. 26, 398–404 (2012)
L. Li, F. Ji, J. Wang, B. Jiang, Y. Li, Y. Bao, Carbohydr. Res. 416, 51–58 (2015)
A. Rajan, J. Sudha, T.E. Abraham, Ind. Crops Prod. 27, 50–59 (2008)
K.G. Desai, J. Biomater. Appl. 21, 217–233 (2007)
J. Mulhbacher, P. Ispas-Szabo, M. Ouellet, S. Alex, M.A. Mateescu, Int. J. Biol. Macromol. 40, 9–14 (2006)
A. Biswas, R. Shogren, S. Kim, J. Willett, Carbohydr. Polym. 64, 484–487 (2006)
Y. Xu, V. Miladinov, M.A. Hanna, Cereal Chem. 81, 735–740 (2004)
Y.J. Wang, L. Wang, Starch-Stärke. 54, 25–30 (2002)
A. Gunaratne, H. Corke, Food chem. 105, 917–925 (2007)
D.S. Roesser, S.P. McCarthy, R.A. Gross, D.L. Kaplan, Macromolecules. 29, 1–9 (1996)
T. Yoshimura, R. Yoshimura, C. Seki, R. Fujioka, Carbohydr. Polym. 64, 345–349 (2006)
M.N. Tahir, A. Adnan, P. Mischnick, Process Biochem. 44, 1276–1283 (2009)
C.G. Lopresto, V. Calabrò, J.M. Woodley, P. Tufvesson, J. Mol. Catalysis B: Enzymatic. 110, 64–71 (2014)
M. Kačuráková, R. Wilson, Carbohydr. Polym. 44, 291–303 (2001)
Q. Sun, H. Fan, L. Xiong, Carbohydr. Polym. 106, 359–364 (2014)
M. Pervaiz, P. Oakley, M. Sain, Int. J. Compos. Mater. 4, 204–212 (2014)
A. Gumel, M. Annuar, T. Heidelberg, Y. Chisti, Process Biochem. 46, 2079–2090 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gill, A.N., Iftikhar, A., Rashid, A. et al. Lipase-catalyzed green synthesis of starch–maleate monoesters and its characterization. J IRAN CHEM SOC 15, 1939–1945 (2018). https://doi.org/10.1007/s13738-018-1391-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1391-2