Abstract
Adsorption characteristics and doxycycline (DC) removal efficiency of Fe3O4 magnetic nanoparticles as adsorbents have been determined by investigating the effects of pH, concentration of the DC, amount of adsorbents, contact time, ionic strength and temperature. The mechanism of adsorption was also studied. The adsorption of DC to the Fe3O4 magnetic nanoparticles could be described by Langmuir-type adsorption isotherms. Short contact time between the reagents, reusability of Fe3O4 for three times after recycling of the nanoparticles, good precision and accuracy, wide working pH range and high breakthrough volume are among the highlights of this procedure. The proposed extraction and determination procedure based on magnetic nanoparticles as adsorbent was successfully applied to the determination of DC spiked in honey and various water samples. The method presented here is fast, simple, cheap and robust, and it does not require the use of organic solvents. Also, the method needs only a magnet and can be performed in any laboratory without sophisticated equipment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics are an important group of pharmaceuticals that are released to the aquatic environment in different pathways and have been detected in various aquatic environments such as ground water, agricultural wastewater and in drinking water [1–3]. The animal excreta are the major source of contamination, as the most of these substances end up in manure that are either stored or applied as fertilizers. They are generally weakly absorbed by the human body and thus defecated either unchanged or altered, via urine and feces [4]. There is an increasing interest about their existence, persistence and fate in the environment because low levels of antibiotics can help the proliferation of antibiotic-resistant bacteria.
Tetracyclines (TCs) constitute one of the most important antibiotic families, ranking second in production and usage worldwide [5]. Up to date TCs have been broadly used in pharmaceuticals and food additives. The bee farmers have fed bees with TCs to strengthen their resistance to disease but traces of tetracycline would remain in the honey. Therefore, it is important to understand the occurrence, fate and effects, and control of TCs in the environment. Among 25 antibiotics, tetracycline was the most commonly detected antibiotic, being present in 80 % of the wastewater influent and sewage. Doxycycline (DC) is one of the kinds of antibiotics of the TC family containing the β-diketonate configuration. Its curative effect is superior to that of TC. Doxycycline hydrochloride, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2- naphthacene carboxamide monohydrate, is one of the TC derivations, which has a wide range of antibacterial activity. DC has broad-spectrum antibacterial function and is active against both Gram-negative and Gram-positive bacteria. DC is preferred to other TCs in the treatment of specific infections because of its fairly reliable absorption and its long half-life, which permits less frequent dosage.
It is critical to develop reliable, rapid, precise and economical analytical procedures for monitoring TCs that are harmless to the environment as well. In this regard, a tremendous technique for separation and removal of TCs has been reported [6–9]. Some researchers have proposed the adsorption of TCs on oxide minerals [10, 11], Fe–Mn binary oxide [12], sediments [13], humic substances [14] and clay minerals [15]. But the problems for application of these adsorbents are their reusability, removal efficiency, adsorption capacity and/or long equilibrium time; therefore, a reliable and well-defined method is still in need despite a few methods currently available.
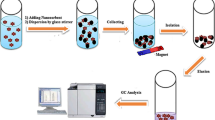
Fe3O4 magnetite nanoparticles have shown great potential for many nanotechnology applications, including effective adsorbents for removal of undesirable contaminants in water treatment [16]. This nano-adsorbent has a high surface area and a small particle size. Especially, the superior magnetic property of these adsorbents makes them to be simply recovered by magnetic separation after adsorption or regeneration, which overcomes the drawback of separation difficulty of usual adsorbents [17]. The application of magnetic nanoparticles as an adsorbent has received considerable attention in recent years [18–21].
To date, most studies on antibiotics in wastewater treatment plants (WWTPs) focused on their occurrences and concentrations in influent, effluent and sludge. However, the percent removals obtained using different methodologies differ greatly with each other, for example, from 11.6 [22] to 85.4 % [23] for TC. In addition, although some antibiotics were removed to some degree using wastewater treatment procedures, the removal routes (biodegradation, adsorption, volatilization, or hydrolysis) are still not very clear. To investigate the removal routes, a few works have been conducted for TC antibiotics [23, 24].
The objective of this study is: (1) to measure the removal efficiency of DC using magnetite nanoparticles (Fe3O4) as new adsorbents; (2) to investigate the mechanism and predominant factors controlling the adsorption of DC onto Fe3O4 nanoparticles; and (3) to study the separation and determination of the DC in real water samples and honey.
Materials and methods
Materials
Analytical grades DC hyclate, sodium hydroxide solution (1.5 mol L−1), hydrochloric acid, Ca(NO3)2.4H2O, Mg(NO3)2.6H2O, Al(NO3)3.9H2O, Cu(NO3)2.3H2O, Ni(NO3)2.6H2O, Zn(NO3)2.6H2O, KNO3, NaNO3, Na2SO4, NaCH3COO, KBr, KCl, FeCl3.6H2O and FeSO4 .7H2O were purchased from Merck. Glucose, lactose, maltose, fructose were purchased from Fluka. Stock solution (1,000 mg L−1) of DC hyclate was prepared. The pH adjustments were performed with HCl and NaOH solutions (0.01–1.0 mol L−1).
Instrumentation
A UV–visible spectrophotometer (Pharmacia Biotech) equipped with a 1-cm quartz cell was used for recording the visible spectra and absorbance measurements. A Metrohm 780 pH meter was used for monitoring the pH values. A water ultrasonicator (Model CD-4800, China) was used to disperse the nanoparticles in solution and a super magnet Nd–Fe–B (1.4 T, 10 × 5 × 2 cm) was used. All measurements were performed at ambient temperature. Transmission electron microscope (Philips CM 10TEM) was used for recording TEM images.
Preparation of Fe3O4 nanoparticles
The nanoparticles of Fe3O4 were synthesized by mixing ferrous sulfate and ferric chloride in NaOH solution with constant stirring speed of 1,500 rpm as reported in the literature [25]. To obtain maximum yield for magnetic nanoparticles during co-precipitation process, the molar ratio of Fe2+/Fe3+ was about 0.5. The precipitate was heated at 80 °C for 30 min and was sonicated for 20 min, then washed three times (50 mL in each time) with distilled water. Figure 1 shows a typical TEM photograph of the Fe3O4 nanoparticles. The average diameter of Fe3O4 nanoparticles was about ~10 nm.
Adsorption experiments
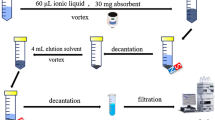
Adsorption experiments with the synthesized Fe3O4 nanoparticles were carried out using batch method under magnetic stirring. Each experiment consisted of preparing a 5 mL of DC solution with a desired initial concentration (2–250 mg L−1) and pH (3.0–10.0), by diluting with distilled water, and transferring it into the individual beaker on the magnetic stirrer. A known dosage of nano-Fe3O4 in the range of 5.0–25.0 mg was then added to the solution. After the mixing time elapsed (2.0–15.0 min), the magnetic particles were separated magnetically. The DC concentration in the supernatant was measured by UV–Vis spectrometer; the absorbance before and after adsorption of the DC were measured. The percent adsorption of DC was calculated using the following equation:
where C 0 and C f, respectively, represent the initial and final (after adsorption) DC concentrations (in mg L−1) in the aqueous phase. All tests were performed in duplicate at ambient temperature. The absorption spectrum of the DC solution, before and after addition of the magnetic nanoparticles, is shown in Fig. 2.
Results and discussion
Effect of nanoparticle dosage
The percent adsorption of DC on nano-Fe3O4 was studied at different adsorbent doses (5.0–25.0 mg), DC concentration of 20.0 mg L−1, pH 3.0 and agitation time of 5 min. The percent adsorption increased from 85.8 to 99.9 % as the adsorbent dose was increased from 5.0 to 25.0 mg. Figure 3 shows that by increasing the amount of adsorbent up to 15.0 mg, the percent adsorption of DC increased. This could be explained by this fact that more adsorbent became available for adsorbing the DC molecules. Further addition of the adsorbent did not show any significant change in percent adsorption of DC. Thus, 20.0 mg of the nanoparticles was chosen as the optimum amount for performing the following steps of the optimization procedure.
Effect of solution pH
pH is one of the most important parameters controlling the adsorption process. The effect of pH on the removal of DC is shown in Fig. 4. The experiment was carried out for adsorption of 20.0 mg L−1 DC on 20.0 mg Fe3O4 nanoparticles as adsorbent for a contact time of 5 min. The pH of the sample solution was adjusted by HCl and NaOH solutions. The DC adsorption was quantitative with percent adsorption range of 98.95–99.95 % indicating that the adsorption is independent of pH in the studied pH range of 3.0–10.0. These results indicate that the Fe3O4 nanoparticles is suitable as adsorbent under moderately acidic, neutral and moderately alkaline conditions. It should be mentioned that the experiment was not performed at pH values lower than 2.5 as the nanoparticles dissolve in this pH and lower.
Sorption mechanism is based on complexation of iron sites of nanoparticles with DC molecules as reported in the literature [11, 26]. Since DC shows three pK a values of approximately 3.4, 7.7 and 9.3, adjustment of pH must be an important step in the separation process of DC. The pH is usually adjusted to value of ≤3.0 to assume that DC would be in its cationic form (pK a1 = 3.4) which is important for obtaining an optimal extraction. Because DC tends to form complexes with divalent metal ions such as Ca2+ and Mg2+, these ions can prevent effective extraction of DC.
Werner et al. [27] reported the aqueous speciation of tetracycline over a range of pH and by association with Ca2+ and Mg2+ ions; the ionization and complexation processes are shown as follows:
where M = Ca2+ or Mg2+ and L = TC. Complexation of TC with Ca2+ and Mg2+ ions occurs predominantly under basic condition [28]. Lambs et al. [29] reported that only at pH ≥ 9, complexation of TC with Ca2+ occurred significantly. To avoid similar complexations between DC and divalent metal ions such as Ca2+ and Mg2+, pH 3.0 has been chosen for the following experiments.
Effect of doxycycline concentration
To find the required concentrations of DC that must be used in the optimization procedure, the adsorption of different initial concentrations of DC, in the range of 2–250 mg L−1, was studied at pH 3.0. The decision to use the concentration of 2 mg L−1, as the lower limit of the DC concentration, was because of the limitations in sensitivity of UV–Vis absorption measurements.
The experimental results of this study, shown in Fig. 5, reveal that the adsorption sites of the nanoparticles became saturated almost at DC concentrations of more than 200.0 mg L−1, while the reported experimental conditions were considered. The DC concentration of 20.0 mg L−1 was selected for following the optimization procedure.
Effect of contact time
The effect of contact time between DC and the adsorbent on the adsorption of DC was studied to determine the time taken by magnetic nanoparticles to remove 20.0 mg L−1 DC solution at pH 3.0. A 20.0 mg of magnetic nanoparticles was added into a 5.0 mL of DC solution. Variation of absorbance of the solution at 345 nm as a function of stirring time was determined to monitor the DC concentration changes in the solution. The percent extraction of DC as a function of stirring time (with a speed of 500 rpm) in the time period of 2.0–15.0 min indicated that the adsorption process started immediately upon addition of Fe3O4 to the sample solution containing DC and 98.7 % of the DC was adsorbed within 2.0 min. A contact time of 5.0 min was selected for further works. The contact time obtained in this study was found to be shorter than most of the reported values by the other adsorbents [9, 13].
Effect of solution temperature
The effect of temperature on the adsorptions of 20.0 mg L−1 of DC solution was investigated at pH 3.0 while a stirring time of 5.0 min was performed. The results showed that the percent adsorption of DC as a function of temperature in the range of 278–308 K was not significantly affected by temperature.
Effect of solution ionic strength
The effect of ionic strength on the percent adsorption of DC was investigated under the optimum experimental conditions in batch technique. A selected concentration of KCl, in the range of 0.10–1.0 mol L−1, was added into individual beakers containing 5.0 mL of 20.0 mg L−1 DC solution. After the mixing time elapsed, the Fe3O4 nanoparticles were magnetically separated and the solution was analyzed for the residual DC. The percent adsorption of DC was not significantly affected by increasing KCl concentration.
Breakthrough volume and preconcentration factor
To find the breakthrough volume (i.e., the volume of the most diluted solution containing a fixed amount of DC that could be handled by a fixed given mass of sorbent for quantitative adsorption of DC), 5.0 mL of 20.0 mg L−1 DC solutions was diluted with water to different volumes of 5, 50, 100, 150, 200, 250, 300 and 350 mL to have a fixed amount of DC (0.10 mg) in each solution. The recommended procedure was performed for each solution under the optimum experimental conditions. As seen in Fig. 6, the percent adsorption of DC was quantitative up to a volume of 300 mL and then decreased when higher volumes were tested. Thus, it was realized that the sorbent cannot quantitatively adsorb the DC from solutions with volumes more than 300 mL at the specified experimental conditions. Considering the final elution volume of 3.0 mL, as discussed later, and the breakthrough volume of 300 mL, a preconcentration factor of 100 was easily achievable.
Interference study
The effect of some cations, anions and sugars, known as common coexisting substances, on the determination of DC antibiotics by UV–visible spectrophotometer was investigated. The results showed that, interferences of metal ions (K+, Na+, Ca2+, Mg2+, Al3+, Zn2+, Cu2+, Ni2+), anions (Cl−, Br−, CH3COO−, SO4 2−) and sugars (glucose, lactose, fructose, maltose) were tolerable. They do not interfere with the determination of DC up to the values less than 100 times the amounts of DC. It should be noted that the formation of complex between DC and iron was effective in achieving a selective separation by magnetic nanoparticles as discussed in Section “Effect of solution pH”.
Desorption and reusability studies
To make the adsorption process more economical, it is necessary to regenerate the Fe3O4 nanoparticles. The DC desorption from the nanoparticles was conducted by washing the DC-loaded Fe3O4 nanoparticles using 0.05 mol L−1 Ca (NO3)2. The nanoparticles were collected magnetically from the solution. The concentration of DC in the desorbed solution was measured spectrophotometrically. The reusability of the adsorbent for DC in several successive separation processes was tested and the result showed that the Fe3O4 nanoparticle can be reused for three times without any reduction in its removal efficiency.
Adsorption isotherms
The adsorption isotherm reveals how the adsorbate molecules dispense between the liquid phase and the solid phase when the adsorption process reaches an equilibrium condition.
Adsorption isotherm study is performed on two popular isotherms, Langmuir and Freundlich. In the present investigation, the equilibrium data are analyzed using the Langmuir and Freundlich isotherm expressions given by Eqs. (2) and (3), respectively [30, 31]. The Langmuir isotherm theory supposes monolayer coverage of adsorbate over a uniform adsorbent surface, i.e., the surface comprise of identical sites, equally available for adsorption and with equal energies of adsorption. Thus, at equilibrium, a saturation point is achieved where no more adsorption can happen. Adsorption is assumed to take place at specific homogeneous sites with the adsorbent and once an antibiotic molecule occupies a site, no further adsorption can take place at the site. The qm is the surface concentration at monolayer coverage in mg g−1 and illustrates the maximum value of q e that can be attained as C e is increased. The b parameter is a coefficient related to the energy of adsorption and increases with increasing strength of the adsorption bond. Langmuir constants q m and b can be determined from the linear plot of C e/q e versus C e, which has a slope of 1/q m and an intercept of 1/bq m. The linear form of Langmuir equation is given by:
The experimental DC uptake values obtained have also been analyzed using Freundlich equation. In Freundlich adsorption isotherm, the model assumes that the adsorbent consists of a heterogeneous surface composed of different classes of adsorption sites. The Freundlich constants n and k are obtained from the linear regression analysis of the equation:
where q e is the maximum uptake capacity and C e is the equilibrium concentration. Plot of log q e versus log C e should give a straight line with a slope of 1/n and intercept of log K F. K F and n are the constants from the Freundlich equation representing the capacity of the adsorbent for the adsorbate and the reaction order, respectively.
In the present investigation, the equilibrium data were analyzed using the Langmuir and Freundlich isotherm expression. The calculated values of the Langmuir and Freundlich parameters are given in Table 1. The correlation coefficient (R 2) of the Langmuir model suggests a better fit for the experimental equilibrium adsorption data than that of Freundlich model. This indicates monolayer coverage of the surface of Fe3O4 nanoparticles by DC molecules.
Calibration curve and real samples analysis
For constructing the calibration curve (Fig. 7), standard DC solutions with different concentrations were prepared and their absorbances were measured by UV–Vis spectrometer at 345 nm.
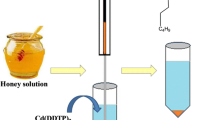
Applicability of the proposed method to real samples was evaluated by extraction and determination of spiked DC in honey (Khomein company, Iran), tap water (Shiraz city, Iran), well water (Kavar region, Fars, Iran) and agricultural water (Marvdasht region, Fars, Iran) as reported in Table 2.
For the analysis of water samples, all water samples were filtered through a 0.2-μm syringe filter. For the honey sample, 5.0 g of the sample was dissolved in hot water and the solution was stirred for 5 min using a vibratory stirrer at 1,000 rpm and then filtered. After adjustment of the pH of all samples at 3.0, each sample was extracted via the proposed method under the optimum experimental conditions. Table 2 shows that the results of three replicate analyses for each real sample, based on the proposed method, are in satisfactory agreement with the spiking amounts. In all cases, the RSD was <3 %.
Table 3 compares the performance characteristics of the present method with some methods, previously reported for determination of DC. When compared with other methods, shown in Table 3, the proposed work performed very well. A preconcentration factor of 100 and a concentration range of 2–250 were achieved which are significantly better than preconcentration factor and concentration range reported with dispersive liquid–liquid microextraction (DLLM). Also, in terms of percent recovery and RSD, the results are significantly better than DLLM. The value of the obtained RSD is comparable with RSD reported for HPLC coupled with resonance Rayleigh scattering. When compared with carrier-mediated hollow-fiber liquid-phase micro extraction, the percent recovery and RSD of the proposed technique are incredibly better but the preconcentration factor is lower. The obtained linear range is comparable with those reported for most HPLC techniques; however, the proposed technique is better than them in terms of cost and simplicity of the procedure.
Conclusions
It is clear from the data obtained in this study that Fe3O4 nanoparticles are robust adsorbents for DC antibiotics in water treatment systems. Mechanism and predominant factors controlling the adsorption of DC onto Fe3O4 nanoparticles and the separation and determination of the DC in real samples (water samples and honey) were investigated.
On the basis of these results, Fe3O4 nanoparticles could become more popular for treating agricultural, well and tap waters soon because the magnetic separation process can be operated easier, faster, environmental friendly with cheaper handling and operation under high salt concentration conditions, as compared to conventional methods.
It should be realized that, in addition to the aqueous concentrations of pollutants, attention also is requested in monitoring and modeling studies to the role of adsorbent in the retention of pollutants in the water environment. The adsorption of DC to the Fe3O4 magnetic nanoparticles could be described by Langmuir-type adsorption model.
References
D. Perret, A. Gentili, S. Marchese, A. Greco, R. Curini, Chromatographia 63, 225 (2006)
A.A.M. Stolker, W. Niesing, E.A. Hogendoorn, J.F.M. Versteegh, R. Fuchs, U.A.T. Brinkman, Anal. Bioanal. Chem. 378, 955 (2004)
Z. Ye, H.S. Weinberg, M.T. Meyer, Anal. Chem. 79, 1135 (2007)
C.S. McArdell, E. Molnar, M.J.F. Suter, W. Giger, Environ. Sci. Technol. 37, 5479 (2003)
S.B. Levy, The Antibiotic Paradox (Perseus Publishing, Cambridge, MA, 2002)
L. Ji, W. Chen, J. Bi, S. Zheng, Z. Xu, D. Zhu, P.J. Alvarez, Environ. Toxicol. Chem. 29, 2713 (2010)
L. Ji, W. Chen, L. Duan, D. Zhu, Environ. Sci. Technol. 43, 2322 (2009)
P. Oleszczuk, B. Pan, B. Xing, Environ. Sci. Technol. 43, 9167 (2009)
W. Yang, F. Zheng, Y. Lu, X. Xue, N. Li, Ind. Eng. Chem. Res. 50, 13892 (2011)
R.A. Figueroa, A.A. MacKay, Environ. Sci. Technol. 39, 6664 (2005)
C. Gu, K.G. Karthikeyan, Environ. Sci. Technol. 39, 2660 (2005)
H. Liu, Y. Yang, J. Kang, M. Fan, J. Qu, J. Environ. Sci. 24, 242 (2012)
X.R. Xu, X.Y. Li, Chemosphere 78, 430 (2010)
C. Gu, K.G. Karthikeyan, S.D. Sibley, J.A. Pedersen, Chemosphere 66, 1494 (2007)
L. Aristilde, C. Marichal, J. Miėhė-Brendlė, B. Lanson, L. Charlet, Environ. Sci. Technol. 44, 7839 (2010)
A. Khodabakhshi, M.M. Amin, M. Mozaffari, Iran. J. Environ. Health. Sci. Eng. 8, 189 (2011)
J. Qu, J. Environ. Sci. 20, 1 (2008)
M. Ghaemi, G. Absalan, Microchim. Acta 181, 45 (2014)
A. Afkhami, R. Moosavi, J. Hazard. Mater. 174, 398 (2010)
S. Qadri, A. Ganoe, Y. Haik, J. Hazard. Mater. 169, 318 (2009)
S. Pirillo, M. Ferreira, E.H. Rueda, Ind. Eng. Chem. Res. 46, 8255 (2007)
A.L. Spongberg, J.D. Witter, Sci. Total Environ. 397, 148 (2008)
A.L. Batt, S. Kim, D.S. Aga, Chemosphere 68, 428 (2007)
B. Li, T. Zhang, Environ. Sci. Technol. 44, 3468 (2010)
D.K. Kim, Y. Zhang, W. Voit, K.V. Rao, M. Muhammed, J. Magn. Magn. Mater. 225, 30 (2001)
I. Ihara, K. Toyoda, N. Beneragama, K. Umetsu, J. Phys. Conf. Ser. 156, 012034 (2009)
J.J. Werner, W.A. Arnold, K. McNeill, Environ. Sci. Technol. 40, 7236 (2006)
J.M. Wessels, W.E. Ford, W. Szymczak, S. Schneider, J. Phys. Chem. B 102, 9323 (1998)
L. Lambs, B. Decocklereverend, H. Kozlowski, G. Berthon, Inorg. Chem. 27, 3001 (1988)
H.M.F. Freundlich, Z. Phys. Chem. 57A, 385 (1906)
I. Langmuir, J. Am. Chem. Soc. 40, 1361 (1918)
P.J. Ramesh, K. Basavaiah, K. Tharpa, K.B. Vinay, H.D. Revanasiddappa, J. Pre-Clin. Clin. Res. 4, 101 (2010)
L. Wang, J. Peng, L. Liu, Anal. Chim. Acta 630, 101 (2008)
S.S. Mitić, G.Ž. Miletić, D.A. Kostić, D.Č. Nasković-Ðokić, B.B. Arsić, I.D. Rašić, J. Serb. Chem. Soc. 73, 665 (2008)
S. Shariati, Y. Yamini, A.J. Esrafili, Chromatogr. B 877, 393 (2009)
D. Hou, Y. Guan, X. Di, Chromatographia 73, 1057 (2011)
Acknowledgments
The authors wish to acknowledge the support of this work by Shiraz University Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghaemi, M., Absalan, G. Fast removal and determination of doxycycline in water samples and honey by Fe3O4 magnetic nanoparticles. J IRAN CHEM SOC 12, 1–7 (2015). https://doi.org/10.1007/s13738-014-0450-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-014-0450-6