Abstract
The current work develops a new green methodology for the separation/preconcentration of cadmium ions (Cd2+) using room temperature ionic liquid-dispersive liquid phase microextraction (RTIL–DLME) prior to analysis by flame atomic absorption spectrometry with microsample introduction system. Room temperature ionic liquids (RTIL) are considered “Green Solvents” for their thermally stable and non-volatile properties, here 1-butyl-3-methylimidazolium hexafluorophosphate [C4mim][PF6] was used as an extractant. The preconcentration of Cd2+ in different waters and acid digested scalp hair samples were complexed with 1-(2-pyridylazo)-2-naphthol and extracted into the fine drops of RTILs. Some significant factors influencing the extraction efficiency of Cd2+ and its subsequent determination, including pH, amount of ligand, volume of RTIL, dispersant solvent, sample volume, temperature, and incubation time were investigated in detail. The limit of detection and the enhancement factor under the optimal conditions were 0.05 μg/L and 50, respectively. The relative standard deviation of 100 μg/L Cd2+ was 4.3 %. The validity of the proposed method was checked by determining Cd2+ in certified reference material (TM-25.3 fortified water). The sufficient recovery (>98 %) of Cd2+ with the certified value. The mean concentrations of Cd in lake water 13.2, waste water 15.7 and hair sample 16.8 μg/L, respectively and the developed method was applied satisfactorily to the preconcentration and determination of Cd2+ in real samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd2+) is a non-essential element and plays no positive nutritional or biometallic role for humans or animals. Contamination of water by Cd is a great concern due to ecological and occupational health hazard effects even at very low concentrations [1]. The most significant anthropogenic sources of Cd2+ are emission from industrial plants, such as steel works, zinc smelters, power stations, and incinerators [2]. The intake of Cd2+ by humans takes place by respiration of polluted air or through ingestion of different foods [3]. Prolonged consumption of drinking water containing Cd at higher level than 3.0 μg L−1 can cause deleterious effect on different organs of the human body [4]. According to the US Environmental Protection Agency, Cd is classified as a human carcinogen at low-level exposure into the environment [5]. Due to these adverse effects, monitoring of Cd2+ in environmental and biological samples is very important to estimate health risks. For that purpose, flame atomic absorption spectrometry (FAAS), electrothermal atomic absorption spectrometry (ETAAS) [6, 7], and inductively coupled plasma optical emission spectrometry (ICP-OES) [8, 9] have been proposed for routine metal determinations. However, aforementioned methods except FAAS involve a greater expenditure and enhanced instrumentation complexity. FAAS is still being used due to swift analysis time, ease of operation and a cheaper cost [10, 11]. Because many samples have lower analyte levels than the detection limits of FAAS, precise analysis requires a procedure that does not affect the sensitivity and accuracy of the selected analytical method in various environmental and biological matrices.
The development of economically or environmental friendly and practical sample pre-treatment methods are essential robust analysis. However, many analytical methods for the trace and ultra-trace determination of Cd2+, such as liquid–liquid extraction (LLE) [12], coprecipitation [13], ion exchange [14], cloud point extraction (CPE) [15] and solid phase extraction [16, 17].
LLE has been used for several years, but this technique is usually time-consuming and requires large quantities of high purity solvents. In order to reduce the amount of hazardous organic solvents in sample preparation methods, Assadi and co-workers [18] developed a novel microextraction technique, termed dispersive liquid–liquid microextraction (DLLME). However, the use of environmental damaging and hazardous organic solvent is a common [19–22]
Recently, there has been interest in using room temperature ionic liquids (RTILs) as environment-friendly solvents to replace traditional volatile organic solvents in various areas of chemistry. They are salts, liquids over a wide temperature range including room temperature, and prepared by the combination of bulky, non-symmetrical organic cations with various anions. RTILs have some unique physico-chemical properties, such as negligible vapor pressure, nonflammability, as well as good extractability for various organic compounds and metal ions, which make them an excellent choice for LLE and LPME [23, 24]. Several reports have appeared in which RTILs have been successfully utilized for extraction of metal ions [25–28]. Furthermore, their polarity, hydrophobicity, viscosity, and other chemical and physical properties can be selected by choosing the cationic or the anionic constituent. ILs are regarded as “designer solvents” because of this tunable nature, which increases their potential applications in analytical chemistry [23].
The objective of the present study is to develop a fast, novel, and miniaturized sample pre-treatment analytical procedure coupled with FAAS. The applicability of the proposed room temperature ionic liquid-based dispersive liquid phase microextraction (RTIL–DLME) method has been demonstrated for the determination of Cd2+ in environmental and biological samples. 1-(2-pyridylazo)-2-naphthol (PAN) is used as ligand that can produce a complex with many metal ions. It acts as a ligand with methanol for the enrichment of Cd2+ in sample solutions. Different experimental variables influencing the extraction efficiency of Cd2+ were investigated in detail to examine the extraction behavior of ionic liquids.
Material and Methods
Reagents and Chemicals
All of the solutions were made with water that was purified through reverse osmosis and was metal-free. PAN was obtained from (Merck, Darmstadt, Germany) and its 0.01 % (m/v) solution was prepared by dissolving 0.01 g in 100 mL of ethanol. All reagents used were analytical grade. Concentrated nitric acid (HNO3, purity 65 %) and ethanol were obtained from Merck (Germany) and Sigma Aldrich (St. Louis, USA), respectively. Ionic liquid (IL) 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim][PF6]) was obtained from Merck. Standard solution of Cd2+ was prepared by the dilution of certified standard solution (1,000 mg/L) Merck, Darmstadt, Germany. 0.2 M HNO3 was used for series dilution of the stock standard solution to make working standards. The buffer solutions were prepared according to the literature [29].
Instrumentation
The instrumental detection system used in this work was a Perkin-Elmer Model 3110 flame atomic absorption spectrometer (Norwalk, CT, USA). All measurements were carried out in an air/acetylene flame. A 10-cm-long slot-burner head was employed and a hollow cathode lamp of Cd2+ was used as radiation source at wave length (nanometers) 228.8. The operating parameters for Cd2+ were set as recommended by the manufacturer. All measurements were carried out without background correction. An aliquot of 100 μL of the samples was injected to the nebulizer of FAAS, using the microinjection method. The peak height signals were recorded.
A pH meter, Nel pH-900 (Ankara, Turkey) Model glass-electrode was employed for measuring pH values in the aqueous phase. An ALC PK Model 120 centrifuge machine and Vortex mixer (WIGGEN HAUSER®, Malaysia) was used for thorough mixing of solutions. The pure water used in all experiments was purified in a Human model RO 180 (HUMAN Corp., Seoul, Korea), resulting in water with a conductivity of 1 MΩ cm−1.
Samples Collection
In the present study, surface water sample was obtained from Camlik Lake, Yozgat, Turkey and waste water sample was collected from industrial effluent sites (Kayseri, Turkey). Water samples collected by using rubber-stoppered Von Dorn polyethylene plastic bottles (1.5 mL capacity) which were soaked in 10 % nitric acid for 24 h and before use rinsed with ultra pure water. All water samples were filtered through a 0.45 μm pore size membrane filter (Millipore Corporation, Bedford, MA, USA) to remove suspended particulate matter and were stored at 4 °C.
The samples of scalp hair were collected from male subjects, those were working in the batteries manufacture factories. Hair samples were collected from the nape of head using stainless steel scissors. The scalp hair samples were sealed separately in labeled polyethylene zip lock bags and were not opened until return to laboratory for further treatments. Prior to analysis, all hair samples were cut into 2-cm pieces.
Sample Pre-treatment
The washing procedure of scalp hair samples carried out was that proposed by International Atomic Energy Agency (IAEA) in order to offer an accurate judgment of endogenous metal contents. Washing of samples was carried out with Triton X-100, followed by rinsing with deionized water and acetone, and then drying in an oven at 80 °C. [30]
Samples that weighed approximately 0.5 g of duplicate scalp hair samples of each were placed in PTFE flasks (25 mL in capacity). To each sample, we added 2 mL of a freshly prepared mixture of concentrated HNO3–H2O2 (2:1, v/v) in to each flask and kept for 10 min at room temperature, then heated on an electric hot plate till semi dried mass was obtained. The semidried mass dissolved in 5 mL of 0.1 M HNO3 and filtered through a Whatman no. 42 filter paper and diluted with deionized water up to 10 mL in volumetric flask [31].
RTIL–DLME Procedure
For Cd2+ separation/preconcentration, 10-mL aliquots containing 10–200 μg/L of Cd2+ was taken in a 50 mL glass conical tube with screw cap, then 2 mL of different buffers were added to adjust the pH to a range of 4–9. The desired pH of the solution was adjusted by the addition of 1 M NaOH and/or HCl. After adjusting pH, 200–1000 μL of 0.01 % (m/v) PAN solution, 100 μL of each IL [C4MIM][PF6] and methanol were added as extractant and disperser solvents, respectively. The tube was capped and shaken carefully on vortex mixer for 10 s at 2,800 rpm until a cloudy solution formed. Cd2+–PAN chelate was extracted into fine tiny droplets of [C4MIM][PF6]. After that, the resulting cloudy mixture was centrifuged at 3,500 rpm for 10 min in order to attain phase separation. After enrichment, the IL phase was sedimented at the bottom of the tube and the upper aqueous phase was decanted. To decrease the viscosity IL phase, we added 0.5 mL of concentrated HNO3 prior to its analysis by FAAS. A blank submitted to the same procedure was measured parallel to the calibration solutions of standards, SRM and real samples.
Results and Discussion
ILs are gaining widespread recognition as novel solvents in chemistry. RTILs and dispersive liquid phase microextraction procedures substantially increase the contact area between extraction solvent and analytes, which also enhances the recovery of analytes. In addition, ILs are widely considered as an alternative to classical organic solvents in RTILs as compared to dispersive DLLME.
Optimization of RTIL–DLME Parameters
Some significant (pH, IL, ligand volume, disperser solvent, sample volume, centrifugation time, and rate) factors that influence the efficiency of proposed microextraction procedure were assessed in order to find the optimized experimental conditions.
Effect of pH
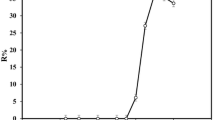
The pH plays an idiosyncratic role on metal-chelate formation and subsequent extraction, this factor proved to be a main all are parameter, this is one that exerts control. The effect of pH on microextraction procedure of Cd2+ was investigated at the pH range of 4–9 keeping other parameters constant. The results illustrated in Fig. 1, show that the extraction recovery of Cd2+ occurred at pH 8. Phosphate buffer of pH 8 was chosen as the optimum for consequent experiments.
Effect of PAN Volume
In this study, PAN was used as the ligand to the high hydrophobic nature of its metal chelates. The effect of 0.01 % (m/v) PAN concentration on extraction recovery was examined in the range of 200–1,000 μL, results are shown in Fig. 2. The extraction efficiency of Cd2+ increases up to 800 μL of 0.01 % PAN, reaching a plateau, indicated that this amount of PAN is sufficient for total complexation and maximum extraction recovery, while the addition of more PAN has no any significant effect on the extraction recovery.
Effect of IL Volume
The amount of IL is a considerable factor to obtain high recoveries of the analyte. For this reason, the microextraction procedure was examined with care, to define the lowest possible IL volume required for achieving the highest enrichment factor. In the present work, [C4mim][PF6] was chosen as the extraction solvent due to its good thermal stability, hydrophobicity and negligible vapor pressure. The variations in recovery against IL volume were studied in the range of 60–250 μL. We noticed that the recovery of the analyte was affected by the IL volume. Figure 3 shows that IL quantitatively extracts Cd2+ when its volume was 100 μL. No significant changes were observed in recovery at higher IL volume. For that reason, in order to get better enrichment factor, 100 μL of IL was found to be optimum.
Selection of Disperser Solvents and their Effect
The miscibility of the disperser solvent in both IL phase and aqueous phase is critical for the selection of disperser solvents. Therefore, methanol, acetone, ethanol, and acetonitrile were selected as disperser solvents. The influence of these solvents on the extraction efficiency of RTILs was determined using different volumes of each disperser solvent with 100 μL of [C4mim][PF6] and 0.01 % PAN. Figure 4 shows that methanol has higher compatibility with aqueous solution than other disperser solvents. Therefore, methanol was selected as the disperser solvent for further experiments.
The influence of methanol volume on the extraction efficiency of Cd2+ was also studied in the range of 50–200 μL. The results indicated in Fig. 5 that the extraction efficiency increased with an increase to the volume of methanol up to 100 μL, then reduced with a further increase in methanol volume. However, the solubility of the Cd2+–PAN complex in aqueous phase increased also, thereby, extraction efficiency decreased. For further studies, 100 μL of methanol was chosen as the optimum volume.
Sample Volume
The effects of sample volume with a constant amount of 100 μL of [C4mim][PF6] and methanol on the extraction of Cd2+ was studied from 10–50 mL. By increasing the sample volume to >15 mL, the extraction efficiency of the respective analytes decreased.
Effect of Centrifugation Time and Centrifugation Rate
Centrifuge time and rate are also among the key variables which influence the separation of IL and aqueous phase. Recovery of Cd2+ was monitored between 5 and 25 min as centrifugation time. We observed maximum recovery when the centrifugation time was up to 10 min. When the centrifugation time was at its low or high value, the recoveries were both lower. The lower centrifugation time may not assure the complete phase separation, and higher centrifuge time may provoke the IL to get dissolve back in aqueous phase. Additionally, the centrifugation rate was studied between 2,000 and 4,000 revolutions per minute (rpm). At centrifugation rate of 500 rpm, we observed lower recovery, while increasing centrifugation rate above 500 rpm, gradual increase was noticed. We attained maximum recovery at 3,500 rpm, while no further increase in recovery was observed with increase in centrifuge rate, so 10 min and 3,500 rpm were chosen as optimum values for further study.
Effect of Interference
In the present study, the extraction recovery of Cd2+ ions by interaction with PAN could be affected by different interfering ions. Experiments were carried out to find the extent to which our RTIL microextraction procedure is influenced by coexisting ions (results are shown in Table 1). To perform this study, 10 mL solution containing 100 μg/L of Cd2+ and interfering ions in different analyte ratios were subjected to the complete procedure. The tolerance limit is defined as the ion concentration causing a relative error smaller than ±5 % related to the preconcentration and determination of Cd2+. The observed Cd2+ recoveries in the presence of these interfering ions were above 96 %. Cd2+ was almost quantitatively recovered in the presence of all interfering ions which prove the applicability of our procedure for Cd2+ determination in different samples.
Analytical Figures of Merit
The calibration graph using the preconcentration system for Cd2+ was linear in the range of 10–200 μg/L and described by the following equation, y = 0.058[Cd2+] + 0.0005 with a correlation coefficient of 0.9982, where y is the absorbance signal and concentration of Cd2+ is expressed as micrograms per liter. The limit of detection (LOD) calculated 0.05 μg/L as three times the standard deviation of the blank signals. The obtained LOD value for cadmium was sufficiently low as compared to reported work [32] and to be valuable for detecting Cd2+ in different real samples. The enrichment factor was 50, as the original volume used in the present experiment was 10 mL and a final ionic liquid phase volume of 0.2 mL. The reproducibility was calculated as % relative standard deviation for 6 replicates containing 100 μg/L of Cd2+ obtained 4.3 % for FAAS.
Analysis of Real Samples
Finally, the proposed method was applied to the determination of the Cd2+ in real samples. Prior to RTIL–DLME, each sample was adjusted to pH 8. Results are given in Table. 2. For recovery experiments, different amounts of Cd2+ were added to the sample solutions and subsequently analyzed by the developed procedure. These results reveal the validity of the combined methodology for analysis of Cd2+ in environmental and biological samples. Moreover, we also checked the applicability of our methodology by the analysis of certified reference material (TM-25.3 fortified water) and standard additions method at three concentration levels, 5.0, 10, and 20 μg/L (Table. 2). We found concordant results of Cd2+ with the certified values (Table. 3). The obtained LOD, relative standard deviation (RSD) and EF of the present study are comparatively better than other reported preconcentration methods [33–40] for Cd2+ determinations are listed in Table 4. Thus, it was concluded that our RTIL microextraction procedure can be successfully applied to real samples without any systematic errors.
Conclusion
The experimental results of this new extraction clearly shows the utility and validity of developed methodology coupled with FAAS for the preconcentration and determination of Cd2+ exhibited many virtues such as excellent enrichment factor, sensitivity, simplicity, cost-effectiveness and reduced the consumption of organic solvents. Therefore, a simple as well as environmentally friendly analytical methodology is achieved. Its high tolerance to coexisting ions and good analytical performance indicated that this proposed method was a good alternative for the determination of Cd2+ in environmental and biological samples.
References
Fan Z, Zhou W (2006) Dithizone–chloroform single drop microextraction system combined with electrothermal atomic absorption spectrometry using Ir as permanent modifier for the determination of Cd in water and biological samples. Spectrochim Acta B 61:870–874
Manzoori JL, Zadeh HA, Amjadi M (2007) Ultratrace determination of cadmium by cold vapor atomic absorption spectrometry after preconcentration with a simplified cloud point extraction methodology. Talanta 71:582–587
Aydin HH, Coker C, Ersoz B (2001) In vivo interaction between cadmium and essential trace elements copper and zinc in rats. Turk J Med Sci 31:127–130
World Health Organization (2008) Guidelines for Drinking-water Quality, Volume 1 Recommendations, 3nd edition, Geneva
US Environmental Protection Agency (1999) Integrated Risk Information System (IRIS) on Cadmium. National Center for Environmental Assessment, Washington, DC
Naseri MT, Milani HMR, Assadi Y, Kiani A (2008) Rapid determination of Lead in water samples by dispersive liquid-liquid microextraction coupled with electrothermal atomic absorption spectrometry. Talanta 75:56–62
Maltez F, Borges DLG, Carasek E, Welz B, Curtius AJ (2008) Single drop micro-extraction with O,O-diethyl dithiophosphate for the determination of lead by electrothermal atomic absorption spectrometry. Talanta 74:800–805
Srogi K (2008) Developments in the determination of trace elements by atomic spectroscopic techniques. Anal Lett 41:677–724
Koksal J, Synek V, Janos P (2002) Extraction spectrometric determination of lead in high purity aluminium salts. Talanta 58:325–330
Soylak M, Narin I (2005) An on-line preconcentration system for cadmium determination in environmental samples by flame atomic absorption spectrometry. Chem Anal (Warsaw) 50:705–715
Soylak M (2004) Solid phase extraction of Cu(II), Pb(II), Fe(III), Co(II) and Cr(III) on Chelex 100 Column prior to their flame atomic absorption spectrometric determinations. Anal Lett 37:1203–1217
Babu PR, Naidu DR (1991) Solvent extraction atomic absorption technique for the simultaneous determination of low concentrations of iron, nickel, chromium and manganese in drinking water. Talanta 38:175–179
Soylak M, Kaya B, Tuzen M (2007) Copper (II)-8-hydroxquinoline coprecipitation system for preconcentration and separation of cobalt(II) and manganese(II) in real samples. J Hazard Mater 147:832–837
Kenduzler E, Turker AR, Yalcinkaya O (2006) Separation and preconcentration of trace manganese from various samples with Amberlyst 36 column and determination by flame atomic absorption spectrometry. Talanta 69:835–840
Liang P, Sang H, Sun Z (2006) Cloud point extraction and graphite furnace atomic absorption spectrometry determination of manganese (II) and iron (II) in water samples. J Colloid Interface Sci 15:486–490
Tuzen M, Soylak M, Elci L (2005) Multi-element preconcentration of heavy metal ions by solid phase extraction on Chromosorb 108. Anal Chim Acta 548:101–108
Soylak M, Elci L, Dogan M (2001) Solid phase extraction of trace metal ions with Amberlite XAD resins prior to atomic absorption spectrometric analysis. J Trace Microprobe Techn 19:329–344
Rezaee M, Assadi Y, Hosseini MRM, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9
Berijani S, Assadi Y, Anbia M, Milani Hosseini MR, Aghaee E (2006) Dispersive liquid-liquid microextraction combined with gas chromatography-flame photometric detection. Very simple, rapid and sensitive method for the determination of organophosphorus pesticides in water. J Chromatogr A 1123:1–9
Jahromi EZ, Bidari A, Assadi Y, Milani Hosseini MR, Jamali MR (2007) Dispersive liquid-liquid microextraction combined with graphite furnace atomic absorption spectrometry. Ultra trace determination of cadmium in water samples. Anal Chim Acta 585:305–311
Hemmatkhah P, Bidari A, Jafarvand S, Milani Hosseini MR, Assadi Y (2009) Speciation of chromium in water samples using dispersive liquid-liquid microextraction and flame atomic absorption spectrometry. Microchim Acta 166:69–75
Bidari A, Hemmatkhah P, Jafarvand S, Milani Hosseini MR, Assadi Y (2008) Selenium analysis in water samples by dispersive liquid-liquid microextraction based on piazselenol formation and GC-ECD. Microchim Acta 163:243–249
Han D, Row KH (2010) Recent applications of ionic liquids in separation technology, review. Molecules 15:2405–2426
Liu JF, Jonsson JA, Jiang G (2005) Application of ionic liquids in analytical chemistry. Trends Anal Chem 24:20–27
Wei GT, Yang Z, Chen CJ (2003) Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions. Anal Chim Acta 488:183–192
Gharehbaghi M, Shemirani F, Baghdadi M (2009) Dispersive liquid-liquid microextraction based on ionic liquid and spectrophotometric determination of mercury in water samples. Int J Environ Anal Chem 89:21–33
Baghdadi M, Shemirani F (2009) In situ solvent formation microextraction based on ionic liquids: a novel sample preparation technique for determination of inorganic species in saline solutions. Anal Chim Acta 634:186–191
Vaezzadeh M, Shemirani F, Majidi B, Vaezzadeh M, Shemirani F, Majidi B (2010) Microextraction technique based on ionic liquid for preconcentration and determination of palladium in food additive, sea water, tea and biological samples. Food Chem Toxicol 48:1455–1460
Alothman ZA, Habila M, Yilmaz E, Soylak M (2012) Solid phase extraction of Cd(II), Pb(II), Zn(II) and Ni(II) from food samples using multiwalled carbon nanotubes impregnated with 4-(2-thiazolylazo)resorcinol. Microchim Acta 177:397–403
Khan S, Kazi TG, Kolachi NF, Baig JA, Afridi HI, Shah F (2011) A simple separation/preconcentration method for the determination of aluminum in drinking water and biological sample. Desalination 281:215–220
Khan S, Kazi TG, Baig JA, Afridi HI, Kolachi NF (2011) Separation/preconcentration methods for the determination of aluminum in dialysate solution and scalp hair samples of kidney failure patients. Biol Trace Elem Res 144:205–216
Chamsaz M, Eftekhari M, Atarodi A, Asadpour S, Ariani M (2012) Preconcentration procedure using vortex agitator system for determination of trace levels of cadmium by flame atomic absorption spectrometry. J Braz Chem Soc 23:1630–1635
Afkhami A, Madrakian T, Siampour H (2006) Flame atomic absorption spectrometric determination of trace quantities of cadmium in water samples after cloud point extraction in Triton X-114 without added chelating agents. J Hazard Mater 138:269–272
Costa LM, Ribeiro ES, Segatelli MG, Nascimento DR, Oliveira FM, Tarley CRT (2011) Adsorption studies of Cd (II) on Al2O3/Nb2O5 mixed oxide dispersed on silica matrix and its on-line preconcentration and determination by flame atomic absorption spectrometr. Spectrochim Acta Part B 66:329–337
Bianchin JN, Martendal E, Mior R, Alves VN, Araujo CST, Coelho NMM, Carasek E (2009) Development of a flow system for the determination of cadmium in fuel alcohol using vermicompost as biosorbent and flame atomic absorption spectrometry. Talanta 78:333–336
Carletto JS, Luciano RM, Bedendo GC, Carasek E (2009) Simple hollow fiber renewal liquid membrane extraction method for pre-concentration of Cd(II) in environmental samples and detection by flame atomic absorption spectrometry. Anal Chim Acta 63:45–50
Adam ISI, Anthemidis AN (2009) Flow injection wetting-film extraction system for flame atomic absorption spectrometric determination of cadmium in environmental waters. Talanta 77:1160–1164
Ma JJ, Du X, Zhang JW, Li JC, Wang LZ (2009) Ultrasound-assisted emulsification–microextraction combined with flame atomic absorption spectrometry for determination of trace cadmium in water samples. Talanta 80:980–984
Rajabi M, Kamalabdi M, Jamali MR, Zolgharnien J, Asanjarani N (2013) Application of response surface methodology for optimization of ionic liquid-based dispersive liquid-liquid microextraction of cadmium from water samples. Hum Exp Toxicol 32:620–631
Melek E, Tuzen M, Soylak M (2006) Flame atomic absorption spectrometric determination of cadmium(II) and lead(II) after their solid phase extraction as dibenzyldithiocarbamate chelates on dowex optipore V-493. Anal Chim Acta 578:213–219
Acknowledgments
The authors are grateful to the Scientific and Technological Research Council of Turkey (TUBITAK) for the “2216 Research Fellowship Programme for Foreign Citizens” and financial support. The authors also thank Erkan Yilmaz for help in experimental studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, S., Soylak, M. & Kazi, T.G. Room Temperature Ionic Liquid-Based Dispersive Liquid Phase Microextraction for the Separation/Preconcentration of Trace Cd2+ as 1-(2-pyridylazo)-2-naphthol (PAN) Complex from Environmental and Biological Samples and Determined by FAAS. Biol Trace Elem Res 156, 49–55 (2013). https://doi.org/10.1007/s12011-013-9853-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9853-y