Abstract
The development of nanoparticles (NPs) and their incorporation into the membrane matrix for water and wastewater treatment is an emerging research area in modern times. Greywater (GW) treatment is gaining significant mileage of late owing wide variety of uses in nonpotable domestic and other industrial applications. The present study focuses on the indigenously synthesized titanium dioxide (TiO2) NPs and their amalgamation of 1–5% (wt) into polyphenyl sulfone (PPSU) mixed matrix membranes (MMMs) i.e., PPSU-T0 to T5 for GW purification by ultrafiltration (UF) process. The 3% (wt) of TiO2-loaded MMMs were optimized based on the results obtained from scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), 40.20% of porosity, and 31.14 nm pore size by 49.4° contact angle with an outstanding performance of 16.97 MPa tensile strength, besides 10.71% of elongation-at-break, along with 92.59 L/m2 h permeation and 99.43% of bovine serum albumin (BSA) rejection with an 89.50% of flux recovery. In addition to this, the optimized membrane exhibited a low fouling tendency with a minimal flux declination, which helps in GW treatment. From the experimental results, the PPSU-T3 achieved a maximum flux of 45.63 L/m2 h, with 80% water recovery by the removal of 95.54% turbidity, 94.20% color, 95.54% TSS and complete E. coli bacterial eradication at an applied pressure of 2 bar. Based on these observations, it can be deduced that the UF membrane played a vital role in GW purification, which can be further extended to desalination, surface water purification, protein concentration, and pre-treatment for nanofiltration (NF) and reverse osmosis (RO) applications. The findings of this study; therefore, shall emerge as a viable alternative for freshwater consumption in vulnerable water-scared areas, besides using it in gardening, laundry, washing, and other industrial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanoparticles (NPs) incorporation in the polymeric membranes is growing exponentially, due to their unique characteristics such as nanoscale size, large surface area, high reactivity, and degree of functionalization for water and wastewater treatment [1,2,3]. In this era, most common inorganic NPs, namely silicon dioxide (SiO2), aluminum oxide (Al2O3), ferrous oxide (Fe3O4), graphene oxide (GO), carbon nanotubes (CNTs), silver (Ag), zinc oxide (ZnO), zirconium dioxide (ZrO2), and titanium dioxide (TiO2), are being used for various applications [4, 5]. These NPs are mostly synthesized by sol–gel, sputtering, ultrasound, co-precipitation, inert gas condensation, spark discharge, hydrothermal, green, and biological methods and incorporated into the polymers [6, 7]. Among those, the sol–gel method is extensively used, due to the ease and cost-effectiveness of the process, either by addition or controlled doping to obtain various forms of materials in terms of shape and size. Another important factor is that the desired metal oxide can be synthesized at low temperatures and provides a highly stable surface area [7]. Nowadays, the TiO2 NPs synthesized from the sol–gel method are gaining much attention in the preparation of mixed matrix membranes (MMMs), due to easy availability, affordability, hydrophilicity, stability, antifouling ability, and photocatalytic properties [8,9,10]. Usually the TiO2 available in 8 structures, out of which, four forms can be typically synthesized in laboratories under high pressure, while the other four are natural polymorphs [11,12,13]. The chemically synthesized TiO2 NPs are tremendously applied in the photocatalytic treatment to degrade the organic and inorganic pollutants from the wastewater [14], which is further divided into black and greywater (GW). In India, around 810 MLD of water is used in urban areas like Bengaluru, for domestic usage, i.e., shower, bathing, washing, cleaning, and flushing, etc., from the total supplied water of 1300 MLD. A person can generate around 86.76 L per day of GW, which is only 64% as per the Indian Standards (IS: 1172-1963) [15]. Generally, the composition of GW varies on the basis of the type, quality, place, and household activities, which consist of oils, food particles, soaps, grease, pathogens, hair, and trace elements, etc. [16]. Hence to overcome these problems, the GW treatment would be an excellent strategy to cope up with environmental pollution by reusing the water within the same house through an easy approach, that helps to reduce freshwater consumption besides overcoming water shortage.
To treat the GW, the MMMs are gaining greater attention from the past few years, especially to enhance the flux, permeability, antimicrobial properties, and long-term durability with very minimal polymeric changes in the manufacturing processes [17, 18]. The NPs are broadly used in the ultrafiltration (UF) membranes to mitigate the fouling by low binding foulants, improve the surface hydrophilicity, robustness in thermal, mechanical, and filtration performance [19]. In the current scenario, UF presence is paramount in various applications such as effluent, water, wastewater, desalination, and protein separation methods [20, 21]. This process has a unique advantage in producing high-quality water with the ease of maintenance, compact modular construction, low chemical treatment, and high separation [22]. Various polymers have been tested for the UF process including polysulfone (PSF), polyethersulfone (PES), poly(vinylidene fluoride) (PVDF), and cellulose acetate (CA), etc. From the previously available literature, most of the researchers have used TiO2 NPs in PES [23,24,25] and PVDF [26,27,28] membranes to increase the hydrophilicity along with mechanical, pure water flux (PWF), and antifouling properties. The mechanical and chemical modification approaches were adapted using TiO2 NPs by Razmjou et al. [29]. According to Yang et al. 2% (wt) of TiO2-loaded PSF MMMs shown higher fouling mitigation property and his studies also revealed higher concentration loading can lead to an agglomeration in the matrix [30].
The hydrophobic nature of polyphenylsulfone (PPSU) is one of the most abundantly available polymers with high thermal, mechanical stability, excellent chemical resistance, and chlorine tolerance compared to the more frequently used PSF and PES [31, 32]. However, to overcome the inadequate antifouling ability and enhance the membrane’s performance using multiple constituents [33, 34], there is a vast potential research scope in membrane technology [35,36,37]. Mahendra et al. studied the fabrication of TiO2/GO incorporated PPSU/polyetherimide (PEI) blend membrane of MG-5 for UF application to remove 98.7% of humic acid (HA) from 10 ppm aqueous solutions at pH 7. These membranes showed a greater ability to reduce the irreversible HA fouling and improve the PWF [38]. Arockiasamy et al. synthesized thermally and mechanically stable PPSU/TiO2 hollow fiber membranes and used them for the first time to separate the macromolecular proteins to achieve 90% yield with minimal fouling [36].
From the literature survey, very few studies were reported on a combination of TiO2/PPSU MMMs in UF process. With this research gap, the present study deals with the preparation of TiO2 NPs loaded PPSU MMMs to treat GW through the UF process. Moreover, the motivation of this research work is to fill the gap by treating the GW using low-cost and eco-friendly methods at their homes to produce safe and reusable water for daily needs. As part of this work, the inorganic TiO2 NPs are synthesized by sol–gel method followed by incorporating into PPSU polymer solution by varying the concentrations. The in-situ synthesized membranes were characterized by various tools such as SEM, XRD, FTIR, contact angle, porosity, pore size, mechanical and biofouling properties to evaluate the polymer morphological nature, hydrophilicity, mechanical stability, and fouling resistance, respectively. The TiO2 MMMs at 3% (wt) were optimized with the scope of morphological studies, mechanical properties, permeation factor, and fouling performance. The GW was treated with the optimized MMM by considering the operating pressure and time on flux, percent rejection of TSS, turbidity, color, and bacterial removal under the cross-flow UF test skid.
Experimental
Materials
Titanium tetra-isopropoxide (TTIP) nano-filler was purchased from Spectrochem Pvt., Hyderabad, India. Isopropyl alcohol (IPA) and 1-methyl-2-pyrrolidone (NMP) solvents were procured from Avra Chemicals, Hyderabad, India. The non-woven polyester (PE) fabric support was obtained from Permionics Membranes Pvt., Vadodara, India. Polyphenylsulfone (PPSU), sodium chloride (NaCl), sodium hydroxide (NaOH), potassium dihydrogen orthophosphate (KH2PO4), and potassium chloride (KCl) were acquired from Finar Chemicals, Hyderabad, India. Sodium phosphate dibasic anhydrous (Na2HPO4), bovine serum albumin (BSA) for fouling studies was procured from Molychem, Hyderabad, India. Glassware such as measuring cylinders, petri dishes, burette, conical flasks, etc., were supplied by Borosil Limited, Mumbai, India. Laboratory experimental units comprising of a laminar airflow chamber, autoclave, digital weighing machine, vacuum pump, magnetic stirrer, chemical oxygen demand (COD) digester, colony counter, muffle furnace, and hot air oven were procured from Remi, Hyderabad, India. Ultrapure water (Type-II) was generated in the laboratory (MSL) itself by a cascaded reverse osmosis system.
Methods
Synthesis of TiO2 nanoparticles
TiO2 nanoparticles were synthesized by sol–gel method using Type-II water, IPA, and TTIP as a precursor. In this method, 15 mL of measured TTIP was added slowly to a conical flask containing 100 mL of IPA and stirred for 10 min at 150 rpm using a magnetic stirrer. 10 mL of Type-II water was added drop-wise into the obtained solution using the burette by continuous stirring for 2 h. The mixed solution was stored in the dark place for 24 h without any disturbance, and allowed to form a white color gel at the bottom of the flask. The supernatant layer was separated from the bottom layer through vacuum filtration. The gel was washed thoroughly for 10 min with 500 mL of Type-II water to remove excess impurities, followed by drying at 80 °C using a hot air oven for 24 h. Calcination of the dried TiO2 NPs at 550 °C for 2 h was carried out with a ramping temperature of 5 °C for a minute in a muffle furnace. The synthesis of the TiO2 NPs procedure and reaction mechanism is pictorially depicted in Fig. 1. From the reaction mechanism of TiO2 NPs, the TTIP solid particles in the liquid was transformed into particulate networks to form the gels. Subsequently, the reaction was followed in two steps, i.e., hydrolysis and condensation. In hydrolysis processes, TTIP precursor reacted with four water molecules and produced titanium isopropoxide complex as an intermediate, which was unstable and further converted into tetra hydroxide (TiOH4) [7]. The resultant TiOH4 molecule reacted with another mole of titanium hydroxide, by undergoing condensation in alcoxolation followed by oxolation at 550 °C. This resulted in formation of TiO2 NPs by eliminating the water molecules [4]. All these comprehensive steps constituted the calcination process.
Synthesis of ultrafiltration membrane by doping TiO2
Initially, 18% (wt) of PPSU polymer solution was prepared by dissolving the 18 g of PPSU in 82 g of NMP solvent. In addition to that, various compositions of TiO2, i.e., 1, 2, 3, 4, and 5% (wt) were prepared by dissolving each in NMP solvent corresponding to polymer weight. The uniform distribution of NPs in the solvent was conducted by sonication for 3 h. The weighed PPSU was added slowly to the NPs solution by vigorous stirring for 24 h to form a homogeneous solution, which is further sonicated for 1 h to attain the uniform dispersion of NPs in the polymer solution. The obtained dope solution was completely bubble-free and honey-like viscous. Table 1 provides the detailed description of the synthesized, pristine, and TiO2 (1, 2, 3, 4, and 5% by wt) loaded PPSU membranes by phase inversion technique.
Figure 2 represents the procedure for indigenously synthesized TiO2-loaded PPSU UF membrane. The prepared dope solution was poured onto the PE support fixed on the glass plate and placed over the casting machine. The desired thickness of the membrane was adjusted by a movable metallic bar of the machine. After casting, the membrane was immersed in a coagulation bath which consisted of Type-II water as a non-solvent to obtain a desired porous membrane. The resultant membrane was soaked in a coagulation bath for 24 h, followed by drying at room temperature (25 ± 2 °C) to remove excess solvent/water from the membrane surface [6].
Preparation of BSA solution
A PBS solution was prepared at 10 mM by dissolving 0.2 g of KCl, 0.24 g of KH2PO4, 1.42 g of Na2HPO4, and 8 g of NaCl in 800 mL of Type-II water. The solution pH was adjusted by adding 0.5 M NaOH and made up to 1 L. The 1000 ppm of BSA solution was prepared by dissolving 1 g of BSA in 1000 mL of PBS solution [5, 39]. The obtained solution was used to study the membrane fouling performance in terms of flux and rejection of the BSA solution.
Characterization of nanoparticles and membranes
Scanning electron microscope (SEM)
SEM analysis was performed by a Jeol JSM-6500F model, Japan, to study the morphologies of the NPs, surface, and cross-sectional images of synthesized pristine and MMMs. Before subjection to the SEM, the membrane was fractured under a liquid nitrogen environment and coated with a thin gold layer on top of the membrane and NPs to improve the sample’s conductive nature. The morphological images of the samples received through SEM were highly reliable and provided accurate results, i.e., nanoparticle distribution on the membrane surface and nature of the films.
Fourier transform infrared spectroscopy (FTIR)
The functional groups present in the polymer before and after NPs incorporation were studied by FTIR analysis across the wavenumber range of 400–4000 cm−1 using the KBr pellet method. The instrument procured from Nicolet-740, Perkin-Elmer-283B, MA, USA, was used to improve the signal-to-noise ratios and increase the quality of the spectra under a resolution of 4 cm−1 at an incident laser power of 1 mW.
X-ray diffraction (XRD)
An X-ray diffractometer was used to investigate the nanoparticles and membranes (model Siemens D5000, USA) by the monochromatic Cu-Kα radiation method. All samples were analyzed in a continuous scan mode using Bragg's equation, by varying the 2 \(\uptheta\) values from 2–80° to determine the amorphous/crystalline properties. The Debye–Scherrer equation was used to calculate the TiO2 nanoparticle size based on the XRD data [40].
where the particle size and dimensionless shape factor (0.9) are D and K, respectively, the wavelength of X-ray is 0.154 nm (λ), β is FWHM (0.4867°), and θ is Bragg’s angle (12.62°).
Porosity and pore size
The membrane porosity represents the total void volume present within the matrix, commonly defined as the ratio of pore volume to the membrane total volume. The porosity of the membrane was measured in a gravimetric pat and weigh method. The membranes, by 3 × 3 cm, were immersed in Type-II water for 24 h and weighed immediately after moping the superficial water with filter paper. The wet membrane was placed in a hot air oven at 80 °C for 24 h to dry and then noted the final weight.
The membrane's porosity was calculated using the following equation [41].
where, the membrane porosity is ε (%), Ww and Wd are the membranes wet and dry weights in g, ρw is the density of pure water (0.998 g/cm3), A and δ are the active area (9 cm2), and thickness (0.019 cm) of the membrane, respectively.
The water flow rate method was used to measure the membrane mean pore diameter, and the total mean pore diameter was calculated through Guerout-Elford-Ferry equation [42].
where I is the thickness of the membrane (0.00019 m), η is the viscosity of water (8.9 × 10–4 Pa.s), Q is the permeate flow rate (m3/s), and ΔP is the transmembrane pressure (196,133 Pa).
Hydrophilicity of membranes
The contact angle of the synthesized membrane was measured using a sessile drop method through a contact angle system with IL4201, Kruss GMBH, Germany. Initially, a 2 × 2 cm flat membrane was placed under the needle of the contact angle measuring system, where the water droplet loons on the homogeneous membrane surface and create an angle between the water and membrane. The experiment was repeated five times to reduce the error and find the accurate value, which was found to be ± 0.5.
Mechanical properties of membranes
The membrane mechanical properties are evident by the stress and strain behavior of the polymer matrix. A universal tensile testing machine was used to measure the pristine and TiO2 incorporated PPSU membranes mechanical properties with Shimadzu Model: AGS-10 kNG at room temperature (25 ± 2 °C). A cross-head speed of 5 mm/min and a static load of 1 kN was provided to measure the tensile strength and elongation-at-break (%) of the membrane samples. These samples were placed in the system with a width of 10 mm, a thickness of 0.03 mm, and a length of 80 mm. To reduce the error of the analytical method, the analysis was repeated three times to provide accurate values, where the error was found to be ± 0.1.
Description of UF experimental setup
The schematic representation of the process flow diagram and the photography of the UF experimental setup is shown in Fig. 3. A laboratory typical cross-flow filtration flat sheet UF membrane test skid consisted of a feed tank, permeate tank, low-pressure pump, membrane holder, pressure gauge, valves, and joints, etc. The 2.5 L feed tank contained 1000 mL of the feed solution, which fed the membrane housing through a feed line using a diaphragm pump of 300 gallons per day (GPD) capacity. The membrane housing held the flat sheet membrane in a circular shape of 10.7 cm diameter with an effective filtration area of 0.009 m2, where the hold-up volume of the module was around 10 mL. Membrane housing comprised of a feed stream as an inlet, permeation, and reject streams through outlets. The pressure gauge was directly connected to the reject line to measure the applied pressure on the membrane and it was controlled by a restricting needle valve. The reject line was sent back to the feed tank, and pure water was collected through the permeate line. The streamlines interconnected with the L and T shape joints at desired places.
Experimental procedure
A typical cross-flow filtration experiment was conducted using laboratory flat sheet membrane test skid to determine the membrane performance and GW treatment. Initially, the Type-II water circulates throughout the system to identify the leakages. A 1000 mL of GW was fed through a membrane module in a cross-flow manner using a low-pressure diaphragm pump. The pressure was applied to the membrane by varying the reject flow using a control needle valve. The desired pressure was measured with a pressure gauge and maintained constantly throughout the experiment without any fluctuation. The permeate was collected at regular time intervals from a continuous process, whereas the retentate stream was sent back to the feed tank in the recycle mode. The physicochemical parameters of the feed, permeate, and rejects in terms of total suspended solids (TSS), turbidity, color, conductivity, hardness, chemical oxygen demand (COD), and bacterial contamination, etc., were measured to evaluate the membrane performance.
Analytical procedures
Various instrumental techniques were used to analyze the TDS, conductivity, pH, color, turbidity, COD, hardness, and TSS of feed, permeate, and reject samples. Total dissolved solids (TDS) present in the samples were analyzed by a digital TDS meter (TDS-3, HM Digital, Hyderabad, India) within a limited detection range of 0–5000 ppm. The laboratory conductivity (DCM 900) and pH meters (DPH-500) were used to measure the detection levels ranging from 0 to 200 mS/cm and 0 to 12 pH, respectively, procured from Global Electronics, Hyderabad, India. The color and turbidity of samples were measured using DR 300 pocket colorimeter obtained from HACH, Hyderabad, India. The quantitative analysis of the sample’s hardness was done by a laboratory hardness test kit, supplied by ORLAB, Hyderabad, India. The COD of samples determined by Remi digester, Hyderabad, India, using the American Public Health Association (APHA) 1998 method.
The TSS of organic and inorganic particles with more than 2 μ particle size present in the samples were filtered through glass fibers and dried at 105 °C, followed by the sample's final weight measured in mg/L.
UV–Vis spectrophotometer
The UV–vis spectrophotometer of LAB India, Model UV 3092, was used to measure BSA unknown concentration in the permeate solutions at 280 nm wavelength. While conducting the analysis, clean cuvettes were used to measure the accurate concentration levels of the samples. A typical calibration curve with a known concentration of BSA values was used as a reference by considering the absorbance values. In addition to this, the unknown concentration of the permeate samples was also determined from the calibration curve.
Analytical description for fouling performance
The experiments were conducted in a cross-flow manner with pure water and BSA solution to study the permeation factor of pristine and MMMs. Initially, the pure water was passed through the membrane at the desired pressure (2 bar) and a room temperature of 25 ± 2 °C for about 30 min. The declination of flux was observed after 30 min of the experimental run and attained the steady-state. Further, the experiments were carried out using 1000 ppm of BSA solution by maintaining the same parameters for about 3 h. Finally, the membrane was washed thoroughly with Type-II water for 2 h until the complete removal of BSA solution on the surface. The BSA concentration and permeate samples were analyzed using a UV–vis spectrophotometer.
Bacterial analysis
The EMB agar plate method was used to examine the E. coli bacterial analysis in the samples. EMB agar at 18 g was dissolved in 500 mL of Type-II water and mixed vigorously to form a homogeneous solution. The prepared solution was autoclaved at 15 lbs pressure and maintained the temperature of 120 °C for about 15 min along with pipette tips and petri dishes. The obtained medium was poured into the petri dishes in a laminar airflow chamber under sterile environmental conditions for further solidification [43]. Each sample, at 1 mL, was introduced into a solidified agar plate by “pour plate” method. The prepared sample plates were wrapped with parafilm and incubated at 37 ± 2 °C for about 18–24 h. After the incubation period, the samples were evaluated for the contamination levels by determination of total colony count besides quantitative analysis through the naked eye.
Mathematical tools for fouling performances
The resistance of membranes and percentage of flux recovery (FR) were calculated to estimate the fouling performance [36]:
where JBF and JAF represent the PWF of the membrane before and after fouling, respectively.
The flux declination was determined using the resistance-in-series model, where the water flux was a function of transmembrane pressure (Δ \(p\)), viscosity (μ), and total membrane resistance (Rt) [36].
Total membrane resistances (Rt) values can be calculated from experimental data using the provided Eqs. 6–9 [36, 44]:
where Δp is transmembrane pressure (196,133 Pa), μ is the viscosity of permeate (8.9 × 10–4 Pa.s), JAF is pure water flux after cleaning and JF is BSA filtration flux.
Calculation of BSA rejection
The percentage rejection (R) of BSA was calculated to assess the synthesized membrane performance by considering the following equation [6]:
Here, the feed and permeate concentrations are Cf and Cp, respectively.
The COD present in the sample was estimated using the following equation [45].
where, B and S are the amounts of FAS consumed for blank and feed samples respectively, N is the normality of FAS, V is the total volume of a sample in mL, DF is dilution factor, the equivalent weight of oxygen per L is in the form of multiplication factor i.e., 8000.
The TSS was calculated using Eq. 12 [46].
where, B and A are the filter paper weights before and after filtration (g), and C is the total sample volume (100 mL).
Results and discussion
Characterization of TiO2 nanoparticles by SEM and XRD
The surface morphology of indigenously synthesized NPs and XRD patterns are illustrated in Fig. 4. The synthesized NPs at 5 µm magnification were observed to be spherical in shape [47]. The primary TiO2 particles can accelerate crystal growth, which is further confirmed by the XRD spectrum. The crystalline nature of the peaks are lying at 25.25°, 37.80°, 47.90°, 53.90°, and 62.56° on 2θ scale of spectrum. These patterns confirmed that the TiO2 structure was an anatase form of crystalline [47, 48] with an average particle size of 16.72 nm.
Effect of TiO2 concentration on the membrane morphology
The surface and cross-sectional morphologies of PPSU T0–T5 membranes are provided in Fig. 5. The surface morphology of the PPSU-T0 (pristine) membrane appears to be a uniform distribution of ultra-pores throughout the membrane, whereas the cross-sectional view of PPSU-T0 represents an explicit penetration of the PPSU layer into PE support. From the PPSU T1 to T3 surface morphologies, it can be seen that the spherical shape of TiO2 NPs with anatase phase in the form of dots was randomly distributed throughout the membrane surface without any agglomeration at a 20 µm magnifications. On the other hand, in PPSU T4 and T5, these NPs dispersion was uneven by the formation of large agglomerates on the membrane surface. The reason for this graphical observation is due to the hydroxyl group of TiO2 interactions with the sulfone group of polymer in the matrix, which prevents the NPs aggregation up to 3% (wt) dosage of TiO2. After which the particles overlap each other and form a large agglomerate on the surface, subsequently weaken the interaction with the hydrophobic polymer [49]. Moreover, the higher concentration of nanoparticles in the polymer causes surface roughness which leads to the brittleness of the membrane, due to its high crystallinity [50].
The cross-sectional morphologies of PPSU T0–T3 represent the finger-like macrovoids pore structure, this may be due to the strong interaction of the hydrophilic nature of TiO2 with water molecules during phase inversion [49]. As a result, the formation of more fingers-like macrovoid growth found in the substrate can lead to a subsequent reduction in skin layer thickness. However, these microvoids were entirely blocked by NPs aggregation at high concentrations of PPSU T4 and T5, which showed a negative impact on polymer structure. The overall surface and cross-sectional morphologies reveal that the TiO2 NPs play a key role in membrane synthesis. Other characterization studies also can further confirm the SEM observations. Therefore, TiO2 NPs at 3% (wt) of uniformly dispersed in the membrane formed a clear finger-like structure than other compositions [50].
Effect of nanoparticles concentration on membranes porosity and pore size
The porosity and pore size of the pristine and MMMs calculated from Eqs. 2 and 3 are summarized in Table 2. The PPSU-T0 membrane shows lower porosity and pore size values of 33.99 and 19.05 nm. The incorporation of TiO2 NPs in a polymer matrix increases the porosity and pore size to 40.20 and 31.14 nm for PPSU-T3, after that, gradually decreases to 34.92 and 19.08 nm for PPSU-T5, respectively. The hydrophilic nature of TiO2 enhances the porosity and pore size on the membrane surface from 1 to 3% (wt), due to the higher interaction between the solvent and non-solvent by phase inversion process, which induces the growth of macrovoids [50].
These values decreased significantly at higher concentrations due to the pore blockage by particles overlapping on the surface during the membrane synthesis [51]. Moreover, the polymer solution viscosity at higher concentrations can also reduce the number of pores, leading to a significant decrease in porosity and pore size of the membrane by the eventual formation of a sponge-like structure [24]. Compared to the pristine membrane the optimized nanocomposite exhibited wider and long finger-like structures.
Effect of TiO2 concentration on FTIR spectra
FTIR spectra for pure TiO2 NPs and PPSU-T0 to T5 membranes are provided in Figure S1. The spectrum of TiO2 NPs exhibited three bandwidths. The stretching and bending vibration frequency of the hydroxyl groups of TiO2 broadband which correspond to the peaks at 3421.83–3589.85 cm−1. A second intense peak is observed at around 1608.34–1672.69 cm−1, which correlates to the bending vibrations of Ti–OH. Another important peak of Ti–O bending frequency is seen at 1342.50 cm−1 [52, 53]. The broad stretching band of Ti–O–Ti is found to be in the range of 569.02–516.94 cm−1, and the lattice vibration at 1440.87 cm−1 of TiO2 NPs, respectively [50]. In contrast, the spectrum of the pristine PPSU showed symmetrical and asymmetrical stretching frequencies at 1321.28 and 1155.40 cm−1 that can be attributed to the sulfone groups (SO2) of the polymer, with stretching vibrations seen at 1579.75 and 1483.31 cm−1, along with either linkage of C–O stretching and bending vibrations that can be assigned to peaks at 1247.99 cm−1 and 1105.25 cm−1, respectively. Moreover, the SO2 scissor deformation peaks of S=O and strong respectively. The aromatic C–C and C–S stretching vibration frequencies are obtained in the range of 719.47–609.53 cm−1 and 592.17 cm−1, respectively [53]. After the incorporation of TiO2 NPs into the PPSU membranes, shifting of these deformations along with stretching and bending vibration peaks can be observed, which means there are strong interactions between the NPs and the PPSU membranes. Furthermore, the deformation peak of S=O is slowly varied from PPSU-T0 to T5, where the peak shifts across the wavenumber range of 719.47–555.52 cm−1, which indicates the formation of hydrogen bonding between the -OH groups of NPs and S=O groups of PPSU. Among those, the PPSU T3 membrane exhibited a more intense peak at 555.52 cm−1 [50]. This behavior is attributed to the transmission interactions of intramolecular hydrogen bonding of the O–H group of NPs with the S=O group of PPSU, which is a strong polar group in nature [54].
Hence, the broad stronger peaks of PPSU-T0 to T5 in the range of 3423.76–3421.83 cm−1 represent the hydrophilicity of the nanocomposites, which is improved upon TiO2 loading [31].
X-ray diffraction analysis
The pristine (PPSU-T0) membrane shows a semi-crystalline nature with sharp characteristic peaks around 12.33º, 18.23º, 23.33º, 26.38º, and 29.49º, while the amorphous nature is noticed in the range of 35–80º on the 2θ scale.
From Fig. 6, the shifting of peaks is observed from pristine to 17.39º, 22.38º, 26.30º, 38.60º, 48.40º, and 54.20º in PPSU-T3. The reason behind this is the possible interaction between the hydroxyl groups of TiO2 with sulfonated groups of PPSU on the membrane surface. Further, with an increase in TiO2 NPs concentration (4–5% by wt) in the membrane matrix, the diffraction peaks gradually become wider without disturbing the crystalline nature of the TiO2 [11].
Effect of TiO2 concentration on membrane contact angle
The contact angle mainly depends on the nature, size, and concentration of the NPs in the MMMs. The pristine and TiO2-loaded membranes (PPSU-T1 to T5) contact angles were measured by a goniometer by replicating five times. The strong alliance between the NPs and water molecules causes a reduction in contact angle from 62.80 to 40.4°(Error ± 0.5) for PPSU-T0 to T5, respectively. From Table 2 it can be observed that the lowest contact angle of PPSU-T5 shows higher hydrophilicity among all the membranes. Hence, the increasing TiO2 NPs concentration in the polymer solution leads to a decrease in the contact angle due to the enhanced hydrophilicity of the membrane, which has a great potential in permeation and the separation process along with close relation in morphology [50].
Effect of TiO2 NPs concentration on mechanical properties
TiO2 concentration on tensile strength and percentage elongation-at-break of PPSU-T0 to T5 are tabulated in Table 2. The pristine PPSU contains a tensile strength of 11.6 MPa from which it was gradually increased to 31.23 MPa by raising the NPs concentrations (1–5% by wt). The outstanding performance was achieved by the reinforcement effect of membrane rigidity and uniform dispersion of TiO2 on the membrane surface, which improved the load transfer [7, 50]. A similar trend of percentage elongation-at-break for PPSU-T0 to T5 was found to be 3.87–12.07, due to the typical behavior of nanofillers reinforced composite matrix, which improves the flexibility. Therefore to break down the MMMs, higher energy is required compared to the pristine membrane, inferring greater mechanical stability that encourages a wider spectrum of applications [50, 55].
Effect of TiO2 NPs concentration on pure water flux (PWF)
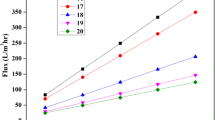
The PWF played a crucial role in membrane performance, which was influenced by various factors such as membrane morphology, porosity, pore size, hydrophilicity, functional layer thickness, etc. In the present study, the permeability test was performed using pristine and MMMs with Type-II water by varying the operating pressures. From Fig. 7, it can be observed that the PWF is consistently increased with increasing the pressure from 1 to 6 bar for PPSU-T0 toT3, which is due to the driving force on the membrane surface, causes the transport phenomena of water molecules on the permeate side [56]. In addition to this, the incorporation of TiO2 NPs into the membrane matrix has resulted in morphological changes and higher hydrophilicity, which affects the permeation factor [44].
From the results, up to 3% (by wt) of TiO2 NPs loaded membranes increase the hydrophilicity and quickly absorb the water molecules into the matrix followed by permeation. Thus, the enhancement of permeability has occurred. The PWF of PPSU-T4 and T5 membranes show lower permeate intension than PPSU-T3 and higher than PPSU-T0 at various operating pressures. Therefore, with the increase in TiO2 concentration, the polymer solution becomes highly viscous and leads to the enhancement of skin layer thickness, which has a negative effect on the membrane surface. This is clearly evident in Fig. 5 cross-sectional morphologies of PPSU T4 and T5 [49]. Moreover, the TiO2 higher load in the composite membrane restricted the spherulite formation and led to reducing the porosity and pore size in the membrane matrix [1, 24].
Effect of NP concentration on BSA flux, rejection and flux recovery (FR %)
Table 3 represents TiO2 nanofillers influence on the membrane surface, which was evaluated from BSA flux, rejection, and % FR. As the TiO2 load increases in membrane composition from PPSU-T0 to T3, subsequently, the BSA flux is increased from 12.83 to 32.46 L/m2 h. After that, a gradual decrease is observed in PPSU-T4 (24.19 L/m2 h) and PPSU-T5 (14.08 L/m2 h). Similarly, the same trend has been followed in BSA rejection, while increasing the TiO2 in PPSU membrane up to 3% (by wt), the percentage rejection increased from 89.56 (PPSU-T0) to 99.43 (PPSU-T3).
The reason was that the TiO2 catalyst firmly holds the water molecules, which forms a hydrated layer and constrains the protein absorption along with other fouling agents on the PPSU composite membrane [39, 40]. Further, the protein rejection through the PPSU membrane was by electrostatic interaction between the charged hydroxyl group of nanoparticles on the membrane surface with solute molecules [5, 6]. The higher percentage of nano-filler accumulation in T4 and T5 affects the membrane thickness, which leads to a decrease in the flux and rejection [36]. Therefore, the % FR of the PPSU-T3 membrane was higher than pristine and other composites. With this background, the % FR observed from Table 3 assured that the membrane life span was enhanced by cleaning with pure water and confirmed by obtaining the 89.50% of FR for PPSU-T3. Among all-composite membranes, 3% (by wt) TiO2-loaded membrane indicated a weak interaction with proteins and exhibited a significant rejection of BSA flux, and rejection with a high % FR.
Effect of TiO2 concentration on membrane fouling performance
The membrane performance was characterized quantitatively by studying the membrane resistance towards foulants. The total filtration resistance (Rt) can be calculated from Eqs. 5–9 by considering the intrinsic (Rm), reversible (Rr), and irreversible (Rir) membrane resistances [36]. The incorporation of TiO2 gradually increased the antifouling property of the PPSU membranes, i.e., PPSU T0 to T5. From Fig. 8, the Rm, Rir, and Rr each resistance of PPSU-T1 to T3 was found to be low, which could be the positive effect of low surface free energy and the hydrophilicity of the membrane along with roughness compared to the pristine. The PPSU MMMs were in strong bonding with negatively charged hydroxyl groups of TiO2, which plays a significant role in antifouling ability. Besides, the resistance gradually increased for PPSU-T4 and T5, which was due to the excessive TiO2 incorporation into membranes leading to pore plugging and providing extra hydraulic resistance [38].
Excessive loading of NPs forms an extensive agglomeration on the surface, which reduces the contact area of the hydroxyl group carried by TiO2 NPs and possibly decreases the repulsion between the protein and membrane surface [57]. From the experimental observations, the 3% (by wt) MMMs showed a higher antifouling property when compared to pristine and other composites due to the hydrolysis of TiO2 particles. This leads to membrane stability, which restricts the possible foulant interactions [36]. The enhancement of interfacial resistance by adding optimized hydrophilic additives to the membrane matrix caused the low-level attachment of the proteins with the membrane [5].
Optimization of TiO2 NP concentration
The optimization of NP in a composition is confirmed by studying the various compositions of MMMs characterization and performance of experimental results. The characterization tools such as SEM, XRD, FTIR, porosity, pore size, contact angle, and mechanical properties revealed that 3% (by wt) of TiO2-loaded membrane has shown higher performance than the pristine and other composites [50]. On the other hand, the experimental studies of PWF, BSA flux, % BSA rejection, and flux recovery of PPSU-T3 achieved 92.59 L/m2 h, 32.46 L/m2 h, 99.43%, and 89.50%, respectively. Moreover, PPSU-T3 has an excellent fouling resistance and water permeation ability than other composites. Based on the overall results, the PPSU-T3 was optimized and continued further experiments for purification of GW.
Effect of optimized NP on low flux declination
The degree of flux declination depends on the extensive fouling on the membrane surface. The experiments further continued using PPSU-T0 and PPSU-T3 to know the membrane performance based on the permeate flux. From the graphical representation (Figure S2), the pure water flux declined from 49.35 to 27.92 L/m2 h with respective time from 20 to 198 min for pristine PPSU membrane, whereas, for PPSU-T3, the negligible declination of flux was observed from 93.63 to 92.59 L/m2 h within 96 min. From these observations, the PPSU-T3 membrane water recovery was around 80% in 96 min, with a minimal flux declination compared to the pristine in 198 min.
This was due to the presence of charged inorganic fillers, which makes the membrane more hydrophilic and increases the pore tendency for free permeation of water molecules [44]. Moreover, TiO2 NPs having excellent antimicrobial and antifouling properties, improves the process efficiency and controlled biofouling for various membrane separation applications, especially in water and wastewater treatment [2, 5].
Experimental results for greywater treatment
Effect of applied feed pressure on average flux with constant water recovery
Usually, UF membranes operate at low pressure, which is sufficient to provide higher flux. Therefore, in the present study, the experiments were conducted at an operating pressure of 1–6 bar. The permeate flux mainly varies on the feed concentration and applied pressure of the membrane. Therefore, by increasing the pressure, the water molecules passed across the membrane faster than the solute molecules, which enhanced the percentage of rejection [56]. In this scenario, the effect of applied feed pressure on average flux was determined with a water recovery of 80%, after that the pressure fluctuations occurred. Hence, to overcome these limitations, the experiments were further carried out by maintaining 80% of water recovery. From Fig. 9, the average permeates flux drastically increased from 27.08 to 62.81 L/m2 h with increasing the applied pressure from 1 to 6 bar, respectively, in which 2 bar was optimized by taking into consideration the quality of permeate (product) [56]. Additionally, the flux enhancement was due to the hydrophilicity, higher porosity, and pore size of the nanocomposite, which allowed water molecules through the solution diffusion mechanism at an applied pressure, as supported by Esfahani et al. [58].
Effect of operating time on permeate flux and water recovery
The membrane operating time is an essential parameter in membrane performance. The effects of operating time on permeate flux and water recovery at an applied pressure of 2 bar depicted in Fig. 9, where the membrane flux decreased from 49.38 to 45.75 L/m2 h in 86 min of operating time and then reached a steady-state at 45.63 L/m2 h. From these results, a negligible declination of flux was observed at 2 bar. On the other hand, the percentage of water recovery simultaneously increased from 10 to 80%, by increasing the operating time from 22 min to 2 h and 35 min. The flux lowered with operating time, was due to the concentration polarization and rapid deposition of rejected solute molecules, which it gradually built up a cake layer structure on the membrane surface, and further restricts the solvent flow [59]. Additionally, the transmembrane pressure (TMP) increased on the membrane surface with time, causing a rise in hydraulic resistance [2]. To control the increasing TMP (≥ 6 bar) in the UF system by removal of the reversible fouling, the membrane had to be backwashed with Type-II water as follows; when the experiment was conducted until 120–150 min, the reversal of permeate flow was provided for about 30 s/cycle at an applied pressure of ≤ 1 bar, with a flow rate of 140 L/m2 h. In this study, one backwash cycle was enough to recover around 95% of flux through the UF membrane. Typically, The backwash frequency mainly varied with raw water quality, production time, and the organic contaminants present [35, 60]. Further, the study revealed that the hydrophilic NPs incorporated MMMs exhibited a negligible declination in flux [61]. Therefore, after each experimental run, the membrane was backwashed followed by mild chemical cleaning to remove the impurities and foulants on the surface, this helped to improve the membrane life span [60].
Water recovery vs. permeate flux in batch operation mode
The permeate flux is always interrelated to the water recovery, which depends on the membrane characteristics. The percentage of water recovery and flux has been studied at an optimized pressure in batch mode operation. As the flux declined linearly from 49.38 to 45.63 L/m2 h for 2 bar pressure, at this point the number of water molecules permeated through the hydrophilic membrane causeing a higher percentage of water recovery from the GW. At the same time, the permeate flux decreases due to the formation of scalants on the membrane surface and rejected the TSS, color, turbidity, hardness, and heavy salts to the feed tank through retentate stream under batch mode operation [15]. This argument was ably supported by the transport mechanism of PPSU-T3 membrane, as shown in Fig. 10, where the TiO2 NPs acted as catalysts for the permeation of pure water along with mono, bivalent ions. On the other hand, it rejected the bacteria, virus, color, turbidity, and high molecular weight components from GW and it was fed back to the feed tank. From the above explanation, when time proceeded, the increased concentration in the feed tank caused the declination of permeate flux and water recovery [43].
Evaluation of physicochemical parameters
Percentage rejection of TSS, turbidity, and color
The physical parameters such as TSS, turbidity, and color of the feed, permeate and reject at a different applied pressure of 1–6 bar, in terms of percentage of rejection was evaluated to understand the membrane performance. The TSS, turbidity, and color of the feed, permeate, and retentate of the present study demonstrated a significant variation. Initially, the TSS, turbidity, and color of the feed were measured as 226.2 mg/L, 112 FAU and 810 Pt–Co, respectively. After membrane treatment, these values decreased drastically by varying applied pressures in permeate samples. From the above experimental observations, the percentage of rejection of TSS was achieved 95.14, 94.92, 94.56, and 94.34% along with percentage of rejection of turbidity 95.54, 95.54, 95.54, and 94.64%, while the percentage of color rejection was 94.20, 91.48, 90.37, and 89.38% at an applied pressure of 1, 2, 4, and 6 bar, respectively. The TDS, COD, conductivity, and hardness were not affected on the membrane by varying pressures, as provided in Table 4. Moreover, the advantage of permeate samples was slightly in alkaline medium, i.e., around 8 pH can be used for other applications. These values can reduce to a minimal extent due to the membrane ultra-pore ranges by incorporating the TiO2 particle [56]. Among those, the maximum reduction of TSS, turbidity, and color obtained using the UF process at 2 bar can be observed from Fig. 11. The reason could be the oxidation properties of nanofillers present in the membrane matrix that shows a higher degradation of solute particulate matter [21]. Besides, after incorporating NPs, the pores on the membrane reached the UF region, which helps to retain and contribute to higher suspended solids, turbidity, and color rejection [56]. The photography of collected feed, clear permeate, and highly concentrated reject are depicted in Fig. 10. The membrane’s active surface layer has effectively removed maximum contaminants from the GW and allowed the clear and transparent permeate [58]. Therefore, an indigenously synthesized PPSU-T3 membrane has effectively removed the maximum percentage of TSS, turbidity, and color. In this context, the permeate water can be reused for further domestic uses, washing, gardening, and other industrial applications by reducing freshwater consumption [1, 2].
Bacterial contamination removal
Figure 12 depicts the bacterial contaminations in terms of colony-forming unit (CFU) of the feed, permeate, and reject samples treated by pristine and PPSU-T3 membranes. The resulting pristine and PPSU-T3 permeate samples showed nil contamination, while the pristine reject contamination level was observed to be 63 CFU, which is higher than that of the feed sample (46 CFU), indicating complete rejection of bacteria by the UF membrane [62]. In the case of PPSU-T3, the NPs degrade the bacteria in the reject stream through the photocatalytic nature of TiO2, leading to a minimal value of 6 CFU, which is negligible and much lesser than the feed. The results implied that TiO2 MMMs played a pivotal role in the bacterial removal capability [50], which was due to the higher interaction between bacteria and nanofillers [63]. The 3% (by wt) TiO2 content incorporated film showed effective microbial growth inhibition, reported by Cijun et al. [64]. As expected, these TiO2 incorporated PPSU MMMs, used in the UF process, are highly significant and are well in agreement with literature data which can be further recommended to remove bacterial and microbial contaminations from wastewater.
Conclusion
The indigenously synthesized TiO2 NPs and pristine along with 1–5% (by wt) NPs loaded PPSU membranes were prepared by sol–gel and phase inversion methods, respectively. The NPs and membranes were characterized by various tools, which revealed the spherical shape of NPs and the random distribution of NPs without any agglomeration on the membrane surface with SEM. The water Ti–OH groups, and the formation of new hydrogen bonding between Ti–OH with S=O groups were exhibited by FTIR, while crystalline nature of NPs and well-defined semi-crystalline nature of the MMMs were demonstrated by XRD, respectively. The PPSU contact angle enhanced from 62.8 to 40.4°, with the outstanding mechanical tensile strength of 11.6–31.23 MPa and 3.87–12.07% elongation-at-break. Among all the composites, the PPSU-T3 membrane exhibited a low fouling tendency with a maximum of 31.14 nm pore size, 40.20% porosity, 92.59 L/m2 h pure water flux, 89.59% flux recovery, and 99.43% BSA rejection. From the experimental and characterization results, the PPSU-T3 membrane demonstrated higher efficiency than other composites. Further, the GW was successfully treated with an optimized PPSU-T3-UF system through which an average flux of 45.63 L/m2 h, efficient removal of TSS 95.14%, turbidity 95.54%, color 94.20%, and E. coli bacteria with an 80% water recovery at an applied pressure of 2 bar was accomplished. The PPSU-T3 exhibited high separation with excellent structural and mechanical properties among the other composites. From the overall experimental observations, it is evident that the TiO2 Nps acted as a catalyst on a membrane surface and formed a strong chemical interaction with sulfone groups of PPSU, which increased the rigidity of the polymer. Furthermore, it can also be concluded that the MMMs are far more suitable for surface water purification, wastewater treatment, and protein purification, etc. These comprehensive results can be further extended to the pilot level and efficient usage of prominent membrane technology for various applications, especially in wastewater treatment. The resultant reclamation water can be used as an alternative to freshwater consumption in the water-scarce regions.
References
Lu H, Wang J, Stoller M, Wang T, Bao Y, Hao H (2016) An overview of nanomaterials for water and wastewater treatment. Adv Mater Sci Eng 2016:10
Bora T, Dutta J (2014) Applications of nanotechnology in wastewater treatment—a review. J Nanosci Nanotechnol 14:613–626
Gupta A, Srivastava P, Bahadur L, Amalnerkar DP, Chauhan R (2015) Comparison of physical and electrochemical properties of ZnO prepared via different surfactant-assisted precipitation routes. Appl Nanosci 5:787–794
Waghmode MS, Gunjal AB, Mulla JA, Patil NN, Nawani NN (2019) Studies on the titanium dioxide nanoparticles: biosynthesis, applications, and remediation. SN Appl Sci 1:1–9
Ying TC, Ebrahim M, Abdul WM, Abdelbaki B, Daniel J, Nidal H (2017) Development of polysulfone-nanohybrid membranes using ZnO-GO composite for enhanced antifouling and antibacterial control. Desalination 402:123–132
Abdel-Karim A, Leaper S, Alberto M, Vijayaraghavan A, Fan X, Holmes SM, Souaya ER, Badawy MI, Gorgojo P (2018) High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. Chem Eng 334:789–799
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications, and toxicities. Arab J Chem 12:908–931
Haider A, Ijaz M, Imran M, Naz M, Majeed H, Khan JA, Ali MM, Ikram M (2020) Enhanced bactericidal action and dye degradation of spicy roots’ extract-incorporated fine-tuned metal oxide nanoparticles. Appl Nanosci 10:1095–1104
Salama A, Mohamed A, Aboamera NM, Osman TA, Khattab A (2018) Photocatalytic degradation of organic dyes using composite nanofibers under UV irradiation. Appl Nanosci 8:155–161
Li X, Fang X, Pang R, Li J, Sun X, Shen J, Han W, Wang L (2014) Self-assembly of TiO2 nanoparticles around the pores of PES ultrafiltration membrane for mitigating organic fouling. J Membr Sci 467:226–235
Kadi MW, Mohamed RM (2020) Pt-decorated CuO nanosheets and their application in the visible light photocatalytic water-splitting reaction. Appl Nanosci 10:4291–4298
Lin F (2006) Preparation and characterization of polymer TiO2 nanocomposites via in-situ polymerization. University of Waterloo, Ontario
Arcadio S, Arman B, Ruixin Z, Patricia L, Jesus MA, Jeonghwan K, Bart VB (2011) Effect of nanoparticle aggregation at low concentrations of TiO2 on the hydrophilicity, morphology, and fouling resistance of PES–TiO2 membranes. J Colloid Interf Sci 363:540–555
Ozkal CB, Meric S (2021) Photocatalytic bacteria inactivation by TiO2-Ag-based photocatalysts and the effect on antibiotic resistance profile. Curr Anal Chem 17:98–106
Sushmitha MB, Chanakya HN, Khuntia HK (2019) Efficient greywater treatment and reuse options for India—a review. Waste Water Recycl Manag 3:143–149
Edwin GA, Gopalsamy P, Muthu N (2014) Characterization of domestic gray water from point source to determine the potential for urban residential reuse: a short review. Appl Water Sci 4:39–49
Nagandran S, Goh PS, Ismail AF, Wong TW, Wan Dagang WRZB (2020) The recent progress in modification of polymeric membranes using organic macromolecules for water treatment. Symmetry 12:239–277
Abdel-Karim A, El-Naggar ME, Radwan EK, Mohamed IM, Azaam M, Kenawy ER (2021) High-performance mixed-matrix membranes enabled by organically/inorganic modified montmorillonite for the treatment of hazardous textile wastewater. Chem Eng J 405:126964
Vetrivel S, Saraswathi MSSA, Rana D, Divya K, Nagendran A (2021) Cellulose acetate ultrafiltration membranes customized with copper oxide nanoparticles for efficient separation with antifouling behavior. J Appl Polym Sci 138:49867
Norman AGF, Li N, Winston Ho WS, Matsuura T (2008) Advanced membrane technology and applications. Wiley, Hoboken
Kim J, Vander B (2010) The use of nanoparticles in polymeric and ceramic membrane structures: review of manufacturing procedures and performance improvement for water treatment. Environ Pollut 158:2335–2349
Lee A, Elam JW, Darling SB (2016) Membrane materials for water purification: design, development, and application. Environ Sci Water Res Technol 2:17–42
Wu G, Gan S, Cui L, Xu Y (2008) Preparation and characterization of PES/TiO2 composite membranes. Appl Surf Sci 254:7080–7086
Jing FL, Zhen LX, Hu Y, Li YY, Min L (2009) Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane. Appl Surf Sci 255:4725–4732
Vatanpour V, Madaeni SS, Khataee AR, Salehi E, Zinadini S, Ahmadi Monfared H (2012) TiO2 embedded mixed matrix PES nanocomposite membranes: influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 292:19–29
Teow YH, Ooi BS, Ahmad AL, Lim JK (2021) Investigation of anti-fouling and UV-cleaning properties of PVDF/TiO2 mixed-matrix membrane for humic acid removal. Membranes 11:16
Zhang F, Zhang W, Yu Y, Deng B, Li J, Jin J (2013) Sol-gel preparation of PAA-g-PVDF/TiO2 nanocomposite hollow fiber membranes with extremely high water flux and improved antifouling property. J Membr Sci 432:25–32
Rabiee H, Davood Abadi Farahani MH, Vatanpour V (2014) Preparation and characterization of emulsion poly(vinyl chloride) (EPVC)/TiO2 nanocomposite ultrafiltration membrane. J Membr Sci 472:185–193
Razmjou A, Mansouri J, Chen V (2011) The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure, and fouling performance of PES ultrafiltration membranes. J Membr Sci 378:73–84
Yang Y, Wang P, Zheng Q (2005) Preparation and properties of polysulfone/TiO2 composite ultrafiltration membrane. J Polym Sci 44:879–887
Xiao S, Yu S, Yan L, Liu Y, Tan X (2017) Preparation and properties of PPSU/GO mixed matrix membrane. Chin J Chem Eng 25:408–414
Sridhar S (2019) Membrane technology: sustainable solutions in water, health, energy, and environmental sectors, 1st edn. Taylor & Francis Group, Oxford
Amoudi AI, Lovitt RW (2007) Fouling strategies, and the cleaning system of NF membranes and factors affecting cleaning efficiency. J Membr Sci 303:4–28
Dehghankar M, Mohammadi T, Moghadam MT, Tofighy MA (2021) Metal-organic framework/zeolite nanocrystal/polyvinylidene fluoride composite ultrafiltration membranes with flux/antifouling advantages. Mater Chem Phys 260:124128
Mansouri J, Harrisson S, Chen V (2010) Strategies for controlling biofouling in membrane filtration systems: challenges and opportunities. J Mater Chem 20:4567–4586
Lawrence Arockiasamy D, Alhoshan D, Alam M, Muthumareeswaran MR, Figoli A, Arun Kumar S (2017) Separation of proteins and antifouling properties of polyphenylsulfone based mixed matrix hollow fiber membranes. Sep Purif Technol 174:529–543
Nasirinezhad M, Ghaffarian SR, Tohidian M (2021) Eco-friendly polyelectrolyte nanocomposite membranes based on chitosan and sulfonated chitin nanowhiskers for fuel cell applications. Iran Polym J 30:355–367
Kumar M, Gholamvand Z, Morrissey A, Nolan K, Ulbricht M, Lawler J (2016) Preparation and characterization of low fouling novel hybrid ultrafiltration membranes based on the blends of GO/TiO2 nanocomposite and polysulfone for humic acid removal. J Membr Sci 506:38–49
Kang Y, Obaid M, Jang J, Ham MH, Kim IS (2018) Novel sulfonated graphene oxide incorporated polysulfone nanocomposite membranes for enhanced-performance in ultrafiltration process. Chemosphere 207:581–589
Chandrashekhar Nayak M, Isloor AM, Moslehyani A, Ismail N, Ismail AF (2018) Fabrication of novel PPSU/ZSM-5 ultrafiltration hollow fiber membranes for separation of proteins and hazardous reactive dyes. J Taiwan Inst Chem Eng 82:342–350
Berta D, Nuria R, Antonio M, Arcadio S, Jose APM, Jesus A, Roberto R (2017) Fouling and biofouling resistance of metal-doped mesostructured silica/polyethersulfone ultrafiltration membranes. J Membr Sci 526:252–263
Nyamukamba P, Moloto MJ, Mungondori H (2019) Visible light-active CdS/TiO2 hybrid nanoparticles immobilized on polyacrylonitrile membranes for the photodegradation of dyes in water. J Nanotechnol. https://doi.org/10.1155/2019/5135618
Vani B, Chandra Sekhar S, Sahu N, Sridhar S (2021) Development of a UV coupled indigenous hydrophilized polyamide membrane system for enhanced shelf life of mature coconut water. J Food Process Eng 44:e13636
Goh PS, Lau WJ, Othman MH, Ismail AF (2018) Membrane fouling in desalination and its mitigation strategies. Desalination 425:130–155
Chandrasekhar SS, Vaishnavi D, Sahu N, Sridhar S (2020) Design of an integrated membrane bioreactor process for effective and environmentally safe treatment of highly complex coffee industrial effluent. J Water Process Eng 37:101436
Branigan J (2013) Development of a field test for total suspended solids analysis. MS Thesis, civil engineering theses, dissertations, and student research, University of Nebraska, Lincoln, p 68
Rzaij JM, Abbas AM (2020) Review on TiO2 thin film as a metal oxide gas sensor. J Chem Rev 2:114–121
Pato AH, Balouch A, Talpur FN, Mahar AM, Shah MT, Kumar A, Qasim S, Gabole AA (2020) Synthesis and catalytic practicality of titania@ITO-grown nanoflakes: an excellent candidate for isopropanol conversion to acetone. Appl Nanosci 10:739–749
Mishra G, Mukhopadhyay M (2019) TiO2 decorated functionalized halloysite nanotubes (TiO2@HNTs) and photocatalytic PVC membranes synthesis, characterization, and its application in water treatment. Sci Rep 9:4345
Diez-Pascual AM, Diez-Vicente AL (2014) Effect of TiO2 nanoparticles on the performance of polyphenylsulfone biomaterial for orthopaedic implants. J Mater Chem B 2:7502–7514
Man HC, Abba MU, Abdulsalam M, Azis RA, Idris AI, Hamzah MH (2020) Utilization of nano-TiO2 as an influential additive for complementing separation performance of a hybrid PVDF-PVP hollow fiber: boron removal from leachate. Polymers 12:2511
Abazovic ND, Comor MI, Dramicanin MD, Jovanovic DJ, Ahrenkiel SP, Nedeljkovic JM (2006) Photoluminescence of anatase and rutile TiO2 particles. J Phys Chem B 110:25366–25370
Mugundan S, Rajamannan B, Viruthagiri G, Shanmugam N, Gobi R, Praveen P (2015) Synthesis and characterization of undoped and cobalt-doped TiO2 nanoparticles via sol-gel technique. Appl Nanosci 5:449–456
Belfer S, Fainchtain R, Purinson Y, Kedem O (2000) Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J Membr Sci 172:113–124
Movsesyan L, Maijenburg AW, Goethals N, Sigle W, Spende A, Yang F, Kaiser B, Jaegermann W, Park SY, Mul G, Trautmann C, Toimil-Molares ME (2018) ZnO nanowire networks as photoanode model systems for photoelectrochemical applications. Nanomaterials 8:693
Shukla AK, Alam J, Alhoshan M, Dass LA, Muthumareeswaran MR (2017) Development of a nanocomposite ultrafiltration membrane based on polyphenylsulfone blended with graphene oxide. Sci Rep 7:1–2
Seyedpour SF, Rahimpour A, Najafpour G (2019) Facile in-situ assembly of silver-based MOFs to surface functionalization of TFC membrane: a novel approach toward long-lasting biofouling mitigation. J Membr Sci 573:257–269
Esfahani MR, Aktij SA, Dabaghian Z, Firouzjaei MD, Rahimpour A, Eke J, Escobar IC, Abolhassani M, Greenlee LF, Esfahani AR, Sadmani A (2019) Nanocomposite membranes for water separation and purification: fabrication, modification, and applications. Sep Purif Technol 213:465–499
Liao Z, Fang X, Xie J, Li Q, Wang D, Sun X, Wang L, Li J (2019) Hydrophilic hollow nanocube-functionalized thin film nanocomposite membrane with enhanced nanofiltration performance. ACS Appl Mater Interf 11:5344–5352
Gao Y, Qin J, Wang Z, Osterhus SW (2019) Backpulsing technology applied in MF and UF processes for membrane fouling mitigation: a review. J Membr Sci 587:117136
Kumar M, Khan MA, Arafat HA (2020) Recent developments in the rational fabrication of thin film nanocomposite membranes for water purification and desalination. ACS Omega 5:3792–3800
Vani B, Kalyani S, Pabba M, Sridhar S (2021) Forward osmosis aided concentration of lycopene carotenoid from watermelon juice. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.6720
Andrew LN (2008) What can be inferred from bacteria–nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles. Ecotoxicology 17:362–371
Shuai C, Shuai C, Feng P, Gao C, Peng S, Yang Y (2018) Antimicrobial capability, physicochemical properties, and biocompatibility of n TiO2 incorporated polymeric scaffolds. Polymers 10:328
Acknowledgements
The first author wishes to acknowledge CSIR-IICT-AcSIR for giving funding (CSIR-SRF), and the opportunity to carry out Ph.D. The authors are thankful to DST, New Delhi for the grant of the Water Technology Initiative (WTI) project under the Center of Excellence in Water, Project Code GAP-789. Authors acknowledge to IICT library for plagiarism checker (Manuscript communicated number: IICT/Pubs./2020/106)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have seen and approved the manuscript being submitted. Hence, the corresponding author states that there is no conflict of interest on behalf of all authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vani, B., Shivakumar, M., Kalyani, . et al. TiO2 nanoparticles incorporated high-performance polyphenyl sulfone mixed matrix membranes for ultrafiltration of domestic greywater. Iran Polym J 30, 917–934 (2021). https://doi.org/10.1007/s13726-021-00945-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-021-00945-6