Abstract
In this work, TiO2 and doped TiO2 photocatalysts (Fe/TiO2 and Cu/TiO2) were synthesized by the sol–gel method. The main objective of this study was to investigate the influence of dopants on the structure, morphology, and activity of the catalysts in powder and immobilized states. XRF, XRD, and SEM methods were used to characterize the catalysts. The structure and phase distribution of the nanocrystalline powders were identified by XRD. Nanoparticles crystallite size and the degree of crystallinity were affected by doping. The anatase contents of catalysts were achieved as follows: TiO2 (5.89 %) < Fe/TiO2 (42.17 %) < Cu/TiO2 (70.28 %). It was indicated that the activity of the catalysts strongly depends on the anatase content. Under the same circumstances, copper-modified TiO2 exhibited a twofold higher photocatalytic activity compared with TiO2. The nanostructured catalysts were immobilized on light expanded clay aggregate (LECA) granules in order to investigate the effect of a novel support on the activity of the catalysts. Morphological changes are recognizable in the SEM images. Activity tests indicated that the best catalytic performance was assigned to Cu/TiO2/LECA. After 120 min of irradiation, 61 % degradation of phenol in synthetic wastewater was achieved. The high photocatalytic activity of Cu/TiO2/LECA confirms that LECA is as an excellent support.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenol is considered as one of the most important organic contaminants; its major sources in aquatic environment are industrial wastewaters including paint, pesticides, coal conversion, polymeric resin, petroleum, and petrochemical industries. Phenol is reputed for its bioresistance and acute toxicity. Among numerous methods that have been recommended for the degradation of phenolic compounds, photocatalytic treatment is the most effective and economical technology [1–3]. Titanium dioxide (TiO2) has attracted considerable attention mainly because of its high photocatalytic activity, environmental-friendly nature, and the relatively low cost [4]. Several modification methods have been applied to enhance the photocatalytic activity of TiO2. The deposition of nanosized metal particles on TiO2 has proven to be beneficial for improving the efficiency of photocatalytic reactions involved in the degradation of organics. Since these nanoparticles act as a sink of electrons, they inhibit the recombination of electron and hole pairs [5, 6]. Lorret et al. [6] reported that the addition of tungsten drastically improved the photocatalytic activity of TiO2, where the activity was measured via degradation of methylene blue (MB) in aqueous solution. Yean Ling Pang and Ahmad Zuhairi Abdullah prepared Fe-doped titanium dioxide nanotubes using sol–gel followed by hydrothermal method; the activity was evaluated based on the oxidation of rhodamine B under ultrasonic irradiation [7]. Huang et al. [8] concluded that CuO/TiO2 is an active and stable catalyst for CO oxidation. In order to have sufficient separation of the catalyst from aqueous solution and to reuse it, the application of nanosized catalyst, which is suspended in solution, is preferred to be limited. In fact, the application of an appropriate support for catalyst immobilization is of high importance. Various materials have been tested as the substrate for catalysts such as glass, silica, pumice granules, perlite stone, and stainless steel [3, 4]. Haileyesus Tedla et al. applied synthetic zeolite as a support for ZnO/Fe2O3/MnO2 composite. They reported high efficiency for the degradation of methylene blue (MB) and real sewage sample [9]. Human society has been using clays and clay minerals since the Stone Age, but recently in line with the rapid advances of nanotechnology, the attention toward clays is focused upon their uses in adsorption, catalysis, and biology [10]. Vaccari [11] stated in 1999 that the application of clays in environmental catalysis would have a promising future. Hassan et al. [12] evaluated the catalytic performance of Ni (II) and Cu (II) phthalocyanines, immobilized on bentonite clay surface. Sharifnia et al. studied the photocatalytic degradation of ammonia using LECA granules as a substrate for their catalyst. The chemical composition of LECA by XRF analysis disclosed that SiO2 is the major mineral and Al2O3 and Fe2O3 are present as minor minerals. Meanwhile, some trace minerals were also detected in this natural material [13]. Zarrabi et al. have used LECA for defluoridation of water and reported some of the properties of lightweight expanded clay aggregate (LECA) such as low density, high porosity, and thermal resistance (up to 1000 °C). The porous structure of LECA along with its low density facilitates its floatability [14].
In this research study, TiO2, Fe/TiO2, and Cu/TiO2 photocatalysts were synthesized by sol–gel method. The structure and morphology of the catalysts were scrutinized by XRF, XRD, and SEM techniques. Moreover, the activity of the catalysts was compared in suspended and immobilized modes. Light expanded clay aggregate (LECA) was applied as a support to immobilize TiO2, Fe/TiO2, and Cu/TiO2 photocatalysts.
Materials and methods
Materials
To prepare Cu/TiO2 and Fe/TiO2 photocatalysts, titanium isopropoxide (98 %), Cu (NO3)2·3H2O (99 %), Fe (NO3)3·9H2O (98 %) were used as the precursors for the titanium, copper, and iron, respectively. LECA is a special type of clay, which has been pelleted and fired in a rotary kiln at a very high temperature (with grain size of 4–10 mm). LECA is considered a silicate compound. It was provided by Azarbayjani Company, Kermanshah, Iran. It was crashed and washed with tap water in order to remove the dust. Subsequently, it was heated at 100 °C, until the weight became constant. Moreover, ethanol (96 %) and nitric acid (65 %) were used for the synthesis of the catalysts and H2O2 (30 wt%) and phenol (99 %) were used in the phenol photodegradation experiments. The chemicals were purchased from Merck Company and used without further purifications. Distilled water was used through the experiments.
Methods
Synthesis of Cu/TiO2 and Fe/TiO2 photocatalysts
Step 1: Based on sol–gel method, titanium isopropoxide (TIP) was added to double-distilled water with the molar ratio of 1:100 (TIP to water). The mixture has been stirring and heating up to 85 °C. Step 2: At that temperature, a specified amount of nitric acid was added so that the molar ratio of TIP to nitric acid was 1:0.07. Meanwhile, a solution of 2 wt% of Cu, which was prepared by dissolving Cu (NO3)2·3H2O in ethanol, was added. In case of Fe/TiO2 photocatalyst, a solution of 2 wt% of Fe, which was obtained by dissolving Fe (NO3)3 9H2O in ethanol, was used. Step 3: The mixture was kept under reflux at 85 °C for 24 h. Step 4: The obtained gel was dried at 100 °C. Step 5: The dried gel calcination was conducted at 600 °C for 2 h. The experimental data on the choice of calcination temperature are given in [15]. Finally, the powder was crushed and sieved into 60–90 μm.
Immobilization
LECA was applied as the support for TiO2, Fe/TiO2, and Cu/TiO2 photocatalysts. The procedure of Cu/TiO2/LECA preparation is presented in Fig. 1. The first three steps of Cu/TiO2/LECA preparation method, which consist of the preparation of a homogenous mixture from the precursors, the hydrolysis of Ti alkoxide, and the formation of sol, were the same as the synthesis method of Cu/TiO2 photocatalyst. Afterward, LECA granules were added to the obtained sol. Step 4: the LECA granules, which had adsorbed sufficient sol, were filtered and dried at 100 °C. Step 5: Finally, the calcination process was carried out at 600 °C for 2 h. Hereby, TiO2, Fe/TiO2, and Cu/TiO2 were immobilized and coated on LECA.
Characterization
Copper and iron content of the catalysts were measured by X-ray fluorescence (XRF) spectrometer (Spectro X-Lab Pro). TiO2 and modified TiO2 powders were characterized by X-ray diffraction (XRD) using X’Pert MPD diffractometer with Cu Kα radiation at 40 kV and 40 mA. The XRD patterns were collected from 5° to 80° in 2θ at a scan rate of 0.2°/s. The morphology of the catalysts was investigated by field emission scanning electron microscopy of FESEM of the TSCAN Company on the acceleration voltage of 15.0 kV. The porosity of the catalysts was investigated using Micrometrics ASAP 2010. Before measuring nitrogen adsorption, the catalyst was degassed at 300 °C for 6 h.
Photocatalytic experiments
The catalyst was added to a phenol solution (200 ppm), which was selected as the synthetic wastewater. Hydrogen peroxide (H2O2: 12.5 ml, 30 wt%) was added to the solution as an oxidizing agent. The amounts of catalyst loading, initial phenol concentration, and H2O2 dosage were investigated and optimized in one of our previous works [16]. From our optimization experiments for Cu/TiO2 [17], it can be concluded that the optimum Cu/TiO2 dosage is 0.5 g/l. In addition, according to another work of us [16], the optimum dosage for Fe/TiO2 dosage is 0.5 g/l. Accordingly, based on the results obtained by the two papers, the dosages of Cu/TiO2 and Fe/TiO2 in suspended photocatalytic experiments were determined to be 0.5 g/l.
To be able to have a rational judgment on the competitive performance of the catalysts in suspended and immobilized states, we decide to adjust the dosages of Cu/TiO2/LECA and Fe/TiO2/LECA catalysts so as to obtain 0.5 g/l dosage for Cu/TiO2 in Cu/TiO2/LECA (likewise for Fe/TiO2 in Fe/TiO2/LECA). It means that LECA granules were weighed before absorbing sol and after the calcination process. The difference between these two values reveals the Cu/TiO2 amount, which is immobilized on LECA (likewise for Fe/TiO2 in Fe/TiO2/LECA). Since in each experiment 200 ml of phenol solution is used, 0.1 g of catalyst is applied to attain 0.5 g/l dosage. To immobilize 0.1 g of Fe/TiO2 on LECA 3.96 g of the substrate was needed, while for Cu/TiO2 the required amount of LECA is lower (3.58). This shows superior immobilization of Cu/TiO2 on LECA.
A magnetic stirrer was used to provide uniform conditions in the reacting solution. The illumination is conducted by a UV lamp (with light intensity of 757.38 mW/cm2), which was fixed 19.5 cm over the batch photoreactor of cylindrical shape (Pyrex vessel capacity: 2L). The setup was in a chamber, and it was shielded by aluminum foil during the reaction time to prevent the interference from outside light.
Analytical methods
All the experiments were carried out at ambient temperature. After 2 h of reaction, the mixture was centrifuged and the absorbance of the supernatant was measured at 270 nm using a Specord 210 UV spectrometer. The experiments should be repeated for the blank. All the conditions in the blank were the same as the sample, but the blank had no catalyst. The activity of photocatalysts was evaluated by determining the phenol degradation percentage. The concentration of phenol in the solution was measured using the Beer–Lambert law, and the degradation of phenol was calculated using the following equation [16]:
where A 0 and A are absorbance of blank and sample, respectively.
Mechanism
In the photocatalytic degradation process, an organic pollutant is destroyed in the presence of an oxidizing agent and a semiconductor, which is irradiated by an energetic light source. As the result of sufficient illumination with UV lamp, an electron from the valence band migrates to the conduction band leaving behind an electron vacancy (hole) in the valence band. The generated hole in the valence band can produce hydroxyl radicals, and subsequently, OH radicals oxidize the organic material. The photogenerated e− and h+ are vigorous oxidizing and reducing agents, and their reactions are, respectively, as follows:
Equation (2) expresses the hydroxyl radicals generation by the oxidation step, in which positive holes react with absorbed water. The reduction step leads to the hydroxyl radical generation through direct and indirect routes; the direct way, which is stated in Eq. (3), is the reaction of electrons with H2O2. The indirect way, which is summarized in Eq. (4), composes of two steps: First, e − is transferred to the adsorbed oxygen of the modified catalyst (Cu/TiO2, Fe/TiO2), which produces superoxide anions and then O2 − reacts with H2O2 resulting in ·OH generation. When reduction and oxidation do not advance simultaneously, there would be an electron accumulation in the conduction band. Thereby, this can increase the rate of recombination of e − and h +. Since the recombination causes energy waste, it should be prevented to ensure efficient photocatalysis. The role of dopant in photocatalysis is in keeping the charges separate through trapping the electrons of TiO2 conduction band and consequently avoiding the recombination of electrons and holes [2, 18]. Cu2+ and Fe3+, which are absorbed on the TiO2 surface, can act as electron trapping centers. Thus, they increase the electron–hole pair separation efficiency.
Results and discussion
XRF
Chemical composition of the doped catalysts, which was evaluated by XRF analysis, is indicated in Table 1. Given that the method of synthesis, all the materials used and their quantities, is the same for the two catalysts (Cu/TiO2 and Fe/TiO2), only the type of dopant is different (a solution of 2 wt% of Cu was used in step 2 of Cu/TiO2 synthesis, and likewise a solution of 2 wt% of Fe for Fe/TiO2 synthesis). Under the current circumstance, chemical composition of the doped catalysts indicates that the ratio of CuO/TiO2 is 0.24 % and the ratio of F2O3/TiO2 is 0.2 %. Higher CuO loading in Cu/TiO2 suggests better embedment of the dopant in the TiO2 lattice, and accordingly, it possesses more active sites.
XRD
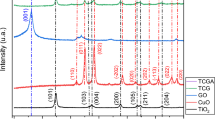
The X-ray powder diffraction was used to determine the crystalline phases present in the samples. XRD patterns of pure TiO2, Fe/TiO2, and Cu/TiO2 are shown in Fig. 2. In the XRD pattern of pure titanium dioxide, the peak at 29.5090° is attributed to the anatase phase (JCPDS 21-1272), and the peaks at 32.0095°, 42.1696°, and 64.0611° are attributed to rutile (JCPDS 21-1276). These results imply that both rutile and anatase phases exist in pure TiO2. In the XRD pattern of Cu/TiO2, in addition to the peaks, corresponding to rutile and anatase phases (JCPDS 21-1272 and 21-1276), there is a peak at 63.52°, which corresponds to CuO (JCPDS 48-1548). Additionally, in the pattern of Fe/TiO2, along with the peaks belonging to rutile and anatase phases, two peaks at 56.51° and 65.02° are recognized, which correspond to Fe2O3 (JCPDS file no. 39-1346). Phase distribution of samples is estimated by Spurr–Myers correlation where the weight percent of anatase is given by:
where “X” denotes the weight fraction, “I” denotes the intensity of the strongest reflection, and the subscripts “A and R” denote the anatase and rutile phases, respectively. The anatase content is estimated by Eq. (5). The rutile content can then be determined by a subtraction [19].
The portions of anatase and rutile phases, which are calculated according to Eq. (5), are presented in Table 2. As can be seen, more than 90 % of titanium dioxide crystals are in the rutile phase, while Fe/TiO2 is composed of 42.17 % of anatase and Cu/TiO2 is mainly made up of anatase structure (70.28 %). Thus, the crystallization of a catalyst, especially the ratio of anatase to rutile, is affected by dopants. Moreover, it is observed that the existence of dopants decreases anatase to rutile transformations. The XRD patterns enunciate that the catalysts are composed of both anatase and rutile phases. Juang et al. indicated that high catalytic activity is associated with the crystalline structures containing both anatase and rutile. In other words, the co-existence of the two phases leads to a synergistic effect [20]. A higher content of rutile phase is related to a lower photocatalytic activity [2, 21, 22]. Another important point, which lies in the XRD patterns, is that as the result of copper oxide incorporation into the crystalline structure of TiO2, the intensity of anatase peak is increased and the intensity of rutile peaks is abated. It can be observed that the dopants retard anatase to rutile transformations. A higher anatase content indicates higher catalytic activity. Accordingly, Cu/TiO2 that is primarily composed of highly active anatase structure has the potential to be an outstanding photocatalyst. The experimental results confirm the existence of a relationship between anatase content and catalytic activity.
The Scherrer formula is used to estimate the crystallite size of the nanoparticles, where “D” is the crystallite size, “K” is a correction factor, which is related to shape, taken as 0.89, “λ” is wavelength of X-ray radiation used (0.15406 nm), “β” is the line width at half-maximum peak height, after subtraction from equipment broadening, and “θ” is the diffraction angle [23].
Since the samples are made up of both anatase and rutile phases, crystallite sizes of samples should be the average according to the following equation:
Crystallinity degree is defined as the crystalline portion of a sample divided by the sum of both crystalline and amorphous portions.
where “C” is the crystallinity degree and “\(I_{\text{C}}\) and \(I_{\text{A}}\)” are diffracted X-ray radiations of crystalline and amorphous portions of the samples, respectively [24].
Nanoparticles crystallite size based on Eq. (7) and the degree of crystallinity based on Eq. (8) are calculated and given in Table 2. From the results of Table 2, it is inferred that the crystallinity and likewise the crystallite size are reduced as the dopant is embedded into the TiO2 lattice. It is originated from the fact that the order of TiO2 lattice is disturbed by the dopant, in fact because of the different ionic radius of Ti4+ and Fe3+ or Cu2+. The source for numerical values of ionic radii is the paper by Shannon [25]. According to the so-called database, most reliable numerical value for ionic radii of Fe3+, Cu2+, and Ti4+ is 0.645, 0.57, and 0.605, respectively. Thereupon, the disorder imposed to the lattice because of dopants incorporation leads to the formation of defects. Defects are considered as amorphous. The more defects form, the less crystalline phases exist, and crystallinity degrees of the samples are decreased. In addition, XRD results, which are presented in Table 2, indicate that the existence of dopants retards the crystallization of samples decreasing anatase to rutile transformations. Thus, the crystallinity degree is reduced as the dopant is embedded into the TiO2 lattice.
SEM
Figures 3, 4b, and 5 illustrate the scanning electron micrographs (SEM) of Fe/TiO2, Cu/TiO2, and Cu/TiO2/LECA photocatalysts, respectively. It is quite evident that the surface morphology of iron-modified TiO2 is different from that of copper-modified TiO2. Since the synthesis methods are identical, it can be concluded that dopants are responsible for the morphological changes. The scales of the images of Fe/TiO2 in Fig. 3 range from 200 nm to 2 µm. Figure 3a displays that the Fe2O3 nanoparticles are distributed evenly on the TiO2 smooth surface. Bright spots, which are representative of iron oxide particles, are marked by white rings. Figure 3b, c, and d images exemplify the porosity of the catalyst. The images of Fig. 4, ranging from 100 nm to 1 µm, correspond to Cu/TiO2. The brilliant spots are representative of copper oxides, which are homogenously incorporated into the TiO2 lattice. It is of high importance that the morphology of CuO nanoparticles in Cu/TiO2 photocatalyst is different from Cu/TiO2/LECA photocatalyst. Therefore, SEM images of Cu/TiO2/LECA were studied over a wider range (500 nm–50 µm). It is indicated from the images of Fig. 5a–d that Cu/TiO2 covers the LECA surface with a uniform flaky layer. Active sites of the catalyst are depicted as brilliant spots, which are representative of CuO particles marked by white rings. Moreover, the microporous structure of LECA granules is depicted in the images of Fig. 5e–h. The high porosity of LECA suggests that it can be applied as an appropriate support. The copper-modified titanium dioxide photocatalyst, which was coated on LECA, simultaneously benefits from the high activity of Cu/TiO2 and the high porosity of LECA.
The SEM images are different regarding dopant covering and loading in TiO2 structure. CuO is found more abundantly in SEM images compared to Fe2O3; the existence of bright spots is more frequent in Cu/TiO2, but in one aspect they all are alike each other and this is the fact that the nanocatalysts (Cu/TiO2 and Fe/TiO2) are porous materials. Specifically, the microporosity of LECA and mesoporosity of Cu/TiO2 and Fe/TiO2 are evident in the SEM images. In the SEM images of Cu/TiO2/LECA, pores in the micrometers scale are obviously detectable, and the SEM images of Cu/TiO2 and Fe/TiO2 pores in nanodimensions are well depicted.
N2 adsorption/desorption isotherm
Nitrogen adsorption/desorption isotherm for Fe/TiO2 catalyst is shown in Fig. 6. The isotherm shape confirms that Fe/TiO2 catalyst is mesoporous. According to IUPAC classification, Fe/TiO2 corresponds to type IV, which represents mesoporous solids, and regarding hysteresis, it belongs to type H2. Catalysts with H2-type hysteresis have inkbottle pores [26]. As it is noted in “Synthesis of Cu/TiO2 and Fe/TiO2 photocatalysts” section, the titanium precursor and the synthesis method for Cu/TiO2 and Fe/TiO2 photocatalysts are the same and the whole quantities and ratios of materials kept constant. In summary, all the conditions, which affect porosity, are the same. It can be concluded that Fe/TiO2 and Cu/TiO2 nanocatalysts are porous materials. The results are in agreement with [27].
LECA (the applied substrate in this work) is a commercial product, which is manufactured worldwide, and accordingly, its properties such as low density, high porosity, and high surface area are well known. Furthermore, there are numerous articles [28–34], which support its porosity and high surface area.
Activity tests of suspended catalysts
Recently, many researchers have focused on enhancing the photocatalytic activity of TiO2. As an illustration, doping TiO2 with nonmetals like nitrogen and carbon was reported. Moreover, metallic nanoparticles have been used to improve the catalytic activity of TiO2 [6, 35]. A couple of studies reported high photocatalytic activity of Cu-doped TiO2 [36–39]. Tryba [40] observed that photoactivity of Fe–C–TiO2 was higher than TiO2. In this work, phenol photocatalytic degradation was experimentally investigated to evaluate the activity of TiO2, Fe/TiO2, and Cu/TiO2 photocatalysts. The catalysts were applied in the powder form, and the powders have been suspended in the phenol solution with the aid of magnetic stirrer. The catalyst loading was the same for each experiment (0.5 g/l). The results are given in Fig. 7. Phenol degradation efficiency was 19.98 % for TiO2, 27.87 % for Fe/TiO2, 43.21 % for Cu/TiO2. The results assert that introduction of iron and copper in the titanium dioxide lattice results in enhancing its photocatalytic activity. It is notable that copper has a more pronounced effect compared to iron. Cu-loaded TiO2 exhibits a twofold higher photocatalytic activity compared with TiO2 under the same conditions, which can be attributed to the chemical synergy, which is originated from the interaction between CuO and TiO2. This brings about suitable incorporation of copper oxide particles in the TiO2 lattice.
The XRD, XRF, SEM are consistent with the experimental results asserting higher photocatalytic activity of Cu/TiO2. First, XRD results validate the higher catalytic activity of Cu/TiO2. Higher anatase content of Cu/TiO2 enunciates that it has higher catalytic activity in comparison with Fe/TiO2. In addition, it is evident in the XRD pattern that anatase peak has intensified more noticeably once TiO2 is doped with Cu. Second, XRF analysis also confirms higher catalytic activity of Cu/TiO2. Chemical composition of the doped catalysts indicates that the ratio of CuO/TiO2 is 0.24 % and the ratio of F2O3/TiO2 is 0.2 %. The higher CuO loading in Cu/TiO2 suggests better incorporation of the dopant in the TiO2 lattice, and accordingly, Cu/TiO2 has more active sites, and thus, it validates the higher catalytic activity of Cu/TiO2. As the final clue in this section, in the SEM images CuO is found more abundantly compared to Fe2O3; the existence of bright spots representing active sites are more frequent in Cu/TiO2.
This finding is consistent with the work of Suen and Adesina [41], who reported excellent activity of CuO–TiO2 catalyst in the degradation of 4-hydroxy nitrobenzene. They attributed the enhanced performance of the catalyst to the co-operative interaction of CuO surface and TiO2 lattice. They enunciated that this synergetic effect leads to create more active sites. Sato and Taya [37] studied the sterilization of microbial cells, and they concluded that copper component in solid phase (Cu/TiO2 film) is responsible for the promotion of •OH generation. On the other hand, Shan et al. [42] notified that among the three crystalline forms of TiO2, anatase tends to be the most stable form at low temperatures (<700 °C). The XRD results confirm that Cu/TiO2 owing to its higher anatase content can be a more effective catalyst than TiO2 and Fe/TiO2.
Activity tests of immobilized catalysts
The application of catalysts in powder form has some drawbacks such as the loss of catalyst and the need for separation technologies to regenerate the catalyst, which are both time- and money-consuming. Moreover, the catalyst loading should be limited; the turbidity of the solution, which is a result of catalyst overuse, leads to scatter the UV light, and thus less sites of catalyst can be activated. Less available surface area, poor reusability, and low adsorption ability are some other disadvantages of suspended photocatalysis. To overcome these problems, the immobilization of the catalyst on an appropriate support is recommended [43–45]. The activity of the catalysts in suspended and immobilized states was experimentally compared. The results are presented in Fig. 8. It is observed that the photocatalysts, which are immobilized on LECA, have better performance in phenol degradation in comparison with powder photocatalysts. As an instance, phenol degradation efficiency for Cu/TiO2 in its powder form is 43.21 %, while the efficiency of 61 % is attained for the LECA-supported Cu/TiO2. The higher efficiency can be attributed to the enhanced accessible reaction surface provided by the porous structure of LECA. Shan et al. considered the high surface area of a support as a key property for enhancing the activity of a catalyst [41]. Furthermore, floatability of LECA, which is originated from its high porosity, enables the TiO2/LECA, Fe/TiO2/LECA, and Cu/TiO2/LECA photocatalysts to be exposed to the UV light for a longer time. This causes the activation of more catalytic sites. On the other hand, porous structure can trap light facilitating the photocatalyst activation. It is noted in “Photocatalytic experiments” section that to coat the same amount of catalysts on LECA, for Cu/TiO2 less LECA was used. The fact that with lower substrate amount, better performance in phenol degradation is observed supports the higher photocatalytic activity of Cu/TiO2/LECA.
Conclusion
Nanocrystalline powders were successfully synthesized by sol–gel method. The XRD results enunciate that the catalysts are composed of both anatase and rutile phases and anatase is the significant phase of Cu/TiO2. It has been shown that the presence of cation dopants such as Fe3+ and Cu2+ in TiO2 lattice is followed by these structural consequences:
-
1.
Decelerating anatase to rutile transformations (anatase peaks have intensified, while rutile peaks have abated in the XRD patterns of the doped catalysts.)
-
2.
Decreasing the crystallite size of the nanoparticles
-
3.
Reducing the nanoparticles crystallinity degree
-
4.
The existence of copper oxide (CuO) in Cu/TiO2 and Fe2O3 in Fe/TiO2 is confirmed by their corresponding peaks in the XRD patterns. The XRF results reveal that the ratio of CuO/TiO2 is 0.24 % and F2O3/TiO2 is 0.2 %. The SEM images of Fe/TiO2 and Cu/TiO2 catalysts declare that the morphological changes arise from difference of the dopants. Moreover, the microporosity of Cu/TiO2/LECA photocatalyst and mesoporosity of Cu/TiO2 and Fe/TiO2 are evident in the corresponding SEM images. The experimental results assert that doping TiO2 with copper will have an outstanding impact on enhancing its photocatalytic activity. The high activity of Cu/TiO2 is considered to arise from high content of anatase and better incorporation of the dopant in the TiO2 lattice. To overcome the problems regarding suspended photocatalytic reactions, the photocatalysts were immobilized on LECA. The high porosity and low density of LECA empower it to be an ideal substrate for photocatalytic processes. It was observed that the photocatalysts which were coated on LECA showed superior catalytic activity compared to the suspended photocatalysts.
References
E. Grabowska, J. Reszczynska, A. Zaleska, Mechanism of phenol photodegradation in the presence of pure and modified-TiO2: a review. Water Res. 46, 5453–5471 (2012). doi:10.1016/j.watres.2012.07.048
S. Ahmed, M.G. Rasul, W.N. Martens, R. Brown, M.A. Hashib, Heterogeneous photocatalytic degradation of phenols in wastewater: a review on current status and developments. Desalination 261, 3–18 (2010). doi:10.1016/j.desal.2010.04.062
T. Luenloi, B. Chalermsinsuwan, T. Sreethawong, Photodegradation of phenol catalyzed by TiO2 coated on acrylic sheets: kinetics and factorial design analysis. Desalination 274, 192–199 (2011). doi:10.1016/j.desal.2011.02.011
S. Souzanchi, F. Vahabzadeh, S. Fazel, S.N. Hosseini, Performance of an annular sieve-plate column photoreactor using immobilized TiO2 on stainless steel support for phenol degradation. Chem. Eng. J. 223, 268–276 (2013). doi:10.1016/j.cej.2013.02.123
S. Oros-Ruiz, R. Zanella, B. Prado, Photocatalytic degradation of trimethoprim by metallic nanoparticles supported on TiO2-P25. J. Hazard. Mater. 263, 28–35 (2013). doi:10.1016/j.jhazmat.2013.04.010
O. Lorret, D. Francova´, G. Waldner, N. Stelzer, W-doped titania nanoparticles for UV and visible-light photocatalytic reactions. Appl. Catal. B 91, 39–46 (2009). doi:10.1016/j.apcatb.2009.05.005
Y.L. Pang, A.Z. Abdullah, Effect of low Fe3+ doping on characteristics, sonocatalytic activity and reusability of TiO2 nanotubes catalysts for removal of Rhodamine B from water. J. Hazard. Mater. 235–236, 326–335 (2012). doi:10.1016/j.jhazmat.2012.08.008
J. Huang, S. Wang, Y. Zhao, X. Wang, S. Wang, W. Shihua, S. Zhang, W. Huang, Synthesis and characterization of CuO/TiO2 catalysts for low-temperature CO oxidation. Catal. Commun. 7, 1029–1034 (2006). doi:10.1016/j.catcom.2006.05.001
H. Tedla, I.D.T. Kebede, A.M. Taddesse, Synthesis, characterization and photocatalytic activity of zeolite supported ZnO/Fe2O3/MnO2 nanocomposites. J. Environ. Chem. Eng. 3, 1586–1591 (2015). doi:10.1016/j.jece.2015.05.012
C.H. Zhou, J. Keeling, Fundamental and applied research on clay minerals: from climate and environment to nanotechnology. Appl. Clay Sci. 74, 3–9 (2013). doi:10.1016/j.clay.2013.02.013
A. Vaccari, Clays and catalysis: a promising future. Appl. Clay Sci. 14, 161–198 (1999). doi:10.1016/S0169-1317(98)00058-1
S.A. Hassan, F.Z. Yehia, A.A. Hamed, A.A. Zahran, S.M. Solyman, Interaction characteristics controlling catalytic performances of Ni (II) and Cu (II) phthalocyanines immobilized on bentonite clay surface in redox-initiated polymerization of methyl methacrylate in aqueous medium. J. Porous Mater. 18, 1–11 (2011). doi:10.1007/s10934-009-9350-z
Y. Shavisi, S. Sharifnia, M. Zendehzaban, M.L. Mirghavami, S. Kakehazar, Application of solar light for degradation of ammonia in petrochemical wastewater by a floating TiO2/LECA photocatalyst. J. Ind. Eng. Chem. 20, 2806–2813 (2014). doi:10.1016/j.jiec.2013.11.011
M.N. Sepehr, H. Kazemian, E. Ghahramani, A. Amrane, V. Sivasankar e, M. Zarrabi, Defluoridation of water via light weight expanded clay aggregate (LECA): adsorbent characterization, competing ions, chemical regeneration, equilibrium and kinetic modeling. J. Taiwan Inst. Chem. Eng. 45, 1821–1834 (2014). doi:10.1016/j.jtice.2014.02.009
S. Sohrabi, F. Akhlaghian, The effect of fe-loading and calcination temperature on the activity of Fe/TiO2 in phenol degradation, Iran. J. Chem. Chem. Eng. 35(2), 43–50 (2016)
F. Akhlaghian, S. Sohrabi, Fe/TiO2 catalyst for photodegradation of phenol in water. Int. J. Eng. IJE Trans. A Basics 28, 499–506 (2015). doi:10.5829/idosi.ije.2015.28.04a.02
S. Sohrabi, F. Akhlaghian, Modeling and optimization of phenol degradation over copper-doped titanium dioxide photocatalyst using response surface methodology. Process Saf. Environ. Prot. 99, 120–128 (2016). doi:10.1016/j.psep.2015.10.016
L. Sun, J. Li, C.L. Wang, S.F. Li, H.B. Chen, C.J. Lin, An electrochemical strategy of doping Fe3+ into TiO2 nanotube array films for enhancement in photocatalytic activity. Sol. Energy Mater. Sol. Cells 93, 1875–1880 (2009). doi:10.1016/j.solmat.2009.07.001
M.S. Nahar, J. Zhang, K. Hasegawa, S. Kagaya, S. Kuroda, Mater. Sci. Semicond. Process. 12, 168–174 (2009). doi:10.1016/j.mssp.2009.09.011
C.-H. Chiou, R.-S. Juang, Photocatalytic degradation of phenol in aqueous solutions by Pr-doped TiO2 nanoparticles. J. Hazard. Mater. 149, 1–7 (2007). doi:10.1016/j.jhazmat.2007.03.035
W. Qiu, Y. Zheng, A comprehensive assessment of supported titania photocatalysts in a fluidized bed photoreactor: photocatalytic activity and adherence stability. Appl. Catal. B 71, 151–162 (2007). doi:10.1016/j.apcatb.2006.08.021
A. Fernández, G. Lassaletta, V.M. Jiménez, A. Justo, A.R. González-Elipe, J.-M. Herrmann, H. Tahiri, Y. Ait-Ichou, Preparation and characterization of TiO2 photocatalysts supported on various rigid supports (glass, quartz and stainless steel). Comparative studies of photocatalytic activity in water purification. Appl. Catal. B Environ. 7, 49–63 (1995). doi:10.1016/0926-3373(95)00026-7
J.G. McEvoya, W. Cuib, Z. Zhang, Degradative and disinfective properties of carbon-doped anatase–rutile TiO2 mixtures under visible light irradiation. Catal. Today 207, 191–199 (2013). doi:10.1016/j.cattod.2012.04.015
D.B. Black, E.G. Lovering, Estimation of the degree of crystallinity in digoxin by X-ray and infrared methods. J. Pharm. Pharmacol. 29, 684–687 (1977). doi:10.1111/j.2042-7158.1977.tb11435.x
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A32, 751–767 (1976). doi:10.1107/S0567739476001551
G. Leofanti, M. Padovan, G. Tozzola, B. Venturelli, Surface area and pore texture of catalysts. Catal. Today 41, 207–219 (1998). doi:10.1016/S0920-5861(98)00050-9
S. Rahimnejad, S. Rahman Setayesh, M.R. Gholami, A credible role of copper oxide on structure of nanocrystalline mesoporous titanium dioxide. J. Iran. Chem. Soc. 5(3), 367–374 (2008). doi:10.1007/BF03245990
E.M. Kalhori, K. Yetilmezsoy, N. Uygur, M. Zarrabi, R.M.A. Shmeis, Modeling of adsorption of toxic chromium on natural and surface modified lightweight expanded clay aggregate (LECA). Appl. Surf. Sci. 287, 428–442 (2013). doi:10.1016/j.apsusc.2013.09.175
L. Amado, A. Albuquerque, A.E. Santo, Influence of stormwater infiltration on the treatment capacity of a LECA-based horizontal subsurface flow constructed wetland. Ecol. Eng. 39, 16–23 (2012). doi:10.1016/j.ecoleng.2011.11.009
H. Amiri, N. Jaafarzadeh, M. Ahmadi, S.S. Martínez, Application of LECA modified with Fenton in arsenite and arsenate removal as an adsorbent. Desalination 272, 212–217 (2011). doi:10.1016/j.desal.2011.01.018
S.B. Bonabi, J.K. Khabushan, R. Kahani, A.H. Raouf, Fabrication of metallic composite foam using ceramic porous spheres “Light Expanded Clay Aggregate” via casting process. Mater. Des. 64, 310–315 (2014). doi:10.1016/j.matdes.2014.07.061
R.K. Tabase, D. Liu, A. Feilberg, Chemisorption of hydrogen sulphide and methanethiol by light expanded clay aggregates (Leca). Chemosphere 93, 1345–1351 (2013). doi:10.1016/j.chemosphere.2013.07.068
M.A. Nkansah, A.A. Christy, T. Barth, G.W. Francis, The use of lightweight expanded clay aggregate (LECA) as sorbent for PAHs removal from water. J. Hazard. Mater. 217–218, 360–365 (2012). doi:10.1016/j.jhazmat.2012.03.038
M. Malakootian, J. Nouri, H. Hossaini, Removal of heavy metals from paint industry’s wastewater using Leca as an available adsorbent. Int. J. Environ. Sci. Technol. 6, 183–190 (2009). doi:10.1007/BF03327620
S.-Y. Lee, S.-J. Park, TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 19, 1761–1769 (2013). doi:10.1016/j.jiec.2013.07.012
H.J. Choi, M. Kang, Hydrogen production from methanol/water decomposition in a liquid photosystem using the anatase structure of Cu loaded TiO2. Int. J. Hydrog. Energy 32, 3841–3848 (2007). doi:10.1016/j.ijhydene.2007.05.011
T. Sato, M. Taya, Copper-aided photo-sterilization of microbial cells on TiO2 film under irradiation from a white light fluorescent lamp. Biochem. Eng. J. 30, 199–204 (2006). doi:10.1016/j.jeurceramsoc.2009.04.016
H.W.P. Carvalhoa, A.P.L. Batistab, P. Hammer, T.C. Ramalho, Photocatalytic degradation of methylene blue by TiO2–Cu thin films: theoretical and experimental study. J. Hazard. Mater. 184, 273–280 (2010). doi:10.1016/j.jhazmat.2010.08.033
T. Sreethawong, S. Yoshikawa, Immobilization of TiO2 and Fe–C–TiO2 photocatalysts on the cotton material for application in a flow photocatalytic reactor for decomposition of phenol in water. Catal. Commun. 6, 661–668 (2005). doi:10.1016/j.catcom.2005.06.004
B. Tryba, Immobilization of TiO2 and Fe–C–TiO2 photocatalysts on the cotton material for application in a flow photocatalytic reactor for decomposition of phenol in water. J. Hazard. Mater. 151, 623–627 (2008). doi:10.1016/j.jhazmat.2007.06.034
C.L. Suen, A. Adesina, Aqueous phase oxidative degradation of 4-hydroxy nitrobenzene over a CuO–TiO2 Catalyst. Asia Pac. J. Chem. Eng. 6, 85–100 (1998). doi:10.1002/apj.5500060106
A.Y. Shan, T.I.M. Ghazi, S.A. Rashid, Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: a review. Appl. Catal. A Gen. 389, 1–8 (2010). doi:10.1016/j.apcata.2010.08.053
S. Singh, H. Mahalingam, P.K. Singh, Polymer-supported titanium dioxide photocatalysts for environmental remediation: a review. Appl. Catal. A Gen. 462–463, 178–195 (2013). doi:10.1016/j.apcata.2013.04.039
A. Nezamzadeh-Ejhieh, Z. Salimi, Heterogeneous photodegradation catalysis of o-phenylenediamine using CuO/X zeolite. Appl. Catal. A 390, 110–118 (2010). doi:10.1016/j.apcata.2010.09.038
Z. Sun, Y. Yan, G. Zhang, W. Zhaoyang, S. Zheng, The influence of carriers on the structure and photocatalytic activity of TiO2/diatomite composite photocatalysts. Adv. Powder Technol. 26, 595–601 (2015). doi:10.1016/j.apt.2015.01.007
Acknowledgments
The financial support from University of Kurdistan is gratefully acknowledged. In addition, the authors would like to thank “Dr. Sharam Sharifnia, Assistant Professor, Department of Chemical Engineering, Faculty Engineering, Razi University, for providing LECA” and “Mr. Mohamad Rahmani, Forest Biology Laboratory instructor and supervisor of the Department of Natural Resources of University of Kurdistan, for his kind cooperation.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sohrabi, S., Akhlaghian, F. Light expanded clay aggregate (LECA) as a support for TiO2, Fe/TiO2, and Cu/TiO2 nanocrystalline photocatalysts: a comparative study on the structure, morphology, and activity. J IRAN CHEM SOC 13, 1785–1796 (2016). https://doi.org/10.1007/s13738-016-0896-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-0896-9