Abstract
Phospholipase A2 receptor (PLA2R) is the most common primary target antigen of idiopathic membranous nephropathy (MN) although PLA2R antibodies are also reported to be present in malignancy-associated MN. However, a case of PLA2R-positive MN secondary to PLA2R-positive carcinoma has not been reported. A 26-year-old Japanese woman presented with general fatigue, fever, and nonproductive cough. Computed tomography demonstrated a left kidney mass with pathologic diagnosis of Xp11.2 translocation renal cell carcinoma (RCC). After the second time of administration with Sunitinib, the patients exhibited significant proteinuria and hypoalbuminemia. Renal biopsy revealed a diagnosis of diffuse MN secondary to RCC. Immunofluorescence staining showed granular patterns positive for immunoglobulin (Ig) G, IgA, and C3c. PLA2R and IgG1-3 were positive, while IgG4 was negative. For the treatment of severe nephrotic syndrome, we attempted steroid therapy without any clinical improvement. Open nephrectomy was performed and surprisingly, RCC was stained for PLA2R with polarity for the basal side. At outpatient follow-up, 4 months after the operation, urinary protein had still persisted, although serum albumin was slightly increased. We report a case of PLA2R-positive MN secondary to PLA2R-positive RCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Membranous nephropathy (MN) is the most common type of malignancy-associated glomerular lesions [1, 2]. The most common carcinomas causing malignancy-associated MN are lung and gastrointestinal carcinomas and cases associated with renal cell carcinoma (RCC) are rare [2,3,4]. In MN, phospholipase A2 receptor (PLA2R) is recently identified as one of the target antigens [4], accounting for 70–80% of idiopathic MN cases. Although PLA2R is expected as a noninvasive tool to diagnose idiopathic MN [5], some reports noted the existence of PLA2R antibodies in malignancy-associated MN [6,7,8]. Thrombospondin type I domain-containing 7A (THSD7A) is another target antigen of idiopathic MN [9]. While cases of THSD7A-positive MN secondary to THSD7A-positive cancer have been reported [10,11,12,13], PLA2R-positive MN secondary to PLA2R-positive carcinoma has not been reported.
Herein, we reported the case of PLA2R-positive renal cell carcinoma (RCC)-associated MN where RCC also expressed PLA2R.
Case presentation
A 26-year-old Japanese woman without any medical, surgical histories, or relevant family history had been healthy until February 2016, when she first noticed symptoms, such as general fatigue, fever at 38 ℃, and a nonproductive cough. Her home doctor prescribed antibiotics, but her symptoms did not improve and she was referred to the community hospital. Computed tomography (CT) and positron emission tomography revealed a left kidney mass and left subclavicular and periaortic lymphadenopathies. Percutaneous left renal tumor needle biopsy was performed and diagnosis of Xp11.2 translocation RCC was made. Because of lymph node metastasis, Sunitinib was administered at a cycle of 37.5 mg/day for 4 weeks followed by 2 weeks of rest. After the second cycle, the patient had >10 g/day proteinuria and an acutely decreased serum albumin level, indicating the nephrotic syndrome. Judging from the clinical course, Sunitinib was suspected to cause the nephrotic syndrome, and it was stopped and oral methylprednisolone (32 mg daily) was begun for the treatment of nephrotic syndrome. However, this treatment did not resolve proteinuria completely and methylprednisolone was tapered gradually to 6 mg daily. She was referred to our hospital for further treatment.
At admission, the patient (height 150 cm, weight 42.9 kg) had a blood pressure of 118/83 mmHg, pulse 101/min, and temperature 36.9 °C. Physical examination revealed left subclavian lymphadenopathy and mild abdominal distension. Urinalysis demonstrated protein level 3 + and microscopic hematuria (50–100 red blood cells per high power field). The 24 h urine protein excretion was 4.8 g/day, β-2 microglobulin level was 452 μg/L (reference range < 230 μg/L), and N-acetylglucosamine level was 91.3 U/L (reference range < 7.0 U/L). Urine electrophoresis revealed no abnormalities. Her serum creatinine (sCr) level was 0.42 mg/dL and blood urea nitrogen was 5.9 mg/dL. Liver function tests were normal. Serum albumin was 1.4 g/dL (reference range 3.9–5.2 g/dL), calcium 7.8 mg/dL (reference range 8.5–10.2 mg/dL), C-reactive protein (CRP) 8.19 mg/dL (reference range < 0.35 m g/dL), immunoglobulin G (IgG) level 813 mg/dL (reference range 870–1700 mg/dL), IgA level 355 mg/dL (reference range 110–410 mg/dL), and IgM level 145 mg/dL (reference range 46–260 mg/dL). Complement (C3, C4, and CH50) levels were normal. Antinuclear antibody, double-stranded DNA antibody, anti-GBM antibody, PR3-antineutrophil cytoplasmic antibody (ANCA), and MPO-ANCA were negative. Serum electrophoresis showed no abnormalities. Complete blood count was normal except for decreased red blood cell count to 4.08 × 106/μL.
Computed tomography scan revealed a 35 mm left heterogeneous mass and another 17 mm mass in the left lower renal pole. There were several lymphadenopathies around the aorta, celiac artery, and bilateral common iliac arteries, as well as a 33 mm supraclavicular lymphadenopathy that encased the left vertebral artery. Surgical treatment against the lesion was deferred due to the severe nephrotic syndrome and left kidney needle biopsy was performed. In renal histological, light microscopy showed diffuse spikes and a bubbly appearance on periodic Schiff-methenamine (PASM) staining and fuchsinophil subepithelial deposits on Masson-trichrome staining (Fig. 1a, b), but no mesangial expansion, hypercellularity, tubular atrophy, or interstitial fibrosis was observed. Arteries were almost intact. Electron microscopy showed subepithelial electron dense deposits and foot processes effacements (Fig. 1c). Immunofluorescence (IF) staining revealed a granular pattern positive for IgG, IgA, and C3c (Fig. 2a). Immunohistochemical studies revealed that PLA2R, IgG1, IgG2, IgG3 were granularly positive along with the capillary wall, but IgG4 and THSD7A were negative (Figs. 2b, c).
Histological findings of kidney biopsy specimen from the patient. a PASM staining showed spikes (arrow) and bubbly appearance (arrowhead) on the subepithelial side of the glomerular basement membrane. Scale bar: 10 μm. b Masson-Trichrome stain showed fuchsinophilic deposits (arrow) on the subepithelial side of the glomerular basement membrane. Scale bar: 10 μm. c Electron microscopy showed subepithelial electron dense deposits (arrow) and foot processes effacements. Scale bar: 2 μm
Immunofluorescence and immunohistochemistry of kidney biopsy specimen from the patient. a Immunofluorescence showed a granular staining pattern positive for IgG, IgA, and C3c. b PLA2R staining was positive in glomeruli, while THSD7A staining was negative in glomeruli. c IgG1-3 colocalized with PLA2R
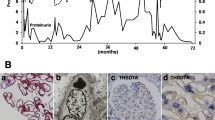
Based on the pathology and clinical course, diagnosis of the nephrotic syndrome caused by secondary MN due to RCC was made. Because her nephrotic syndrome was too severe for surgery, we attempted pulse steroid therapy for 3 days followed by oral systemic prednisolone 30 mg daily. However, the nephrotic syndrome did not improve. Therefore, open nephrectomy was performed. Histologic findings of the nephrectomy specimen revealed that RCC cells were stained for PLA2R with polarity for the basal side. RCC cells were not stained positive for IgG or THSD7A. Glomeruli around RCC were stained positive for IgG (Fig. 3a–d). Four months after surgery, urinary protein level was 2.7 g/gCr and slightly decreased compared with its value before surgery (5.5 g/gCr) and serum albumin level was slightly increased (2.3 g/dL) compared with its value before surgery (2.0 g/dL). They had not completely remitted.
Discussion
We report a case of malignancy-associated MN secondary to Xp11.2 translocation RCC. Our case had two features: (1) PLA2R was colocalized with IgG1, 2, and 3 staining on the subepithelial area. (2) Both glomeruli and RCC were stained positive for PLA2R.
We observed PLA2R expressions in the subepithelial area with IgG1, 2, and 3 co-localization. In general, PLA2R staining and IgG subclasses, as well as subendothelial deposits, mesangial matrix expansion, and C1q staining are used to differentiate secondary from idiopathic MN. Although PLA2R staining and the serum anti-PLA2R antibody test are used clinically to diagnose idiopathic MN, the sensitivity and specificity of PLA2R staining for idiopathic MN are modest (75% and 83%, respectively) [6]. Glomerular PLA2R staining showed 56% and approximately 19–25% positive rates for idiopathic and malignancy-associated MN, respectively [6, 7]. IgG subclasses are another clue to distinguish idiopathic from secondary MN. PLA2Rs are usually IgG4 in patients with idiopathic MN [14]. One study reported that 81.7% of idiopathic MN cases were IgG4 dominant [8]. Therefore, an IgG4-dominant staining pattern implies idiopathic MN, and some even claim that IgG4 negativity may indicate malignancy-associated MN [15]. In addition, a retrospective study reported that IF intensities of IgG1 and IgG2 were more dominant in the malignancy than in the idiopathic group [16]. This difference in IgG subclass staining patterns between idiopathic and secondary MN has been explained by T helper type 1 (Th1) or Th2 involvement. IgG1 and IgG2 are associated with Th1 and IgG4 with Th2 [16, 17]. Idiopathic MN may be involved mainly with Th2 [17]. Antigens from malignancy have been reported to activate interleukins stimulating Th1 as well as Th2 [16]. In our case, IgG1, IgG2, and IgG3 colocalized with PLA2R staining on subepithelial deposits, implying that anti-PLA2R antibodies were IgG1, IgG2, and IgG3. According to previous reports, anti-PLA2R IgG1-3 antibody exists [18, 19] and these anti-PLA2R IgG1-3 have reactivity to PLA2R [8]. These reports may also support our hypothesis. Unfortunately, we did not test circulating anti-PLA2R antibody, because her follow-up was finished and she was deceased, but this colocalization of IgG1-3 and PLA2R suggests the presence of circulating anti-PLA2R antibody.
In the present case, PLA2R was overexpressed in RCC. PLA2R is involved in tumor suppressing function. Basic researches have shown that ectopic PLA2R expression lead to apoptosis in breast cancer cell lines [20, 21]. In contrast, PLA2R-knockdown in human kidney cell line, ACHN cells induced malignant transformation [21]. In a clinical situation, PLA2R levels decrease in several carcinomas, including RCC [21,22,23]. On the other hand, PLA2R expression increases in some carcinomas [24, 25]. Inflammation and proinflammatory conditions are stimuli that upregulate PLA2R expression [20]. Overall, PLA2R overexpression in our case may result from the high-inflammatory state associated with RCC.
A case of MN in which PLA2R is positive both for glomerulus and cancer cells has not been reported thus far. Cases with another antigen of idiopathic MN, THSD7A, provided us with some information. THSD7A also is involved in angiogenesis and is associated with tumor progression [26,27,28]. Like our case, several reports have shown malignancy-associated MN with THSD7A double-positive for MN and cancer [10,11,12,13]. These investigators hypothesized that an antibody to THSD7A on tumor cells is produced primarily and then this antibody recognizes THSD7A on podocytes, leading to the onset of MN. This hypothesis could be the case with our case. Expressed PLA2R on tumor cells by treatment with Sunitinib was sensitized and antibodies were produced, which caused secondary MN. This mechanism resembles the animal Heymann nephritis model [29].
In our case, proteinuria was slightly decreased, but had not completely recovered after surgery. We speculate that one possible reason may be the remained metastasis in several lymph nodes. Hoxha reported that antigen-presenting cells of metastasized lymph nodes were positive for THSD7A [10]. Therefore, antigen-presenting cells in lymph nodes might contribute to the persistent activity of membranous nephropathy in our case.
Conclusion
This is the first report of PLA2R-positive MN secondary to PLA2R-positive RCC.
References
Jhaveri KD, Shah HH, Calderon K, Campenot ES, Radhakrishnan J. Glomerular diseases seen with cancer and chemotherapy: a narrative review. Kidney Int. 2013;84(1):34–44.
Ronco PM. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int. 1999;56(1):355–77.
Bacchetta J, Juillard L, Cochat P, Droz JP. Paraneoplastic glomerular diseases and malignancies. Crit Rev Oncol Hematol. 2009;70(1):39–58.
Leeaphorn N, Kue-A-Pai P, Thamcharoen N, Ungprasert P, Stokes MB, Knight EL. Prevalence of cancer in membranous nephropathy: a systematic review and meta-analysis of observational studies. Am J Nephrol. 2014;40(1):29–35.
Bobart SA, De Vriese AS, Pawar AS, et al. Noninvasive diagnosis of primary membranous nephropathy using phospholipase A2 receptor antibodies. Kidney Int. 2019;95(2):429–38.
Larsen CP, Messias NC, Silva FG, Messias E, Walker PD. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013;26(5):709–15.
Lönnbro-Widgren J, Ebefors K, Mölne J, Nyström J, Haraldsson B. Glomerular IgG subclasses in idiopathic and malignancy-associated membranous nephropathy. Clin Kidney J. 2015;8(4):433–9.
Qin W, Beck LH, Zeng C, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22(6):1137–43.
Beck LH. PLA2R and THSD7A: Disparate paths to the same disease? J Am Soc Nephrol. 2017;28(9):2579–89.
Hoxha E, Wiech T, Stahl PR, et al. A mechanism for cancer-associated membranous nephropathy. N Engl J Med. 2016;374(20):1995–6.
Zhang C, Zhang M, Chen D, et al. Features of phospholipase A2 receptor and thrombospondin type-1 domain-containing 7A in malignancy-associated membranous nephropathy. J Clin Pathol. 2019;72(10):705–11.
Taguchi S, Koshikawa Y, Ohyama S, Miyachi H, Ozawa H, Asada H. Thrombospondin type-1 domain-containing 7A-associated membranous nephropathy after resection of rectal cancer: a case report. BMC Nephrol. 2019;20(1):1–6.
Zhang Z, Gong T, Rennke HG, Hayashi R. Duodenal schwannoma as a rare association with membranous nephropathy: a case report. Am J Kidney Dis. 2019;73(2):278–80.
Huang CC, Lehman A, Albawardi A, et al. IgG subclass staining in renal biopsies with membranous glomerulonephritis indicates subclass switch during disease progression. Mod Pathol. 2013;26(6):799–805.
Qu Z, Liu G, Li J, et al. Absence of glomerular IgG4 deposition in patients with membranous nephropathy may indicate malignancy. Nephrol Dial Transplant. 2012;27(5):1931–7.
Ohtani H, Wakui H, Komatsuda A, et al. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant. 2004;19(3):574–9.
Holdsworth SR, Kitching AR, Tipping PG. Th1 and th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 1999;55(4):1198–216.
Hofstra JM, Debiec H, Short CD, et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23(10):1735–43.
Kanigicherla D, Gummadova J, McKenzie EA, et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83(5):940–8.
Sukocheva O, Menschikowski M, Hagelgans A, et al. Current insights into functions of phospholipase A2 receptor in normal and cancer cells: more questions than answers. Semin Cancer Biol. 2019;56(July):116–27.
Vindrieux D, Devailly G, Augert A, et al. Repression of PLA2R1 by c-MYC and HIF-2alpha promotes cancer growth. Oncotarget. 2014;5(4):1004–13.
Menschikowski M, Hagelgans A, Nacke B, Jandeck C, Sukocheva O, Siegert G. Epigenetic control of phospholipase A2 receptor expression in mammary cancer cells. BMC Cancer. 2015;15(1):1.
Bernard D, Vindrieux D. PLA2R1: expression and function in cancer. Biochim Biophys Acta. 2014;1846(1):40–4.
Gorovetz M, Baekelandt M, Berner A, Trope CG, Davidson B, Reich R. The clinical role of phospholipase A2 isoforms in advanced-stage ovarian carcinoma. Gynecol Oncol. 2006;103(3):831–40.
Amin R, Fiancette R, Bordessoule D, et al. Phospholipase A2 receptors in human leukemic blasts. Leuk Lymphoma. 2011;52(5):908–9.
Kuo MW, Wang CH, Wu HC, Chang SJ, Chuang YJ. Soluble THSD7A is an N-glycoprotein that promotes endothelial cell migration and tube formation in angiogenesis. PLoS ONE. 2011;6(12):e29000.
Stahl PR, Hoxha E, Wiech T, Schröder C, Simon R, Stahl RAK. THSD7A expression in human cancer. Genes Chromosom Cancer. 2017;56(4):314–27.
Hou Z, Abudureheman A, Wang L, et al. Expression, prognosis and functional role of Thsd 7a in esophageal squamous cell carcinoma of Kazakh patients Xinjiang. Oncotarget. 2017;8(36):60539–57.
Heymann W, Hackel DB, Harwood S, Wilson SG, Hunter JL. Production of nephrotic syndrome in rats by Freund’s adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med Soc Exp Biol Med (New York, NY). 1959;100(4):660–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
As the patient was deceased, written informed consent was obtained from the mother of the patient for publication of this case report. A copy of the written consent is available for review by the Editor of this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yasuda, I., Tokuyama, H., Hashiguchi, A. et al. Malignancy-associated membranous nephropathy with PLA2R double-positive for glomeruli and carcinoma. CEN Case Rep 10, 281–286 (2021). https://doi.org/10.1007/s13730-020-00556-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-020-00556-9