Abstract
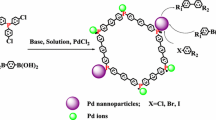
A new nitrogen-containing phosphinite ligand based on modified Merrifield resin was synthesized and characterized. This ligand was combined with PdCl2 to afford efficient catalyst for Heck and Suzuki cross-coupling reactions. The catalyst was highly efficient for cross-coupling reactions of aryl iodides, bromides and also chlorides with olefinic compounds. Aryl chlorides are usually inactive in coupling reactions, but with this catalytic system, excellent result was gained in the presence of tetrabutylammonium bromide. Transmission electron microscopy of catalyst shows that palladium was well dispersed and of typical diameter of 8–30 nm. The X-ray powder diffraction pattern of the Pd catalyst was consistent with the metallic Pd (0). Elemental analysis of Pd by ICP-OES and hot filtration test shows low leaching of the metal into solution from the supported catalyst which confirms the full heterogeneous character of the catalytically active species. Short reaction times, excellent yields and recyclability are among the advantages of this polymeric catalytic system. The catalyst can be reused for many times without loss in its efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Insoluble polymer-supported catalysts are among the most powerful and convenient tools in organic synthesis. The main advantage of using such heterogeneous catalysts is their easy separation from the reaction mixture. In the past decades, much attention has been paid to the development of polymer supported metal complexes as catalysts in organic reactions [1–3].
A large number of organic polymers have been used as supports for reagents and catalysts. One of such polymers is polystyrene due to its ready availability, inexpensiveness, mechanical robustness, chemical inertness and facile functionalization [4]. Polystyrene-based catalysts for organic synthesis have been widely reported. Merrifield resin is one of the derivatives of polystyrene that has been modified by functional groups. The functional groups of these modified polymers act as surface active sites for metal complexes with high suitable catalytic properties [1–7].
Efficient assembly of ligands on a solid support is a possible way towards the successful preparation of polymer immobilized transition metal catalysts [8–10]. The choice of ligand allows the tuning of the catalytic activity and selectivity. In general, these catalysts are positioned on the support activated by ligands such as diphosphines, phosphines, phosphinite and carbenes [11–15]. High cost of the precious metal catalysts has led to the development of the supported complexes that can be recovered from the reaction mixtures and can be recycled and reused. Many efforts have been focussed on the development of new ligand-derivatised polymeric supports for the attachment of metals [16].
Transition metal-mediated catalytic cross-coupling reactions are among the most powerful tools for modern organic synthesis [17]. The palladium-catalyzed Heck reaction, involving cross-coupling of aryl halides with unsaturated compounds [18–20] and coupling reaction of aryl halides with organo boronic acids (Suzuki–Miyaura reactions) [21–23] have been used in the simplest way to obtain C–C bound formations. The important goals in these reactions are the synthesis of chemicals, natural products, bioactive compounds, and advanced materials [24]. These reactions are carried out in the presence of Pd catalyst involving ligands such as phosphines [1], carbon [14] and phosphinite [25]. Phosphinites are efficient ligands for generation of the C–C bonds because they can stabilize palladium and increase its reactivity [26–31]. In continuation of our previous studies on heterogeneous Pd catalyst based on polymeric support [32] and polymer-grafted silica [33, 34], herein, we report the synthesis and application of stable and active heterogeneous palladium catalysts containing phosphinite ligand based on modified Merrifield resin to be used in Heck and Suzuki reactions.
Experimental
General
Substrates were purchased from Aldrich, Fluka and Merck companies. All products were characterized by comparison of their FT-IR and NMR spectra and physical data with those reported in the literature. All yields refer to the isolated products. Progress of reactions were followed by TLC on silica-gel Polygram SILG/UV 254 plates or by GLC on a Shimadzu model GC 10-A instrument with hydrogen flame ionization detector. IR spectra were run on a Shimadzu FT-IR-8300 spectrophotometer. The 1H NMR and 13C NMR spectra were recorded on a Bruker Avance DPX instrument (250 MHz). The Pd analysis and leaching test were carried out by inductively coupled plasma analyzer (ICP-OES) (Varian, Vista-Pro). CHN analysis was carried out on Thermo Finningan FIASHEA 1112 series instrument. X-ray diffraction data obtained with XRD, D8, Advance, Bruker, axs. Transmission electron microscope (TEM) analyses were performed on a Philips model CM 10 instrument. Scanning electron micrographs were obtained by, XL-30 FEG SEM, Philips, at 20 kV.
Preparation of polystyrene immobilized ethanolamine (II)
Trioxane (0.9 g, 10 mmol) and chlorotrimethylsilane (3.8 mL, 30 mmol) were dissolved in chloroform (10 mL). Polystyrene (1 g, 2 % Cross-linked) and SnCl4 (0.5 mL, 4.3 mmol) were added to the solution. The mixture was stirred at 0 °C for 30 min and then for 2 h at room temperature. The resin was washed with methanol, THF, and water, successively to obtain white powdery beads of chloromethylated polystyrene containing 3.21 meq of Cl/g. FT-IR ν: 1263.3 cm−1 (C–Cl), CHN Anal. Found: C. 81.65; H. 6.85; Cl.11.50.
Chloromethylated resin (1 g) was suspended in DMF (10 mL) and to this were added ethanolamine (0.23 mL, 4 mmol) and triethylamine (0.48 mL, 4 mmol). The mixture while stirred was heated at 90–100 °C for 17 h. It was cooled, filtered and washed thoroughly with water, DMF and acetone and dried under vacuum overnight to give the desired product. The hydroxyl group capacity of the polymer was determined by CHN analysis and was found to be 2.3 mmol/g of polymer.
Synthesis of polystyrene immobilized β-aminophosphinite ligand (III)
Polystyrene immobilized β-aminoalcohol (II) (1 g) and triethylamine (0.54 mL, 4.6 mmol) were suspended in THF (10 mL) at 0 °C. A solution of chlorodiphenylphosphine (0.82 mL, 4.6 mmol) in THF (2 mL) was added dropwise in the period of 20 min while stirring. The reaction mixture was then stirred for additional 10 h at R.T, and filtered. The solid was washed with water, THF, ethanol and dried under reduced pressure. Phosphorous content of the polymer was determinated by iodometric titration method and was found to be 1.6 mmol/g of the polymer.
Preparation of the Pd catalyst (IV)
The polystyrene supported phosphinite ligand (1 g) was treated with a solution of PdCl2 (0.14 g, 0.8 mmol) in DMF (15 ml) at 100 °C for 18 h. The mixture was filtered and washed thoroughly with DMF, water and acetone. It was then conditioned for a total of 18 h (3 × 2 h) each refluxing in water, ethanol and acetone and finally dried under vacuum. This was done to remove any physisorbed palladium. To determine the Pd content of the black catalyst, a mixture of concentrated H2SO4 and HNO3 was added to the catalyst and filtered. The filtrate was diluted to 50 cm3 with distilled water and subjected to ICP determination using calibration curve method. The Pd capacity of catalyst was determined by elemental analysis. ICP analysis revealed that the complex contained an average of 0.19 mmol Pd/g of polymer.
General procedure for Heck cross-coupling reactions
Aryl halide (1 mmol), n-butyl acrylate or styrene (1.2 mmol), K2CO3 (0.27 g, 2.0 mmol), Pd catalyst (0.016, 0.3 mol%) and DMF (3 mL) were mixed in a flask. The mixture was heated with stirring at 100 °C for the appropriate time. Progress of the reaction was followed by TLC (or GC if necessary). On completion of the reaction, the catalyst was easily recovered by filtration and the residue diluted with EtOAc and the organic layer was washed with water and brine (10 mL). The organic phase was dried over Na2SO4, and evaporated under vacuum. The mixture was then purified by column chromatography (n-hexane/ethyl acetate 7:3) over silica gel or recrystallization to afford the product with high purity. Characterization of the product was performed by comparison of its FT-IR, 1H-NMR, 13C-NMR, and physical data with those of the authentic sample.
General procedure for Suzuki cross-coupling reactions
Aryl halide (1 mmol), phenylboronic acid (0.14 g, 1.2 mmol), K2CO3 (0.27 g, 2.0 mmol), Pd catalyst (0.016 g, 0.3 mol%) and DMF (3 mL) were mixed in a flask. The mixture was heated with stirring at 80 °C for the appropriate time. The reaction was followed by TLC (or GC if necessary). After completion of the reaction, the procedure outlined above was followed.
Catalyst recycling
After completion of reaction, the catalyst was isolated by filtration. It was washed with acetone and water and dried in air. The resulting solid catalyst was charged into another batch for repeating cycle.
Results and discussion
The synthesis of Pd catalyst was accomplished according to Scheme 1. Simple and efficient process for the chloromethylation of cross-linked polystyrenes (Merrifield resin I) involves the reaction of cross-linked polystyrene (2 %) with trioxane and chlorotrimethylsilane in the presence of stannic chloride in chloroform [35]. The IR spectrum of polymer showed the characteristic absorption peak of (C–Cl) at 1263.3 cm−1. Polymer-bound β-aminoalcohol (II) was synthesized according to the literature procedures by nucleophilic substitution reaction of β-aminoalcohol with the Merrifield resin [10]. IR spectrum of the modified polymer showed a broad band at 3420 cm−1 due to overlapping of OH stretching and amine NH. The appearance of these bands suggests that the reaction have occurred successfully. The hydroxyl group capacity of II was determined by CHN analysis and was found to be 2.3 mmol/g of polymer. β-Aminophosphinite ligand was prepared on modified polystyrene by the reaction of II with ClPPh2 in THF [36]. The phosphorous content of the III was determinated by iodometric titration method and was found to be 1.6 mmol/g. The complexion between obtained polymer and palladium chloride was carried out in DMF to obtain black polymeric Pd (0) complex (IV). Two P atoms coordinated to Pd (П) center and followed by the reduction to Pd (0) by another phosphinite group. This reduction pathway is generally known for Pd (П) compounds [26–28, 37]. The Pd capacity of catalyst was determined by elemental analysis. ICP analysis revealed that the complex contained an average of 0.19 mmol Pd/g. XRD pattern of the Pd catalyst is presented in Fig. 1. It shows the crystallographic planes of the Pd (0) nanoparticles at (111), (200), (220) and (311). [38]. Transmission electron microscopy (TEM) image of Pd catalyst is shown in Fig. 2. TEM image shows that particles are dispersed in the polymer matrix with a size in the range of 10–30 nm. Scanning electron microscopy (SEM) images of Pd catalyst is shown in Fig. 3. SEM image shows the morphology of the nanoparticles of Pd.
The activity of catalyst was first tested in the Heck reaction. Coupling of iodobenzene with n-butyl acrylate was initially studied as a model reaction. The reaction condition was optimized for the Pd catalyst and results are presented in Table 1. The amount of catalyst can be reduced down to 0.01 mol% of Pd by prolonging the reaction time to 10 h. Generality of this reaction system was shown when coupling reactions of n-butylacrylate or styrene as olefinic substrate, with other aryl halides were carried out (Scheme 2). The results are presented in Table 2. Electron-neutral, electron-rich and electron-poor aryl iodides and bromides reacted with olefins very well. Aryl halides with electron-withdrawing groups were excellent for Heck reaction (entries 4–7, 12–14 and 20–23). In general, the reaction with styrene proceeds slower compared to n-butyl acrylate. Less reactive aryl chlorides have not been employed much, in palladium-catalyzed coupling reactions because the oxidative addition of C–Cl bond to Pd (0) species is usually difficult [39, 40]. Only a few heterogeneous Pd catalysts have been reported to convert activated aryl chlorides [26, 30, 32, 41], the reaction with chlorides had to be run in the presence of tetrabutylammonium bromide (TBAB) (Jeffery catalyst) as an additive [42]. Polymer catalyst was also examined for the Suzuki reaction which is one of the most powerful methods for generation of new biaryls (Scheme 3). We optimized the reaction condition similar to Heck reactions, the best result was obtained with 0.3 mol% of catalyst in the presence of K2CO3 as a base and DMF as a solvent at 80 °C. As shown in Table 3 different aryl halides react with phenylboronic acid to give the corresponding biaryl. Aryl chlorides can also give the coupling products in the presence of TBAB.

Heterogeneity test and catalyst reuse
The recovery and reusability of the supported catalyst was investigated using reaction of iodobenzene with n-butyl acrylate as a model reaction. The recovered catalyst was successfully used in 6 subsequent reactions without change in its efficiency (Table 4). To find the amount of palladium leaching in our system, the filtrate of model reaction was analyzed by ICP in six repeating cycles. Palladium leaching was neglected.
Hot filtration test was performed to understand the catalyst behavior in a truly heterogeneous manner or whether it is merely a reservoir for more homogenous active form of Pd. In a model reaction, Pd complex (0.3 mol%) iodobenzene (1 mmol) n-butyl acrylate (1.2 mmol), K2CO3 (2 mmol) and DMF (5 ml) were stirred at 100 °C for 30 min (25 % conversion). The catalyst was filtered off and the residue was stirred at 100 °C for another 24 h. There was no increase in the product concentration as is evidence from the GC analysis. The heterogeneous character of the catalytically active species is illustrated.
Conclusions
A new palladium catalyst based on modified Merrifield resin carrying phosphinite ligand was synthesized and characterized. Through TEM and XRD we can observe high Pd dispersion and small particle sizes in nano scale. The catalyst exhibits excellent activity and stability in Mizoroki–Heck and Suzuki–Miyaura cross-coupling reactions. Short reaction times, high yields, easy purification, recyclability and very low Pd leaching are the main characteristics of the process. The electron-rich, electron-poor and electron-neutral aryl halides showed good yields with high TON. Excellent results were gained with inactive aryl chlorides in the presence of TBAB.
References
C.A. McNamara, M.J. Dixon, M. Bradley, Chem. Rev. 102, 3275 (2002)
B. Clapham, T.S. Reger, K.D. Janda, Tetrahedron 57, 4637 (2001)
M. Lamblin, L. Nassar-Hardy, J.C. Hierso, E. Fouquet, F.X. Felpin, Adv. Synth. Catal. 352, 33 (2010)
J. Lu, P.H. Toy, Chem. Rev. 109, 815 (2009)
C.A. Lin, F.T. Luo, Tetrahedron Lett. 44, 7565 (2003)
C.A. McNamara, F. King, M. Bradley, Tetrahedron Lett. 45, 8239 (2004)
K.E. Elson, I.D. Jenkins, W.A. Loughlin, Tetrahedron Lett. 45, 2491 (2004)
B. Jandeleit, D.J. Schaefer, T.S. Powers, H.W. Turner, W.H. Weinberg, Angew. Chem., Int. Ed. 38, 2494 (1999)
C. Gennari, H.P. Nestler, U. Piazulli, B. Salom, Liebigs Ann., 637 (1997)
A. Mansour, M. Portnoy, J. Chem. Soc., Perkin Trans. 1 952 (2001)
A. Mansour, M. Portnoy, J. Mol. Catal. A: Chem. 250, 40 (2006)
D.E. Bergbreiter, A.M. Kippenberger, G. Tao, Chem. Commun. 18, 2158 (2002)
M. Guino, K.K. Hiib, Tetrahedron Lett. 46, 7363 (2005)
P.D. Stevens, G. Li, J. Fan, M. Yen, Y. Gao, Chem. Commun. 35, 4435 (2005)
R. Lou, A. Mi, Y. Jiang, Y. Qin, Z. Li, F. Fu, A.S.C. Chan, Tetrahedron 56, 5857 (2000)
F. Alonso, I.P. Beletskaya, M. Yus, Tetrahedron 61, 11771 (2005)
M. Beller, C. Bolm (eds.), Transition metal for organic synthesis, 2nd edn (Wiley-VCH, Weinheim, 2004)
I.P. Beletskaya, A.V. Cheprakov, Chem. Rev. 100, 3009 (2000)
L. Brandsma, S.F. Vasilersky, H.D. Verkruijsse, Applications of Transition Metal Catalysts in Organic Synthesis (Springer, Berlin, 1988)
K.C. Nicolaou, E.J. Sorensen, Classics in Total Synthesis (VCH, NewYork, 1996)
F. Diederich, P.J. Stang, Metal-Catalyzed Cross-Coupling Reactions. (Wiley-VCH, Weinheim, 1998)
N. Miyaura, Cross-Coupling reaction. Topics in Chemistry. (Springer, Heidelberg, 2002)
L. Yin, J. Liebscher, Chem. Rev. 107, 133 (2007)
F. Alonso, I.P. Beletskaya, M. Yus, Tetrahedron 64, 3047 (2008)
C.M. Andersson, K. Karabelas, A. Hallberg, J. Org. Chem. 50, 3891 (1985)
N. Iranpoor, H. Firouzabadi, R. Azadi, J. Org. Chem. 13, 2197 (2007)
N. Iranpoor, H. Firouzabadi, R. Azadi, J. Organomet. Chem. 693, 2469 (2008)
H. Firouzabadi, N. Iranpoor, M. Gholinejad, Tetrahedron 65, 7079 (2009)
I.D. Kostas, B.R. Steele, A. Terzis, S.V. Amosova, Tetrahedron 59, 3467 (2003)
A. Naghipour, S.J. Sabounchei, D. Morales, D. Canseco-Gonzalez, C.M. Jensen, Polyhedron 26, 1445 (2007)
Y.-H. Cheng, C.-M. Weng, F.E. Hong, Tetrahedron 63, 12277 (2007)
B. Tamami, S. Ghasemi, J. Molecul, Catal A: Chem. 322, 98 (2010)
B. Tamami, F. Frarjadian, J. Iran, Chem. Soc. 8, 77 (2011)
B. Tamami, H. Allahyari, S. Ghasemi, F. Frarjadian, J. Organomet. Chem. 696, 594 (2011)
S. Itsuno, K. Uchikoshi, K. Ito, J. Am. Chem. Soc. 112, 8187 (1990)
A. Mansour, M. Portnoy, Tetrahedron Lett. 44, 2195 (2003)
D.E. Bergbreiter, P.L. Osburn, A. Wilson, E.M. Sink, J. Am. Chem. Soc. 122, 9058 (2000)
S. Martinez, A. Vallribera, C.L. Cotet, M. Popovici, L. Martin, A. Roig, M. Moreno-Manas, E. Molins, New J. Chem. 29, 1342 (2005)
K. Selvakumar, A. Zapf, M. Beller, Org. Lett. 4, 3031 (2002)
K. Kohler, W. Kkleist, S.S. Prockl, Inorg. Chem. 46, 1876 (2007)
S. Prockl, W. Kleist, M.A. Gruber, K. Kohler, Angew. Chem., Int. Ed. 43, 1881 (2004)
T. Jeffery, Tetrahedron Lett. 26, 2667 (1985)
Acknowledgments
The authors gratefully acknowledge the partial support of this study by Shiraz University Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamami, B., Dodeji, F.N. Synthesis and application of modified polystyrene-supported palladium nanoparticles as a new heterogeneous catalyst for Heck and Suzuki cross-coupling reactions. J IRAN CHEM SOC 9, 841–850 (2012). https://doi.org/10.1007/s13738-012-0101-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-012-0101-8