Abstract
Heavy metals are one of the major toxic pollutants affecting water quality. The higher concentrations of heavy metals in the environment cause water quality deterioration. As these metals are used for various purposes, they enter in the effluent streams of these processes. Their removal and recovery without further contamination would enhance their recyclability and usability in further applications. The use of graphite nanoplatelets (GNPs) with a polysulfone (PSF) based membrane is one potential solution to this issue. This will lead to rejection of heavy metals through the Donnan Exclusion Principle. At the same time, it offers the chemical and mechanical stability of PSF, and GNPs can be chemically modified to provide desired charge to membrane surface for optimum removal of heavy metals. Experiments using ultrafiltration membranes with GNPs anchored on them showed an increase in pore density, hydrophilicity, water flux and permeability transport properties. In addition, experiments involving Mn and Cr rejections revealed 96.97 and 93.07% rejections when 0.2% wt of GNP was included in the PSF based membrane. This highlights the importance of GNP treatment with suitable materials providing lower pore size and increased porosity and rejection of Mn and Cr. Such higher porosity would help to enhance transport rate and rejection properties which is necessity for successful industrial applications.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals are high density (3.5 to 7 g/cm3) components of earth crust (Bellis & Aprile, 2020), with varying composition and availability across the world. Cr and Mn are notable members of heavy metals due to their large applications as machine components, structural supports. They are important members of several alloys and used in other applications due to favorable mechanical, thermal, and chemical properties (Jaishankar et al., 2014). Moreover, they also play an essential role in metabolism of human and animal (Nasir et al., 2018). Despite of their importance in life and growth, these metallic materials affect adversely to human and surrounding life at higher concentrations (Mahajan-Tatpate et al., 2021b; Gholamian et al., 2023; Kravkaz Kusku et al., 2018; Cetin et al., 2022a, b, c). Their exposure and intake can result in large health hazards to humans, animals and flora (Tekin et al., 2022). Its leaching and contamination also affect soil properties (Kravkaz-Kuscu et al., 2018; Çiçek et al., 2022; Pekkan et al., 2021). The soil quality variation also affects vegetation in the area (Cetin et al., 2022a, b, c). Additionally, non-biodegradable nature and bioaccumulation properties of these material would enhance the intensity and severity of these hazards and damage during long and continuous exposure (Gholamian et al., 2023; Mahajan-Tatpate et al., 2021a, b; Verma & Dwivedi, 2013).
Adverse effect of these materials raises a need to control their contamination in food chain and water bodies (Gholamian et al., 2023). Out of several reasons for contamination, effluent and waste disposal are resultant of human activities which can be controlled. An increase in heavy metal contamination in environment due to population increase in Turkey is reported (Cetin et al., 2022a, b, c). This has resulted in posing stringent regulations on disposal of these materials during effluent treatment. This has prompted world health organizations (WHO) to set the permissible limits for different heavy metals to limit their effect on human being e.g., the permissible limits of Chromium (Cr) and Manganese (Mn) are 0.05 and 0.1 (mg/l), respectively (Mahajan-Tatpate et al., 2021a, b).
Separation of heavy metals has been reported by methods of flocculation, chemical precipitation, coagulation, flotation, ion exchange, adsorption, electrochemical treatment and membrane processes (Malik et al., 2017; Agarwal et al., 2015; Mahajan-Tatpate et al. 2021a, b, 2022). Some of these conventional methods have the issues of secondary treatment requirement resulting in sludge generation, change in chemical composition and purity (Mahajan-Tatpate et al. 2021a, b). On the other hand, the membrane-based processes work on physical separation and reported to be one of the effective methods for separation and recovery of metal components (Fadhil, 2023; Khulbe & Matsuura, 2018; Mahajan-Tatpate et al., 2021a, b). These separation systems have benefits of ease of operation, space saving and high efficiency (Ezugbe & Rathilal, 2020). They can be used for continuous separation and recovery of components, and combined with other processes in industry or effluent plant. Other benefits of membrane processes include mild operational conditions, low energy requirements (compared to secondary treatment) and possible linear scale up due to modular nature (Riffat, 2013).
Moreover, membranes transport properties can be optimized for separation of desired components. Application of various membrane processes like Reverse osmosis (RO), Nanofiltration (NF), Electrodialysis (ED) and Ultrafiltration (UF) in heavy metal separation is reported (Abdullah et al., 2019). Here RO, NF and UF works on pressure gradient as driving force, where as ED required electrical potential difference as driving force. This results in higher energy consumption for ED based separations. during the process (Fadhil, 2023; Mahajan-Tatpate et al., 2021a, b; Ezugbe & Rathilal, 2020; Khulbe & Matsuura, 2018). In case of pressure driven systems, RO and NF requires high pressure of more than 50 kg and 20 to 50 kg for efficient operation respectively compared to UF which works on 1 to 5 kg pressure gradient (Mulder, 2012). This provides UF based separations the benefits of low energy consumption along with ease of operation (Mahajan-Tatpate et al., 2021a, b).
The ultrafiltration membranes supported by Micellar and Polymer complexation (MEUF, PEUF) are reported for separation of heavy metals (Lin et al., 2020). Both methods work on addition of complexing agent which forms micelles or complexes with the pollutants. This complex or micelles formation makes direct recycle or use of separated components difficult in further processes. Hence the downstream processing for recovery of heavy metal ions is required. This would generate secondary pollutants which needs further treatment. This limits applicability of these membranes. Hence there is need for defining single membrane system which can work in extreme conditions for removal of furthermost multiple heavy materials.
This requires careful optimization of membranes properties viz., pore size, porosity and providing surface charge to membranes (Dhume et al., 2020; Mahajan-Tatpate et al., 2022; Li et al., 2023; Zhang et al., 2023). These membranes would be helpful in separation of heavy metal ions by Donnan Exclusion principle (Abdullah et al., 2019). Modification of membranes for surface charge can be done by chemical treatment (grafting or surface treatment) or use of additive in dope solution. Chemical treatment modifies polymeric structure and affect membrane stability and applicability. On the other hand, use of carefully selected additive would not affect the chemical structure of base polymer. This would help to maintain the stability, while additive would provide the necessary charge and charge distribution for enhancement in heavy metal removal. Use of carbon based nanomaterial to enhance membrane separation properties is reported (Rajesh et al., 2023).
Considering all these factors, graphite nanoplatelets (GNP) have been used as additives in PSF based membranes in the current work. GNP possesses excellent mechanical properties, and they can be dispersed easily and evenly due to their nanometric sizes. Further GNP possess very high surface area and high number of active sites available for interaction (Herreros-Lucas et al., 2023). Hence they can be charged easily with suitable chemical treatment. This would provide easy method to control the charge and charge density of membranes.
The study is targeted towards about optimization of membranes for removal of heavy metals using GNP as an additive in PSF based membranes. The development of membranes was targeted for separation of multiple heavy metal viz., Chromium (Cr) and Manganese (Mn). These materials are selected due large applicability of Cr and Mn in tanning and metal processing industry. This results in large quantities of effluent possessing these metal salts. The membranes optimized using PSF as base material with PEG as porogenic additive and GNP as surface modifier showed more than 90% removal for these metals.
2 Experimental
2.1 Materials
Polysulfone (PSF) of synthesis grade and a molecular weight (MW) of 35,000 Dalton (Da) was purchased from Otto Chemei Pvt. Ltd. Loba Chemei Pvt. Ltd. supplied N, N’-Dimethyl acetamide (DMAc) of synthesis grade. Nanoshel LLS supplied a synthesis-grade Graphene Nanoplatelets (GNP) with a particle size ranging from 2 to 10 nm, bulk density 0.101 g/cm3 and surface area of 320 m2/g. Polyethylene Glycol (PEG) with molecular weights 200, 400 and 600 Da were obtained from High Purity Lab. Pvt. Ltd. PEG 1500, and 9000 Da, and Acetic Acid, Sodium Hydroxide (NaOH) and Hydrochloric Acid (HCl) were purchased from Loba Chemei Pvt. Ltd., and PEG 6000 and 20000 Da, Potassium dichromate pure (K2Cr2O7) were purchased Sisco Research Lab Pvt. Ltd. They are designated by PEG and MW e.g. PEG 200.
Potassium Iodide (KI) and Iodine (I2) laboratory reagent grades were purchased from ACME Chemicals and Poona Chemical Lab, respectively. Ahlstrom Hollytex provided Nonwoven Polyester backing of grade 3324. Potassium Permanganate (KMnO4) and are Romali Pvt. Ltd., respectively. Sulfuric Acid (H2SO4) was purchased from Fisher Scientific, Nitric Acid (HNO3) was purchased Merck Ltd.
2.2 Solution Preparation
Initially, GNP was treated with some acids and alkalis like Sulfuric Acid (H2SO4), Nitric Acid (HNO3), Hydrochloric Acid (HCl), Acetic Acid, Sodium Hydroxide (NaOH) and Potassium Permanganate (KMnO4). GNP was stirred for 24 h in 1000 ppm acid or alkali solution. It was then filtered out to remove GNP and dry it at 60 °C for 48 h.
The GNP, PEG and PSF dried at 60 °C for 48 h under vacuum conditions were used in formation of dope solution as described earlier (Dhume et al., 2020, 2023). Precisely, pre-measured quantities of dry PSF (23—43%, w/v of DMAc) and PEG (1–10%, w/w of PSF) were added to vessel containing DMAc under a constant stirring to create a dope solution. Subsequently, the solution was added with pre-weighed GNP (0–1%, w/w of PSF) in order to achieve the desired concentration based on w/w ratio with respect to the amount of PSF. The stirring process was continued for 48 h under close observation to ensure the complete dissolution of PSF and PEG in DMAc. This was insured by visual observation, followed by centrifugation of solution which would remove undissolved polymeric materials. Same conditions were maintained for further dissolution. The solution was then subjected to degassing through either probe sonication or a vacuum degassing system depending upon solution viscosity. The resulting solution was utilized for the formation of membranes.
2.3 Membrane Casting
The dope solutions were degassed using a probe sonicator (Johnson Plastosonic, Model No. JP578L) for 2 to 5 min per cycle or controlled vacuum to remove any entrapped air bubbles (Dhume et al., 2020, 2023). It was centrifuged and used for membrane preparation using membrane casting system, following the, methodology as described earlier (Dhume et al., 2020, 2023). A casting system equipped with precise control over parameters such as knife gap, spread time and surface drying time was used to ensure the formation of membranes without irregularities or uneven surfaces.
The non-woven polyester backing of appropriate size was fixed to glass surface attached to casting machine using scotch tape. A doctor blade attached to casting machine is used to spread the casting solution on backing evenly at predefined thickness. This thickness of solution layer was controlled by gap between backing surface and doctor knife. The glass surface, backing and solution was transferred to water bath after a predefined air-drying time of 10 – 30 s. This process lead to membrane formation by gelation. The casted membrane was preserved under formalin at a temperature of 4 °C before further analysis. All the analysis is done in triplicate and best fit data is used in discussion.
2.4 Water Flux Analysis
The water flux of the prepared membrane was measured by using an Amicon type dead end cell with an active membrane area of 13.847 cm2, following the methodology as described earlier (Dhume et al., 2020, 2023). The analysis was conducted at room temperature using distilled water.
The flux (l/m2.h—lmh) was calculated by using Eq. 1, given in Fig. 1,
where, V is volume (L) of water transported across the membrane in time (Δt, h) through the membrane of cross-sectional area (A).
2.5 Analysis of Bubble Point and Pore Size Distribution
2.5.1 Bubble Point Analysis
Bubble point analysis was carried out by using water – air system, combination as described earlier (Dhume et al., 2020). A wet membrane sample was carefully mounted in analysis cell. Dry air was fed into the system, and the upstream pressure was gradually increased at a regular interval until a continuous airflow rate was obtained. The pressure at which this happens was identified as the bubble point pressure, which was used for calculating the maximum pore size using Cantors Equation (Cuperus & Smolders, 1991).
where, σ is surface tension (mN.m-1) of air–water, θ is contact angle and Pi is applied pressure (bar).
2.5.2 Pore Size Distribution
The average pore size of the membranes was determined with the help of both water flux and bubble point analysis by using Cantor’s Eq. (2). Additionally, the number of pores per unit area was calculated using Hagen-Poiseuille’s Equation (Mulder, 2012), as provided below:
In the equation, Ni is the number of pores per unit area, rpi is the average radius of the pore (m), σ is the viscosity of water (P), l is pore length, which is assumed to be equal to the membrane skin layer thickness (µm) and \({J}_{i}\) corresponds to the flux (lmh) measured at the ith increment where applied pressure is Pi (bar).
2.6 PEG Rejection Analysis
The molecular weight cut-off (MWCO) of the resulting membrane was measured using PEG rejection analysis. This analysis was conducted using a dead end cell as described earlier (Dhume et al., 2020, 2023). 0.1% PEG solution was prepared in distilled water and its transport was analyzed. Initially 25 ml of permeate was discarded and further material was considered for analysis. Concentration of PEG in the feed and permeate was measured by using UV analysis (Yusoff et al., 2017). The solution requires treatment for being UV active. For the same, 0.6 ml feed or permeate sample was taken and 3 ml of reagent A (1.27 g I2 in 2% of KI solution) was added to the same with through mixing. They are allowed to interact for 30 min at room temperature, followed absorbance measurement using double beam UV–visible spectrophotometer (UV 3000+, LabIndia Analytical) at 535 nm wavelength.
Rejection (%) was calculated by
where \({C}_{p}\) and \({C}_{f}\) is concentration of permeate and feed concentration, respectively.
2.7 Metal Rejection Analysis
The synthetic solutions of metal salts (Mn and Cr) with a concentration of 1000 ppm (viz., KMnO4 and K2Cr2O7) was prepared in distilled water. These solutions were used for rejection analysis, similar to PEG rejection analysis described in Sect. 2.6. The concentration of the metal salts was determined by using UV analysis at 530 nm and 313 nm wavelengths for Mn and Cr, respectively (Mahajan-Tatpate et al., 2021a, b). A calibration curve is defined and used to analyze unknown concentration of the salt solutions. This analysis was carried out using UV–visible spectrophotometer (UV 3000+) from LabIndia Analytical instruments.
3 Results and Discussion
3.1 Effect of Dope Solution Concentration
Polysulfone (PSF) is widely considered as a preferred material for the formation of porous membrane (Mulder, 2012; Tan & Rodrigue, 2019). This is due to its excellent chemical and thermal stability, as well as its solubility in hydrophilic solvents (Dhume et al., 2020, 2023; Tan & Rodrigues, 2019; Arthanareeswaran & Starov, 2011). These unique properties make it important material for porous membrane formation.
Performance of membrane is majorly defined in terms of its transport and selectivity properties. These properties are dependent upon composition of solution used for membrane formation and the formation or gelation parameters (Mulder, 2012). One of the major parameters is solution composition containing solvent, base polymer, polymer properties and additive (Abdelrasoul et al., 2015). This solution composition in addition of gelation properties would define membrane properties of transport rate and selectivity (Mulder, 2012).
The membrane properties of concern viz., transport rate and selectivity, there is always a trade off (Mahajan-Tatpate et al. 2021a, b). Hence optimization of concentration, gelation conditions are highly essential for membrane properties. One of the method to improve selectivity is an increase in the solution concentration. It would result in low pore size of selective layer (Dhume et al., 2023; Tan & Rodrigues, 2019). This would increase the selectivity while reduction in flux is observed (Mulder, 2012).
Through careful optimization of the solvent and PSF molecular weight (MW), it is possible to achieve high solubility (Dhume et al., 2020). Here by increasing the solution concentration overall membrane transport and rejection properties can be enhanced. Similar increase in membrane properties are reported (Li et al., 2014). In this regard, DMAc was found to be better option due to high solubility of PSF compared to N-methyl pyrrolidone and N,N’-dimethyl formamide. Further DMAc can be used for large scale membrane formation due to its solubility in water, which is commonly utilized as a non-solvent for membrane preparation (Dhume et al., 2020). Moreover the PSF membranes formed with DMAc exhibited remarkable stability properties as observed by linear increase in transport rate with pressure. This indicates the formation of stable membranes without any mechanical pore compaction owing to increased pressure difference.
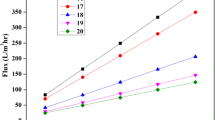
Further the study was initiated for optimization of PSF concentration and its effect on membrane transport properties. A significant exponential decrease in the transport rate (from 622 to 5.91 lmh) with increase in dope solution concentration from 23 to 43% was observed (Fig. 1). This decrease in water flux can be attribute to the decrease in pore size. Such variation is pore size is due to increase in solution viscosity as discussed in section below. This resulted in formation of denser surface layer with lower pore size. It helped to improve the desired selectivity properties. The observed variation in the flux with change in the concentration profile is duly supported by pore size analysis. It is evident that as the concentration of dope solution increased from 23 to 43% there was a decrease in pore size from 1405 to 156 nm.
3.2 Effect of PSF Concentration on Dope Viscosity
Dope solution viscosity is an important parameter during membrane formation and its property optimization. Hence effect of solution viscosity with concentration is analyzed. A linear increase in viscosity from 1400 to 2700 cP with the increase in solution concentration from 33 to 43% was observed (Fig. 2). Such increase in viscosity would restrict the motion of polymeric molecules from dope solution. It would restrict rearrangement and agglomeration of solute particle during casting, air dry and solvent solute demixing in gelation process.
Such restricted motion and agglomeration would affect rearrangement of polymer from solution, resulting in small pore formation in place of solvent removed during gelation and phase inversion (Pal, 2017). This reduction in pore size would lead to increased resistance for the transport. It would result in lower transport rate (flux) through the formed membranes (Fig. 2). Similar reduction in transport rate with increase in dope solution concentration is reported earlier (Dhume et al., 2020, 2023; Ray et al., 2019).
A sharp decrease in transport rate with increase in solution concentration or dope solution viscosity in observed at lower concentration. At higher concentration, to the presence of higher solute content enhances the dope solution viscosity. This higher viscosity reduces the polymeric motions and possibility of realignment. It would reduce the variation in transport properties. Same is observed through the exponential curve in Fig. 1.
3.3 Effect of PEG Molecular Weight
There is always a tradeoff between selectivity and transport rate for membranes. In case of porous membranes this trade off can be suitably modified or optimized by variation in solution composition and membrane formation conditions. The PSF based membranes can be suitably modifies by using polyethylene glycol (PEG) as porogen (Dhume et al., 2020, 2023). PEG is used as polymeric pore forming agent or porogen. It is a material used for modification of morphology of membranes, which is added to solution and subsequently leached out during phase inversion (Rekik et al., 2023). An increase in porosity and transport rate while maintaining the PEG rejection is reported (Dhume et al., 2020; Mahajan-Tatpate et al., 2021a, b). This can be attributed to increase in viscosity of dope solution due to increase in polymer content of solution. It would restrict the rearrangement in polymer while leaching of porogen would enhance porosity which would increase the transport rate.
Further it was thought to optimize the PEG MW to be used as porogen. The effect of different PEG molecular weight in dope solution containing 29% PSF as base material was investigated by analyzing its effect on water flux and pore size of formed membranes.
As seen from Table 1, a reduction in PEG MW in dope solution leads to decrease in pore size from 702.66 to 140.53 nm. This could be attributed to smaller molecular size PEG. The use of PEG as porogen in dope solution would increase the solution viscosity It would restrict base polymer rearrangement during gelation, resulting in smaller pore size.
Further PEG being hydrophilic in nature, it gets dissolved in water and leached from the membrane surface. This would result in smaller size pores with low MW PEG as seen from Table 1. The reduction in pore size would increase resistance for transport through the formed pores. This would reduce the water flux from 541.62 to 282.58 lmh with the variation in PEG MW weight from 6000 to 200 Da at addition of 8% PEG concentration in dope solution.
3.4 Water Flux and Pore Size
As seen from Fig. 3, a sharp decrease in water flux from 622 to 5.91 LMH was observed with the increase in PSF concentration from 23 to 43% in dope solution. This exponential decrease is due to the sharp decrease in pore size with increase in dope solution concentration. Higher dope solution concentration affects the pore formation during gelation resulting in smaller pore size. At high dope solution concentration, rearrangement in pore structure and clustering during gelation reduces and makes the pore size reduction exponential rather linear.
Bubble point method was used to discriminate maximum pore size present in the pore distribution. It is based on minimum pressure necessary to open the pores to transport firstly observed air bubble. A sharp increase in bubble point pressure and reduction in pore size was observed with the increase in dope solution PSF concentration from 33 to 43%. This can be attributed to the rearrangement in polymer during gelation (Dhume et al., 2020, 2023). Resulting high viscosity of higher polymer solution concentration would restrict the movement of polymers and rearrangement in polymers. These restrictions would control the pore formation and result in formation of smaller pores as observed from Fig. 3. This supports the hypothesis of rearrangement of polymer during gelation and thus observed variation in pore size of formed membranes. Such reduction in pore size would lead to separation of low molecular weight solutes and improve the selectivity properties. Similarly they would enhance the resistance towards flow and reduce the membrane flux as observed and discussed above.
3.5 Effect of Graphene Nanoplatelets (GNP) Content in Dope Solution
As seen from the explorations uptill now, it was observed that the PSF based membranes with dope solution containing PEG as porogen agent play major role in obtaining desired transport and selectivity properties. The membranes were further added with graphite nanoplatelets (GNP) to get the desired rejection properties for heavy metals. Selection was GNP was owing to the excellent mechanical and stability properties of GNP (Mohan et al., 2018). Further the thin layered structure of GNP would provide the properties of easy dispersion into the polymer solution (Liang et al., 2018). Additionally, its higher surface area would provide feasibility for optimization of charge on GNP. This charge optimization and ease of dispersion would prove beneficial during the optimization of surface charge of membranes (Perez-Alvarez et al., 2019). It would be beneficial in the optimization of rejection properties for heavy metals. This was investigated to determine composition of PEG and GNP to obtain the desired selectivity and flux properties.
GNP concentration was optimized to obtain the desired transport and selectivity properties. As seen from Fig. 4, the dope solution containing 0.2% GNP gives maximum pore density with minimum pore size. This could be attributed to interactions between PSF, PEG and GNP resulting in lower pore size and higher porosity by leaching of the PEG. Further increase in GNP would lead to agglomeration and cluster formation leading to abnormalities in pore size and porosity. This lower pore size would be beneficial in providing optimum rejection properties, while higher pore density would be helpful to improve membrane flux (Table 2).
Lower flux for membranes based upon dope solution containing 0.2% GNP can be attributed to formation of regular size pores with lower interconnectivity. This would increase the membrane resistance and reduce convective flux (Piry et al., 2011). At the same time, it would enhance rejection properties for all metals investigated and helped to achieve the desired selectivity.
3.6 Fourier Transform Infrared Spectroscopy (FTIR) Analysis
In FTIR analysis infrared radiations were passed through the sample, where some of the radiations will be absorbed by the sample and the rest will pass through it. This absorption and transmittance is dependent upon molecular structure and its electronics activity. Based on transmittance, the graph was plotted between wave number and transmittance for PSF modified membranes is shown in Fig. 5. The membrane shows a variation in FTIR spectra of PSF membrane modified with additive like, PEG/ GNP and GNP nanoparticle treated with KMnO4. There is variation at the presence of IR peaks related to C = C and O–H groups at 1539 and 3820 cm−1, respectively; which were absent in PSF based membranes. This attributes to change in chemical composition (presence of GNP and PEG), and related functional group interactions present in the membrane. Such variation in composition would result in modification surface properties. This change in surface properties would modify the interaction with metal salts. Similar variation in surface properties and interactions has been reported (Enders et al., 2023). These interactions would improve the rejection properties of metal salts by Donnan Exclusion principal. Similar increase in metal rejection with modification in surface composition has been reported (Muthumareeswaran et al., 2017).
3.7 Morphology of Membranes
The surface morphology of the membranes are shown in Fig. 6. The Scanning Electron Microscopy (SEM) of the surface images shows presence of similar size pores in both these membrane with and without porogen. Here the careful selection of PEG as porogen helps to maintain the pore size while enhancing porosity. This PEG is leached during phase inversion process. It increased the voids and reduced the thickness of pore walls. This would increase the permeability of membrane.
The porous layer varied significantly with the addition of GNP, from tighter and void-free pores. Membranes prepared using N,N-dimethylacetamide (DMAc) had higher porosity (89%) as compared to 1-methyl-2-pyrrolidone (NMP) and N,N-dimethylformamide (DMF), which were 83% and 80% respectively. In our study, the use of DMAc as solvent resulting in an asymmetrical porous top layer and pore sizes ranging between 8–19 nm. Similar benefits of DMAc towards formation of PSF based membranes has reported (Ravishankar et al., 2018). This smaller pore size in addition with surface charge would lead to enhanced metal ion rejection and removal by Donnan exclusion principle.
3.8 Heavy Metal Rejection
Rejection of ions can principally be achieved through adsorption, size exclusion, and charge exclusion. In the current investigations GNP is used enhance metal ion rejection by employing Donnan exclusion principle. As seen from, Fig. 7, it was observed that PSF based membranes with dope solution containing PEG as porogen and GNP as modifying agent play major role in obtaining desired rejection properties for heavy metals (Plisko et al., 2021).
The rejection of metal ions at same PSF concentration with different optimization shown in Fig. 8. From the figure, it can be noted that higher rejection was achieved with the 35% of PSF, PEG 400 is 8% and GNP 0.2%. All these membranes demonstrated an increase in rejection when there was a change in membrane content. The membranes based on only PSF gives rejection 26.39 and 42.03% for Mn and Cr ions respectively. A maximum metal rejection of 87.19 and 54.24% of Mn and Cr respectively was achieved using PSF membrane with PEG 400 and GNP content. This can be attributed to the higher interaction and charge on membranes with the presence of GNP. It would result in higher rejection of heavy metals by Donnan exclusion principle. Similar increase in rejection due interaction is reported earlier (Dhume et al., 2020, 2023; Mahajan – Tatpate et al., 2022; Syahirah-Suhalim et al., 2022).
Figure 8 shows the rejection of metal ions at different PSF concentration. From the figure, it can be noted that higher rejection was achieved with the 43% of PSF, PEG 200 is 2% and GNP (KMnO4) 0.2%. All membranes demonstrated an increase in rejection when there was an increase in concentration of PSF, which can be attributed to the smaller pore size. The reduction in pore size would make the membrane more compact and higher repulsive interaction between membrane surface and solute metal ion particles (Costa & de Pinho, 2005). A maximum metal rejection of 96.25 and 93.07% was achieved for Mn and Cr respectively was achieved using 43% PSF membrane containing GNP. This increase in rejection properties can be attributed to the interaction between solute molecules and charged particles on membrane surface.
4 Conclusions
The GNP-anchored PSF ultrafiltration membranes were prepared using DMAc as a solvent. These membranes demonstrated marked improvements in hydrophilicity and porosity, resulting in higher overall flux and permeability when compared to membranes without GNP. The pores of these ultrafiltration membranes have sizes ranging from 8 to 19 nm. Furthermore use of PEG as porogen helped to enhance the membrane porosity while maintaining pore size. This enhanced porosity helped to increase the water flux across membrane. Additionally, the prepared membranes exhibited greater permeability as well as rejection of Mn and Cr ions through tests. The 43% PSF based membranes with 2% PEG (200) as porogen and 0.2 wt % GNP showed the highest rejection rates of 96.97 and 93.07%, and Mn and Cr, respectively. The presence of GNP provides surface charge to the PSF membranes, while still maintaining their stability. Through acid, base or strong chemical reagent treatments (e.g. KMNO4) this surface charge can be modified, along with possible grafting of it onto the GNPs surface. This provides charge similar to that found in salt solution. This resulted in more than 90% retention for Cr and Mn on membranes due to repelling action by similar charge of salt molecules known as Donnan Exclusion, without compromising its stability and permeation performance. These findings emphasize contribution of PEG (200), GNP and DMAc to creating highly porous membranes with improved pore size, for removing heavy metal salts. It would be helpful in water purification by removing heavy metal salts in economical fashion.
Data Availability
The authors declare that all the primary and secondary data in relation with the preparation of manuscript and research considered here is presented in manuscript.
References
Abdelrasoul, A., Doan, H. D., Lohi, A., & Cheng, C.-H. (2015). Morphology control of polysulfone membranes in filtration processes: A critical review. ChemBioEng Reviews, 2, 22–43. https://doi.org/10.1002/cben.201400030
Abdullah, N., Yusof, N., Lau, W. J., Jaafar, J., & Ismai, A. F. (2019). Recent trends of heavy metal removal from water/wastewater by membrane technologies. Journal of Industrial Engineering Chemistry, 76, 17–38. https://doi.org/10.1016/j.jiec.2019.03.029
Agarwal, M., Chaudhry, K., & Kumara, P. (2015). Heavy metal sources impacts & removal technologies. International Journal of Engineering Research and Technology, 3, 1.
Arthanareeswaran, G., & Starov, V. M. (2011). Effect of solvents on performance of polyethersulfone ultrafiltration membranes: Investigation of metal ion separations. Desalination, 267, 57–63.
Bellis, L. D., & Aprile, A. (2020). Editorial for special issue “Heavy metals accumulation, toxicity, and detoxification in plants.” International Journal of Molecular Science, 21, 4103. https://doi.org/10.3390/ijms21114103
Cetin, M., Aljama, A. M. O., Alrabiti, O. B. M., Adiguzel, F., Sevik, H., & Cetin, I. Z. (2022a). Determination and mapping of regional change of Pb and Cr pollution in Ankara City center. Water, Air, & Soil Pollution, 233, 163. https://doi.org/10.1007/s11270-022-05638-1
Cetin, M., Aljama, A. M. O., Muragaa Alrabiti, O. B., Adiguzel, F., Sevik, H., & Cetin, I. Z. (2022b). Using topsoil analysis to determine and map changes in Ni Co pollution. Water, Air, & Soil Pollution, 233, 293. https://doi.org/10.1007/s11270-022-05762-y
Cetin, M., Pekkan, O. I., Ozturk, G. B., Kurkcuoglu, M. A. S., Kucukpehlivan, T., & Cabuk, A. (2022c). Examination of the change in the vegetation around the Kirka Boron mine site by using remote sensing techniques. Water, Air, & Soil Pollution, 233, 254. https://doi.org/10.1007/s11270-022-05738-y
Çiçek, N., Erdogan, M., Yücedağ, C., Yücedağ, C., & Çetin, M. (2022). Improving the detrimental aspects of salinity in salinized soils of arid and semi-arid areas for effects of Vermicompost Leachate on salt stress in seedlings. Water, Air, & Soil Pollution, 233, 197. https://doi.org/10.1007/s11270-022-05677-8
Costa, A. R., & de Pinho, M. N. (2005). Effect of membrane pore size and solution chemistry on the ultrafiltration of humic substances solutions. Journal of Membrane Science, 255, 49–56. https://doi.org/10.1016/j.memsci.2005.01.016
Cuperus, F. P., & Smolders, C. A. (1991). Characterization of UF membranes: Membrane characteristics and characterization techniques. Advances in Colloid and Interface Science, 34, 135–173.
Dhume, S. S., Mahajan-Tatpate, P., & Chendake, Y. J. (2020). Optimization of PSF membrane transport properties with the use of porogenic additive. Journal of Applied Membrane Science and Technology, 24, 57–73.
Dhume, S., Mahajan-Tatpate, P., Chendake, Y. & Chavan, S. (2023). Separation of heavy metals from metal industry effluent for acid recovery. In: Siddiqui, N.A., Baxtiyarovich, A.S., Nandan, A., Mondal, P. (Eds.), Recent advances in recycling engineering. AIR 2021. Lecture notes in civil engineering, vol 275. Springer. https://doi.org/10.1007/978-981-19-3931-0_16. https://springerlink.bibliotecabuap.elogim.com/chapter/10.1007/978-981-19-3931-0_16.
Enders, M., Zhu, C., Kleber, M., Derscheid, G., Berger, R., Bauer, H.-D., & Scheppat, B. (2023). Effects of surface morphology changes on FTIR-ATR spectroscopy with compacted Sodium Alanate (NaAlH4) during cycling. International Journal Hydrogen Energy, 48, 709–722. https://doi.org/10.1016/j.ijhydene.2022.10.014
Ezugbe, E. O., & Rathilal, S. (2020). Membrane technologies in wastewater treatment: A review. Membranes, 10, 89. https://doi.org/10.3390/membranes10050089
Fadhil, S. (2023). Sustainable chromium removal by nanofiltration membranes: Application of pore flow model. International Journal of Environmental Science and Technology, 20, 8391–8398.
Gholamian, F., Karimi, N., Gholamian, F., & Bayat, P. (2023). The effects of some detergents and heavy metals on fucoxanthin yield and phycoremediation potential of Polycladiamyrica. International Journal of Environmental Science and Technology, 20, 8349–8358.
Herreros-Lucas, C., Vila-Fungueirinno, J. M., & Giménez-López, Md. C. (2023). Electrochemically versatile graphite nanoplatelets prepared by a straight forward, highly efficient, and scalable route. ACS Applied Materials & Interfaces, 15, 21375–21383. https://doi.org/10.1021/acsami.2c22495
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7, 60–72. https://doi.org/10.2478/Intox-2014-0009
Khulbe, K. C., & Matsuura, T. (2018). Removal of heavy metals and pollutants by membrane adsorption techniques. Applied Water Science, 8, 19. https://doi.org/10.1007/s13201-018-0661-6
Kravkaz Kuscu, I. S., Cetin, M., Yigit, N., Savaci, G., & Sevik, H. (2018). Relationship between enzyme activity (urease-catalase) and nutrient element in soil use. Polish Journal of Environmental Studies, 27, 2107–2112. https://doi.org/10.15244/pjoes/78475
Kravkaz-Kuscu, I. S., Sariyildiz, T., Cetin, M., Yigit, N., Sevik, H., & Savaci, G. (2018). Evaluation of the soil properties and primary forest tree species in taskopru (kastamonu) district. Fresenius Environmental Bulletin, 27, 1613–1617.
Li, H.-B., Shi, W.-Y., Zhang, Y.-F., Liu, D.-Q., & Liu, X.-F. (2014). Effects of additives on the morphology and performance of PPTA/PVDF in situ blend UF membrane. Polymers, 6, 1846–1861.
Li, Y., Pan, G., Zhang, Y., Wang, J., Yu, H., Zhao, G., Zhao, M., Tang, G., Guo, Y., Wu, C., & Liu, Y. (2023). A new method for tailoring the surface pore size and internal pore structure of ultrafiltration membranes without using additives—Atomization-assisted nonsolvent induced phase separation method. Separation and Purification Technology, 304, 122334.
Liang, A., Jiang, X., Hong, X., Jiang, Y., Shao, Z., & Zhu, D. (2018). Recent developments concerning the dispersion methods and mechanisms of graphene. Coatings, 8, 33. https://doi.org/10.3390/coatings8010033
Lin, W., Jing, L., & Zhang, B. (2020). Micellar-enhanced ultrafiltration to remove nickel ions: A response surface method and artificial neural network optimization. Water, 12, 1269. https://doi.org/10.3390/w12051269
Mahajan-Tatpate, P., Dhume, S., & Chendake, Y. (2021a). Recovery of chromium using membrane containing charged material. IOP Conference Series: Material Science and Engineering, 1146, 012022. https://doi.org/10.1088/1757-899x/1146/1/012022
Mahajan-Tatpate, P., Dhume, S., & Chendake, Y. (2021b). Removal of heavy metals from water: Technological advances and today’s lookout through membrane applications. International Journal of Membrane Science Technology, 8, 1–21.
Mahajan-Tatpate, P., Dhume, S., Chavan, S., & Chendake, Y. (2022). Acid-Modified ZnO nanoparticles embedded polysulfone membranes for separation of copper from industrial wastewater. International Journal of Membrane Science and Technology, 9, 67–76. https://doi.org/10.15379/2410-1869.2022.072
Malik, D. S., Jain, C. K., & Yadav, A. K. (2017). Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Applied Water Science, 7, 2113. https://doi.org/10.1007/s13201-016-0401-8
Mohan, V. B., Lau, K.-T., Hui, D., & Bhattacharyya, D. (2018). Graphene-based materials and their composites: A review on production, applications and product limitations. Composites, Part B: Engineering, 142, 200–220.
Mulder, M. (2012). Basic principles of membrane technology (2nd edn). Springer Dordrecht, Kluwer Academic Press, London. https://doi.org/10.1007/978-94-009-1766-8
Muthumareeswaran, M. R., Alhoshan, M., & Agarwal, G. P. (2017). Ultrafiltration membrane for effective removal of chromium ions from potable water. Scientific Reports, 7, 1–12. https://doi.org/10.1038/srep41423
Nasir, S., Hussein, M. Z., Zainal, Z., & Yusof, N. A. (2018). Carbon-based nanomaterials/allotropes: A glimpse of their synthesis. Properties and Some Applications. Materials, 11, 295. https://doi.org/10.3390/ma11020295
Pal, P. (2017). Water treatment by membrane-separation technology. In Industrial Water Treatment Process Technology (pp. 173–242). https://doi.org/10.1016/B978-0-12-810391-3.00005-9
Pekkan, O. I., Kurkcuoglu, M. A. S., Cabuk, S. N., Aksoy, T., Yilmazel, B., Kucukpehlivan, T., Dabanli, A., Cabuk, A., & Cetin, M. (2021). Assessing the effects of wind farms on soil organic carbon. Environmental Science and Pollution Research, 28, 18216–18233. https://doi.org/10.1007/s11356-020-11777-x
Perez-Alvarez, L., Ruiz-Rubio, L., Moreno, I., & Vilas-Vilela, J. L. (2019). Characterization and Optimization of the Alkaline Hydrolysis of Polyacrylonitrile Membranes. Polymers, 11, 1843. https://doi.org/10.3390/polym11111843
Piry, A., Heino, W., Kuhnl, T., Grein, S., Ripperger, U., & Kulozik. (2011). Effect of membrane length, membrane resistance, and filtration conditions on the fractionation of milk proteins by microfiltration. Journal of Dairy Science, 95, 1590–1602. https://doi.org/10.3168/jds.2011-4292
Plisko, T. V., Bildyukevich, A. V., Zhao, L., Huang, W., Volkov, V. V., & Huang, Z. (2021). Formation of polysulfone hollow fiber membranes using the systems with lower critical solution temperature. Fibers, 9, 28. https://doi.org/10.3390/fib9050028
Rajesh, K. A., Mansour, A., Garudachari, B., Mansour, A. R., & Jibu, P. T. (2023). Ethylenediamine-graphene oxide impregnated thin film nanocomposite membrane for the enhanced boron separation from seawater. Environmental Engineering Research, 28, 220760. https://doi.org/10.4491/eer.2022.760
Ravishankar, H., Christy, J., & Jegathisan, V. (2018). Graphine Oxide (GO) blended polysulfone membranes for Lead ion rejection. Membranes, 8, 77.
Ray, S. S., Chen, S.-S., Nguyen, N. C. & Nguyen, H. T. (2019). Electrospinning: A versatile fabrication technique for nanofibrous membranes for use in desalination. Nanoscale materials in water purification. In Micro and Nano Technologies, Elsevier. https://doi.org/10.1016/B978-0-12-813926-4.00014-8
Rekik, S. B., Gassara, S., & Deratani, A. (2023). Green Fabrication of Sustainable Porous Chitosan/Kaolin Composite Membranes Using Polyethylene Glycol as a Porogen: Membrane Morphology and Properties. Membranes, 13, 378. https://doi.org/10.3390/membranes13040378
Riffat, R. (2013). Fundamentals of wastewater treatment and engineering. In International standard book number-13: 978–0–203–81571–7. CRC Press Taylor & Francis Group.
Syahirah-Suhalim, N., Kasim, N., Mahmoudi, E., Shamsudin, I. J., Mohammad, A. W., Zuki, F. M., & Jamari, N.L.-A. (2022). Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview. Nanomaterials, 12, 437. https://doi.org/10.3390/nano12030437
Tan, X. M., & Rodrigue, D. (2019). A review on porous polymeric membrane preparation. Part I: Production techniques with polysulfone and poly (vinylidene fluoride). Polymers, 11, 1160. https://doi.org/10.3390/polym11071160
Tekin, O., Cetin, M., & Varol, T. (2022). Altitudinal migration of species of fir (Abies spp.) in adaptation to climate change. Water, Air, & Soil Pollution, 233, 385. https://doi.org/10.1007/s11270-022-05851-y
Verma, R., & Dwivedi, P. (2013). Heavy metal water pollution- A case study. Recent Research in Science and Technology, 5, 98–99.
Yusoff, I. I., Rohani, R., & Mohammad, A. W. (2017). Molecular weight cut-off determination of pressure filtration membranes via colorimetric detection method. Malaysian Journal of Analytica Science, 21, 484–495.
Zhang, G., Li, X., Chen, G., Zhang, Y., Wei, M., Chen, X., Li, B., Wu, Y., & Wu, L. (2023). Supramolecular framework membrane for precise sieving of small molecules, nanoparticles and proteins. Nature Communication, 14, 975.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All authors certify that they have no involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhume, S., Chendake, Y., Mahajan-Tatpate, P. et al. Optimization of Polysulfone Based Membranes Using Charged Graphite Nano Platelets for Separation of Manganese and Chromium (VI) From Water. Water Air Soil Pollut 235, 560 (2024). https://doi.org/10.1007/s11270-024-07375-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07375-z