Abstract
A hollow fiber-supported liquid membrane microextraction (HF-SLME) system using diaza-18-crown-6 (DA18C6) in ionic liquid (IL) 1-butyl-3-methylimidazolium hexafluorophosphate ([C4MIM]PF6) as an efficient carrier for the selective transport of cadmium(II) is described. The Cd(II) is ion paired with chlorine ion as a suitable counter into the membrane phase and then complexed with DA18C6; sodium thiosulfate (Na2S2O3) is injected in the lumen of membrane as an acceptor phase to trap the Cd(II) into the acceptor phase. The influence of various experimental parameters in the extraction procedure is discussed using a three-level Box–Behnken experimental design with three factors to optimize. Under the optimum conditions, the selectivity and efficiency of Cd(II) transport from an aqueous sample containing different mixtures of Co2+, Ni2+, Fe3+, Cr3+, Mn2+, and Cd2+ are investigated. The enrichment factor of Cd2+ is 172, the limit of detection is 0.13 ng L−1, and the relative standard deviation is 5.1 %. The method has been successfully applied to the determination of Cd(II) in food and cosmetic samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is known to be an environmental pollutant heavy metal. In the human body, Cd(II) accumulates mainly in the kidneys and results in various diseases. Despite its toxicity, Cd(II) is widely used in different industries such as electroplating, pigmenting, metallurgy and in the fabrication of Ni–Cd batteries [1, 2]. Therefore, separating and determining Cd(II) in different environmental samples are of great importance. Sensitive analytical techniques will be necessary before Cd(II) determination due to its low content and complex matrix in these samples.
Several technologies can be used to remove toxic metals from liquid effluents, including solvent extraction, precipitation, ion exchange, etc. [3, 4]. Most of these methods may result in a large amount of waste solvents. Membrane-based separation processes do not require high energy consumption for their operation; they can be conducted under moderate operating conditions. Accordingly, they have been considered as a promising alternative to overcome the above drawbacks. Among membrane-based separation processes, supported liquid membranes (SLMs) have been used extensively in recent years [5]. Traditional SLMs are tested as flat sheets, but the membrane instability is one of the major disadvantages due to the partition of the organic solvent to the aqueous phases [6]. Some researchers begin testing SLMs with a hollow fiber [7, 8]. The hollow fiber geometry makes it have very high module packing densities, liquid membrane inside can resist great pressure, and the stability of SLMs is improved [9]. In addition, the high surface area of the hollow fiber-supported liquid membrane (HF-SLM) system can achieve high separation rates [10].
In order for selective extraction of the desired substances from the aqueous solution to the acceptor solution, the selection of an appropriate extractant-carrier is very important in the HF-SLM [11]. Macrocyclic compounds such as crown ethers have been well known for selective recognition of specific metal ions since their discovery nearly four decades ago [12]. Compared to alkali and alkaline earth metal cations, the selective transport of heavy metal ions using crown ethers as an ion carrier in liquid membrane is relatively scarce [13]. Some crown ethers containing nitrogen atoms or sulfur atoms, such as aza-crown ethers, seem to be suitable candidates for the selective transport of heavy metal ions [14]. Morteza et al. [15] have described a highly efficient and selective method for the transport of Cd2+ ions through a liquid membrane containing a mixture of aza-18-crown-6 (A18C6) and palmitic acid (PA). Compared with their method, we show here that a mixture of diaza-18-crown-6 (DA18C6) and IL [C4MIM]PF6 was used as the membrane phase in the hollow fiber-supported liquid membrane microextraction (HF-SLME). ILs are considered to be solvents which are beneficial for both the operator and the environment due to their good dissolving capacity for numerous compounds and excellent thermal stability.
In this work, we used DA18C6 and IL [C4MIM]PF6 as an extractant-carrier inside the hollow fiber pores. Cd(II) is ion paired with chlorine ion as a suitable counter into the membrane phase and then complexed with DA18C6. The sodium thiosulfate (Na2S2O3) in the acceptor phase, which had a stronger complexing ability than DA18C6, stripped the Cd(II) to the acceptor phase. The acceptor phase was collected and analyzed by graphite furnace atomic absorption spectrometry (GFAAS) after the extraction process. The mechanism of the HF-SLME was discussed. And the effects of various experimental parameters in HF-SLME, such as DA18C6 concentration, incubation time, and Na2S2O3 concentration, were studied using two optimization methods, one variable at a time and Box–Behnken design (BBD) of the response surface methodology (RSM). Under the optimal conditions, the proposed method was applied to the selective transport of Cd(II) in food and cosmetic samples.
Experimental
Instrumentation
A model TAS-986 graphite furnace atomic absorption spectrophotometer (General Instrument Company, Beijing, China) equipped with deuterium lamp background correction (Hamamatsu Photonics, Japan), a cadmium hallow cathode lamp as a radiation source at the 228.8 nm wavelength with a slit width of 0.4 nm, 2.0 mA current was used for the determination of cadmium [16]. The temperature program used to determine the cadmium by GF-AAS is shown in Electronic Supporting Material (Table S, ESM). The sample solution was stirred by a DF-101S constant temperature magnetic stirring meter (Jintan, Medical Instrument Corporation, Jiangsu, China). 25-μL microsyringes (Anting Corporation, Shanghai, China) were used for sampling and washing the lumen of the hollow fiber.
Reagents and materials
All chemicals and reagents used in this study were of analytical grade, and all of them were purchased from the Shanghai Chemical Reagent Corporation, Shanghai, China, and double distilled water (DDW) was used to dilute all solutions. 1-butyl-3-methylimidazolium hexafluorophosphate ([C4MIM] PF6) was purchased from Chengjie Chemistry Corporation (Shanghai, China). DA18C6 was purchased from J & K Chemical Technology Corporation, Beijing, China. The stock standard solution for Cd(II) (1 mg mL−1) was prepared by dissolving appropriate amounts of CdCl2∙2.5H2O in DDW. Working standard solutions were obtained daily by appropriate dilution of the stock standard solution. 1 mg mL−1 Na2S2O3 was prepared by dissolving moderate Na2S2O3∙5H2O in DDW. Another concentration of Na2S2O3 used in the experiment was diluted with it step by step. [C4MIM]PF6 was used to dissolve DA18C6.
Accurel Q3/2 polypropylene hollow fiber membrane used for the extraction of cadmium was purchased from Membrana (Wuppertal, Germany). Its specifications are 600 µm internal diameter, 200 µm wall thickness and 0.2 µm pore size.
Extraction procedure
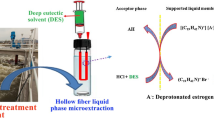
The purchased hollow fiber membrane was cut into 5.7 cm segments by scissors, then immersed in acetone and sonicated for a few minutes to remove the impurities. They were air-dried prior to use. 50 mL sample solution containing a certain amount of Cd2+ was placed in a vial; this vial was put into a constant temperature water bath. The lumen of the hollow fiber was washed with Na2S2O3 contained in the 25-μL microsyringe. After flushing, one end of the hollow fiber was sealed using heated iron. On the other side, 15 μL Na2S2O3 was injected into the lumen, and then the end was sealed in the same way. The hollow fiber was immersed in an ionic liquid [C4MIM]PF6 solution of crown ether for a while to immobilize the solution in the pores of the hollow fiber. The excess solution on the wall of the hollow fiber was washed away with redistilled water. Lastly, the hollow fiber was completely immersed in the sample solution for extraction. The device used for extraction is shown in Fig. 1. Following extraction, the extracted solvent with analytes was put into the graphite furnace tubes for analysis.
Single-factor experiments of HF-SLME
The effects of concentration of DA18C6, sample pH, extraction temperature and stirring rate, extraction time, concentration of Na2S2O3 were studied by a single-factor design as follows: one factor was changed while other factors were kept constant in each experiment. The effect of each factor was evaluated by determining the absorbance of Cd(II).
Experimental design of response surface methodology (RSM)
Optimization of HF-SLME by RSM
Box–Behnken design (BBD) with three independent variables was used for the optimization of the extraction of Cd(II). Experiments were established based on a BBD with three factors at three levels and each independent variable was coded at three levels: −1, 0, and +1. Three parameters including concentration of Na2S2O3 (ng mL−1), concentration of DA18C6 (ng mL−1), and extraction time (min) were chosen as key variables based on the results of single-factor experiments and designated as X 1 , X 2, and X 3, respectively. Absorbance (A) was taken as the response of the design experiments. Table S2 (ESM) lists the ranges of independent variables and their levels. The coding of the variables was undertaken using Eq. (1)
where X i and χ i are the dimensionless and actual values of the independent variable i, respectively, χ 0 is the actual value in the center of the domain, and △χ is the increment of χ i corresponding to a variation of one unit of X i .
The response function (Y) was partitioned into linear, quadratic, and interactive components:
where Y is the dependent variable, β 0 is the constant coefficient, β i is the linear coefficient (main effect), β ii is the quadratic coefficient, and βij is the two-factor interaction coefficient [17].
Verification of the predicted optimized conditions
Triplicate experiments were executed under optimal conditions to confirm the validity of the developed mathematical model. The average value of the experiments was compared to the predicted value of the developed model to ascertain the accuracy and suitability of the optimized conditions.
Sample preparation
Triplicate samples (0.5 g) of milk, honey, hand cream, face cream were accurately weighed in 100 mL conical flasks, respectively. About 10 mL of a freshly prepared mixture of concentrated HNO3-HClO4 (4:1, v/v) was added to each flask and soak overnight. The next day, these samples were heated on a hot plate until releasing largely white smoke. When the heated solutions became clear, samples cooled naturally, then transferred to a 50 mL volumetric flask and diluted to scale with redistilled water. The extracts were filtered with a 0.45 μm membrane filter. The filtered solutions were stored at 4 °C.
Results and discussion
The mechanism of the HF-SLME
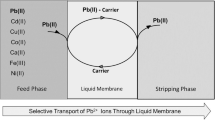
The HF-SLME consists of three phases. The aqueous source phase initially contains chloride ion (Cl−) and all the metal ions (Mn+), the hollow fiber membrane phase involves DA18C6 and [C4MIM]PF6 as the carrier (E), and the aqueous receiving phase Na2S2O3 is present on the lumen of the hollow fiber.
The various steps which characterize the transport of metal species through HF-SLM are described in Fig. 2. Firstly, the metal ions (Mn+) react with the metal carrier (E) after diffusing into the aqueous source phase–HFSLM phase interface. Cl− accompanies the Mn+ into the membrane phase. Secondly, the complex diffuses across the membrane in the lower concentration gradient. Thirdly, at the HFSLM phase–aqueous receiving phase interface, another complexing agent, which has the stronger complexing ability than the carrier, will strip the Mn+ into the receiving phase. Finally, the dissociated free carrier drives to aqueous source phase–HFSLM phase interface in its concentration gradient.
Single-factor tests of HF-SLME
Effect of the concentration of DA18C6
Extractions were carried out at the DA18C6 concentration varying from 100 to 350 ng mL−1, while other extraction variables were set as follows: Na2S2O3 concentration 250 ng mL−1, extraction time 20 min, and the stirring rate 400 rpm. As shown in Fig. 3a, the absorbance of Cd(II) increased with increasing the DA18C6 concentration and reached its maximum absorbance when the DA18C6 concentration was 250 ng mL−1. Therefore, 250 ng mL−1 was selected as the center point for further study.
Effect of the concentration of Na2S2O3
The nature and composition of the ligand in the receiving phase could have an important effect on the selectivity of metal ion. It was reported that the S2O3 2− as a stripping ion had certain selective transport ability for cadmium [15]. In the HF-SLME, Na2S2O3 was used as the stripping ligand in the receiving phase and the effect of different concentrations of Na2S2O3 on the absorbance of Cd(II) is shown in Fig. 3b for the DA18C6 concentration of 250 ng mL−1 (other extraction variables kept constant as described above). It can be seen that the absorbance of Cd(II) increased with the increasing Na2S2O3 concentration from 100 to 250 ng mL−1, then leveled off up to the concentration of 350 ng mL−1. Thus, the Na2S2O3 concentration of 250 ng mL−1 was considered to be optimal in the present experiment.
Effect of the extraction time
Extraction time was another important factor influencing the extraction efficiency and the selectivity of Cd2+. The effect of extraction time on the absorbance of Cd(II) was investigated from 10 min to 50 min, when the other factors kept constant. The results shown in Fig. 3c indicated that the maximum absorbance of Cd(II) was achieved when the extraction time was 20 min. The absorbance of Cd(II) decreased continuously after 20 min due to the loss of ionic liquids over longer extraction time. Thus, the extraction time of 20 min was selected for the following experiment.
Effect of sample pH
The sample pH can affect the absorbance and the selectivity of Cd2+ since different metal ions had their own optimal pH. When the other factors kept constant, the effect of sample pH on the absorbance of Cd(II) was investigated in the pH range of 2–12, as shown in Fig. 3d. It was found that the absorbance of Cd(II) increased continuously at pH 2–7, and when the pH value was greater than 7, the absorbance of Cd(II) was almost constant. The possible reason for this phenomenon was that the NH− of DA18C6 was an electron withdrawing group under the acidic condition, which reduced the complexing ability with Cd2+ [18]. Therefore, the sample pH would not be considered when selecting factors in RSM.
Effect of stirring rate
The stirring rate of the sample solution can reduce the dynamic equilibrium time and accelerate the extraction kinetics. The effect of stirring rate on the absorbance of Cd(II) was investigated in the range of 200–600 rpm. The maximum absorbance of Cd(II) was achieved at 400 rpm, and the absorbance of Cd(II) had no significant change after 400 rpm. A higher stirring rate may generate excess air bubbles on the hollow fiber surface, suspending the diffusion of analyte from the aqueous phase to the organic phase [19]. Thus, the stirring rate of 400 rpm was considered to be optimal in the experiment.
Optimization of HF-SLME procedure by RSM
Analysis of variance (ANOVA) and regression estimation
According to the results of single-factor research, the concentration of DA18C6, the concentration of Na2S2O3, and the extraction time were adopted for RSM experiments. The analyzed results are listed in Table S2 (ESM). The regression analysis was carried out on the experimental data to find an optimal region of factors for the responses studied. The analysis applied allows determination of quadratic statistical models that describe the relation between the responses and factors. Obtained regression models can be described by the following equations:
Significant regression coefficients are determined by the statistical analysis of t test and p values. The estimation value of regression coefficient in the regression equation is shown in Table 1. For the experimental design model, the X 1 , X 2 , X 3 , X 1 X 3 , X 21 , X 22 , X 23 were the significant regression coefficients in the HF-SLME procedure of Cd(II). ANONA results showed that the test model was extremely significant (p < 0.0001), and the R 2 was 0.9969, indicating the goodness-of-fit of the model. The lack-of-fit p value of 0.5891 was insignificant which meant that the model was sufficiently accurate for predicting the relevant response.
Response surface plots and response optimization
RSM was used to determine the optimal response for Cd(II) extraction using HF-LPME. Three-dimensional (3D) surface plots and contour plots for Cd(II) extraction are shown in Fig. 4. The 3D surface plots can be clearly seen the interaction between the other two variables when the third independent variable was fixed at the central experimental level of zero. Each of these plots allows the visualization of the significant factors derived from the statistical analysis [20]. The optimal conditions correspond to the concentration of DA18C6 (261.06 ng mL−1), the concentration of Na2S2O3 (267.40 ng mL−1), extraction time (23.42 min), the maximum absorbance of cadmium (0.093).
Experiments were repeatedly done 6 times under optimal conditions to validate the model prediction. The average absorbance of cadmium was 0.089, which was close to the predicted value. Therefore, the optimization model of the BBD is able to predict the average absorbance of cadmium.
Selectivity of the HF-SLME
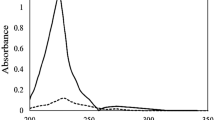
Under the optimum conditions, the selective transport of cadmium(II) from an aqueous sample containing different mixtures of Co2+, Ni2+, Fe3+, Cr3+, Mn2+, Cd2+ was investigated using the DA18C6 as a transport carrier in the HF-SLME. The results are shown in Fig. 5. As can be seen from it, DA18C6 had an efficient selectivity for Cd2+ transport, and other coexistent metal ions could hardly be extracted. It could be explained by the following reasons. On the one hand, the ionic radius of Cd2+ (r = 0.95 Å) was larger than the other coexistent metal ions [Co2+ (r = 0.74 Å), Ni2+ (r = 0.69 Å), Fe3+ (r = 0.55 Å), Cr3+ (r = 0.615 Å), Mn2+ (r = 0.67 Å)], and it was also close to the cavity size of DA18C6 (r = 1.43 Å). On the other hand, based on the Hard and Soft acid–base concept, the Cd2+ as a soft acid had a stronger complex with the nitrogen atom of DA18C6 as a soft base [21, 22].
Evaluation of the method
Various characteristics of this method were investigated under the optimum conditions. The enrichment factor (EF), which was calculated using the ratio of analyte concentration in the acceptor phase after HF-SLME to the initial concentration of analyte in the donor phase, was 172. The calculated calibration was in the range of 6–29 ng L−1 with a correlation coefficient of 0.9991. Limit of detection (LOD) was 0.13 ng L−1. The repeatability of this method expressed as the relative standard deviation (RSD) was 5.1 %.
Comparison of the HF-SLME with other methods for extraction of Cd(II)
To exhibit the merits of the HF-SLME, the proposed method was compared with other methods for extraction of Cd(II). The results are given in Table 2. Although the EF of the proposed method was not the highest, the LOD was lower than other methods. The hollow fiber could effectively avoid the interference of high weight molecular species and particles in the sample solution. In addition, in this experiment, crown ether ionic liquid as the carrier, the proposed method can realize the selective extraction of Cd2+ from the aqueous sample containing different mixtures of Co2+, Ni2+, Fe3+, Cr3+, Mn2+, Cd2+. The stability of HF-SLME was improved using the DA18C6-IL as transport carrier.
Analytical application
Under the optimized conditions, the developed HF-SLME method was applied to preconcentration and selective extraction of Cd(II) in milk, honey, face cream and hand cream. To verify the accuracy of this method, recovery experiments were done by adding different amounts of metal ions into the samples. The analytical results are shown in Table 3. The range of recoveries was 90–110 %, indicating the good reliability and validity of this method.
Conclusions
A three-phase HF-SLME system using DA18C6 in IL as an efficient carrier for the selective transport of Cd(II) in food and environmental sample is developed. DA18C6 as a membrane carrier can be used for the selective extraction of Cd(II) from the source phase containing different mixtures of Co2+, Ni2+, Fe3+, Cr3+, Mn2+, Cd2+. ILs are considered as green solvents, which can improve the stability of the HF-SLME system. Given the advantages of a low detection limit, high selectivity, low cost and environmental friendship of the method presented, it is believed that the proposed method has broad application prospects in the sample pretreatment technology.
References
J.P. Vernet, Heavy metals in the environment (Elsevier, Amsterdam, 1991)
G. Bertin, D. Averbeck, Biochimie 88, 1549–1559 (2006)
M.K. Jha, V. Kumar, R.J. Singh, Resour Conserv Recy 38, 1–22 (2001)
M.S. Safarzadeh, D. Moradkhani, P. Ashtari, Hydrometallurgy 97, 67–72 (2009)
L.J. Lozano, C. Godínez, F.J. Alguacil, Hydrometallurgy 80, 196–202 (2005)
S. Gupta, M. Chakraborty, Z.V.P. Murthy, J Ind Eng Chem 20, 2138–2145 (2014)
S. Wickramanayake, D. Hopkinson, C. Myers, L. Sui, D. Luebke, J Membr Sci 439, 58–67 (2013)
W. Lan, S. Li, J. Xu, G. Luo, Ind Eng Chem Res 52, 6770–6777 (2013)
M. Di Luccio, B.D. Luccio, T. Smith, T.L.M. Kida, C.P. Aves, C.P. Borges, Desalination 148, 213–220 (2002)
N.M. Kocherginsky, Q. Yang, L. Seelam, Sep Purif Technol 53, 171–177 (2007)
M.R. Yaftian, A.A. Zamani, M. Parinejad, Sep Purif Technol 40, 2709–2719 (2005)
C.J. Pedersen, J Am Chem Soc 89, 2495–2496 (1967)
G.H. Rounaghi, M.S. Kazemi, H. Sadeghian, J Inclusion Phenom Macrocyclic Chem 60, 79–83 (2008)
J.M. Izatt, R.S. Bradshaw, S.A. Nielsen, J.D. Lamb, J.J. Christensen, D. Sen, Chem Rev 83, 271–339 (1985)
M. Akhond, M. Shamsipur, J Memb Sci 117, 221–226 (1996)
C.J. Zeng, F.W. Yang, N. Zhou, Microchem J 98, 307–311 (2011)
Y.Q. Xu, L. Zhang, Y. Yang, X.M. Song, Z.Y. Yu, Carbohyd Polym 117, 895–902 (2015)
M.R. Yaftian, A.A. Zamani, M. Parinejad, E. Shams, Sep Purif Technol 42(2), 175–180 (2005)
D.A. Lambropoulou, T.A. Albanis, J Chromatogr A 1072, 55–61 (2005)
N. Khan, I.S. Jeong, I.M. Hwang, J.S. Kim, S.H. Choi, E.Y. Nho, J.Y. Choi, B.M. Kwak, J.H. Ahn, T. Yoon, Food Chem 141, 3566–3570 (2013)
G.H. Rounaghi, M.S. Kazemi, J Incl Phenom Macro 55(3–4), 347–352 (2006)
M. Shamsipur, S.Y. Kazemi, J Chin Chem Soc 54(4), 963–968 (2007)
P.X. Baliza, L.A.M. Cardoso, V.A. Lemos, Environ Monit Assess 184(7), 4455–4460 (2012)
J.N. Bianchin, E. Martendal, R. Mior, V.N. Alves, C.S.T. Araújo, N.M.M. Coelho, E. Carasek, Talanta 78(2), 333–336 (2009)
V.A. Lemos, L.A. Oliveira, Food Control 50, 901–906 (2015)
S. Khan, M. Soylak, T.G. Kazi, Biol Trace Elem Res 156(1–3), 49–55 (2013)
J. Peng, R. Liu, J. Liu, B. He, X.L. Hu, G.B. Jiang, Spectrochim Acta B 62(5), 499–503 (2007)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 31470434, 21406090, 21206059 and 21576124), the Special Financial Grant from the China Postdoctoral Science Foundation (2015T80510), and the Science Foundation of Jiangsu Entry-exit Inspection Quarantine Bureau of China (Nos. 2015KJ27 and 2015KJ28).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mao, Y., Chen, H., Han, J. et al. Selective transport of cadmium(II) through hollow fiber-supported liquid membrane microextraction using diaza-18-crown-6 in ionic liquids as carrier. J IRAN CHEM SOC 13, 403–410 (2016). https://doi.org/10.1007/s13738-015-0767-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0767-9