Abstract
Global resource crisis and severe environmental problems have compelled world scientists to develop sustainable and green chemical materials. In this work, eugenol, a cheap and renewable phenol derivative found in cloves, is successfully utilized to prepare hydroxyl non-isocyanate polyurethanes (HNIPU), with number-average molecular weights (M n) from several to dozens of kilodaltons. First, a diepoxide intermediate is synthesized through three steps in 43.9% total yield. Second, this diepoxide reacts with CO2 at atmospheric pressure to form an intermediate possessing two cyclic carbonate groups in moderate yield. Third, the cyclic carbonate-containing intermediate further reacts, with compounds such as 4,4′-diaminodiphenyl methane, 1,6-hexanediamine and p-xylene diamine by nucleophilic ring-opening to obtain the desired HNIPUs. Furthermore, the diepoxide intermediate and CS2 undergo addition reaction to form cyclic dithiocarbonate intermediate that further reacts with 4,4′-diaminodiphenyl methane to afford polythiourethane (PTU). The resulting PTU contains mercapto groups in its each unit. Number-average molecular weight of PTU is Mn 2800 Da. Finally, crosslinking reactions occur between the mercapto groups of PTU and crosslinkers (1,6-hexanediol acrylate and/or cardanol) by thiol-ene reactions under UV (λ = 365 nm) irradiation conditions, leading to their corresponding crosslinked polymers. The optimized conditions for preparing crosslinked polymers include: PTU/cardanol/1,6-hexanediol acrylate = 1:1:0.05 (mass ratio), and UV irradiation time for 30 min. This work is expected to expand applications of eugenol and to provide a new route to NIPUs and PTUs using various diamines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
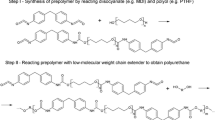
Polyurethanes (PUs) are one of the most important polymers due to their outstanding properties such as good chemical resistance, high elasticity, and strong abrasive performance. Thus, PUs have found versatile applications in coatings, paints, adhesives, foams, elastomers, and so on [1,2,3]. Consequently, global PUs output is expected to reach 18 million tons in 2016 [4]. For example, PUs show the best comprehensive properties in organic coatings in comparison with methacrylate resins, epoxy resins, polyesters, etc. But carbamate groups in PUs are conventionally formed by reactions between diols/polyols and diisocyanates/polyisocyanates. The use of highly toxic isocyanates in PUs manufacturing processes may inevitably affect the health of production workers and limit their wider scale applications in high-safety-required medical or bio-materials. To solve this problem, non-isocyanate polyurethanes (NIPU) are desired and have been studied by chemists and chemical engineers [5,6,7]. There are mainly three approaches for preparation of NIPU: ring-opening reactions between five-membered cyclic carbonates and di/multi-amines, leading to hydroxyl-containing non-isocyanate polyurethanes (HNIPU) [8]; the other is self-polymerization of monomers containing hydroxyl and in situ-forming isocyanate groups [9]; the third is the exchange reactions between carbamates [10, 11]. Among them, the first method plays the most important role due to its advantages such as: (1) green-house gas CO2 is utilized to form cyclic carbonate; (2) other materials such as epoxides and amines are commercially available or can be easily prepared; (3) the resulting HNIPU can form H-bonds, which can help to improve mechanical properties of polymers. For example, Leitsch et al. studied the effect of hydroxyl groups on the properties of HNIPU, and found that hydroxyl groups played critical roles in controlling nano-phase separation, possibly leading to novel broad-temperature-range acoustic and vibration damping materials [12]. In addition to HNIPU obtained from cyclic carbonates and amines, similar polymers can also be obtained by reactions between cyclic dithiocarbonates and amines. This polymer belongs to polythiourethanes (PTUs) group [13,14,15]. In addition to the excellent properties of PUs, PTUs also show outstanding optical properties due to the presence of sulfur, and has found applications in sealing materials, coatings, optical lenses, and so on [16,17,18].
In the second method, isocyanates are usually formed in situ by acyl azides under conditions of Curtius rearrangement [19, 20]. This method can only be conducted in laboratories due to harsh reaction conditions and possible danger of explosion of azides. Recently, our research group reported preparation of ricinoleic acid-based NIPU, in which isocyanate can be formed in situ from amide of ricinoleic acid instead of acyl azide [21].
Conventional fossil resources as well as their derived substances are facing serious crisis, which drive scientists to find and develop alternatives [22,23,24]. Resources from plants or animals such as cellulose, lignin, vegetable oils [25], cardanol [26, 27], and proteins, are renewable and have attracted enormous attention both from academia and industry in recent years [28]. Eugenol, a phenol derivative with an allylic group at para-site of the phenyl ring, is a natural extract of cloves [29, 30]. This essential oil has been studied and found applications in dentistry, medicine, perfume, and functional materials [31,32,33]. For example, an interesting work reported in 2015 by Deng and coworkers indicated that eugenyl methacrylate can form microspheres with diameters ranging from 500 to 800 μm by suspension polymerization [34]. The microspheres showed remarkable ability in large oil absorbency and could be re-used at least five times. More recently, our research group has designed terminal diene derivatives of eugenol and subsequently prepared a self-healable polymer by combination of thiol-ene and thiol-oxidation reactions [35]. Herein, we report preparation and characterization of eugenol-based HNIPUs and non-isocyanate PTUs. Significance of our work is as follows: (1) eugenol, a renewable resource, is used to synthesize polyurethanes; (2) toxic isocyanate is avoided in PU synthesis; (3) green-house gas CO2 is utilized to produce HNIPUs; and (4) cardanol, another renewable resource, and the prepared eugenol-based PTUs are combined to afford UV-crosslinked resins.
Experimental
Material
Commercial epichlorohydrin (99%), 4,4′-diaminodiphenyl methane, 1,6-hexanediamine, p-xylene diamine, meta-chloroperbenzoic acid (m-CPBA, 70–75%), acetic acid, dichloromethane, ethyl acetate, petroleum ether, methanol, tetrahydrofuran, triethyl amine, N-methyl-2-pyrrolidone (NMP), 1,6-hexanediol diacrylate, benzophenone, aqueous dimethyl amine (33%), LiBr, CO2, CS2 were all AR grade purity, and were used straight as received without any treatment. Commercial eugenol and cardanol are industrial grades. Silica gel is CP grade. The above reagents were used directly as received from Shanghai Aladdin Corporation Ltd.
Characterization
Structures of the intermediates were characterized by 1H NMR and 13C NMR spectroscopy techniques on a Bruker AV 400 MHz spectrometer, with tetramethylsilane (TMS) as the internal standard. CDCl3 was used as the solvent in NMR measurement. The Bruker FTIR (V70) spectra were recorded through KBr pellet method. Melting point was measured by an XT-4 melting point meter. Gel permeation chromatography (GPC) was performed on an HP 1100 HPLC, equipped with a Waters 2414 refractive index detector and three Styragel HR 2, HR 4, HR 5 of 300 × 7.5 mm columns (packed with 5 mm particles of different pore sizes). The column packing allowed the separation of polymers over a wide molecular weight range of 500–1000,000. THF was used as the eluent at a flow rate of 1 mL/min at 40 °C. PMMA standards were used as the reference. Elemental analysis was conducted on a Thermo Scientific FLASH 2000 CHNS/O Elemental Analyzer. Hardness of polymer films was measured by standard pencils.
Synthesis of eugenyl acetate
Eugenol (16.4 g, 0.1 mol) was dissolved in acetic acid (15 mL). The solution was heated to 80 °C and kept at this temperature for about 2 h. When TLC indicated complete consumption of eugenol, the reaction mixture was cooled to room temperature, and then poured into an excess of ice-water. The resulting mixture was extracted with 3 × 25 mL of dichloromethane, and the organic phase was combined, washed twice with aqueous sodium bicarbonate, once with brine, and dried on anhydrous sodium sulfate. After filtration, the filtrate was condensed to remove solvent, and the residue was further purified on a silica gel column (petroleum ether/ethyl acetate = 10:1, v/v) to obtain the ester product as a light yellow liquid [17.8 g; 86%; boiling point 162–165 °C (30 mmHg); 1H NMR (400 MHz, CDCl3): δ 6.88–6.86 (m, 1H), 6.74–6.71 (m, 2H), 5.91–5.84 (m, 1H), 5.09–5.01 (m, 2H), 3.75 (s, 3H), 3.34–3.30 (m, 2H), 2.24 (s, 3H)].
Synthesis of epoxide of eugenyl acetate (1)
Eugenyl acetate (10.3 g, 50 mmol) was dissolved in dichloromethane (20 mL) with stirring, followed by adding dropwise m-CPBA (17.2 g, 75 mmol) in dichloromethane (180 mL). After addition, the reaction mixture was stirred at room temperature overnight. The reaction mixture was washed three times with 10% aqueous NaHSO3, then washed with saturated NaHCO3 and brine, respectively, and dried on anhydrous sodium sulfate. After filtration, the filtrate was distilled under reduced pressure to remove dichloromethane, and the residual crude product was purified by silica gel chromatography (eluent: petroleum ether/ethyl acetate = 5:1) to afford 1 as a white crystal [9.0 g; 81%; melting point 58–59 °C; 1H NMR (400 MHz, CDCl3): δ 6.99–6.95 (m, 1H), 6.87–6.83 (m, 2H), 3.84 (s, 3H), 3.17–3.14 (m, 1H), 2.86–2.84 (m, 2H), 2.83–2.81 (m, 1H), 2.58–2.53 (m, 1H), 2.32 (s, 3H)].
Synthesis of eugenol-derived diepoxide compound (2)
Epoxide of eugenyl acetate (1) (11.1 g, 50 mmol) was dissolved in epichlorohydrin (19.5 g, 0.2 mol). NaOH (4.0 g, 0.1 mol) dissolved in EtOH (20 mL) was added dropwise to the previous solution. The mixture was refluxed for 4 h. When the reaction was completed, excess EtOH and epichlorohydrin were distilled off under reduced pressure. To the residue were added dichloromethane and water, and the organic phase was separated, washed with brine, and dried on anhydrous sodium sulfate. After silica gel chromatography (eluent: petroleum ether/ethyl acetate = 5:1) a white crystal was obtained [7.4 g; 63%; melting point 55–57 °C (Ref. [32] 57.5 °C); 1H NMR (400 MHz, CDCl3): δ 6.90-6.85 (m, 1H), 6.81–6.76 (m, 2H), 4.27–4.20 (m, 1H), 4.07–3.99 (m, 1H), 3.88 (s, 3H), 3.41–3.37 (m, 1H), 3.17–3.11 (m, 1H), 2.92–2.88 (m, 1H), 2.83–2.78 (m, 3H), 2.76–2.73 (m, 1H), 2.57–2.52 (m, 1H)].
Synthesis of eugenol-derived dicyclic carbonate (3)
The diepoxide 2 (2.36 g, 10.0 mmol) and LiBr (43 mg, 0.5 mmol) were dissolved in NMP (10 mL), then CO2 (1 atm) was bubbled into the solution. The reaction mixutre was heated to 100 °C and kept at this temperature for 5 h. After completion of the reaction, the mixture was poured into ice-water, followed by extraction with ethyl acetate. The organic phase was washed again with water, and dried with anhydrous Na2SO4. The crude product was purified on a silica gel column (eluent: petroleum ether/ethyl acetate = 1:1) to give 3 as a white solid [2.26 g; 70%; melting point 71–73 °C; IR (KBr): ν 1802 cm−1. 1H NMR (400 MHz, CDCl3): δ 6.91–6.87 (m, 1H), 6.78–6.74 (m, 2H), 5.04–5.01 (m, 1H), 4.95–4.90 (m, 1H), 4.63–4.60 (m, 2H), 4.49–4.44 (m, 1H), 4.25–4.11 (m, 3H), 3.85 (s, 3H), 3.12–2.91 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 154.5, 150.6, 147.1, 129.5, 122.1, 116.8, 114.0, 73.5, 68.5, 67.9, 66.1, 56.0, 39.3. Calcd for C15H16O8: C, 55.56; H, 4.97; Found: C, 55.58; H, 4.96].
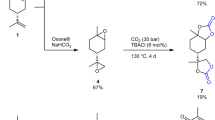
Synthesis of eugenol-derived dicyclic dithiocarbonate (4)
The diepoxide 2 (2.36 g, 10.0 mmol) and LiBr (43 mg, 0.5 mmol) were dispersed in tetrahydrofuran (10 mL) and cooled in an ice bath. Carbon disulfide (2.4 mL, 29.8 mmol) was added dropwise into the reaction mixture within 2 h. After addition, the resulting mixture was stirred at room temperature for 24 h. Excess water was poured into the mixture and then extracted with ethyl acetate. After similar workup, product 4 was obtained as a light yellow solid [3.20 g; 79%; melting point 79–81 °C; IR (KBr, cm−1): ν 1031, 1181; 1H NMR (400 MHz, CDCl3): δ 6.91–6.88 (m, 1H), 6.78–6.73 (m, 2H), 5.49–5.44 (m, 1H), 5.30–5.26 (m, 1H), 4.34–4.32 (m, 2H), 3.87–3.94 (m, 4H), 3.78–3.73 (m, 1H), 3.58–3.51 (m, 1H), 3.45–3.40 (m, 1H), 3.17 (d, J = 44.2 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 212.0, 149.5, 147.16, 129.1, 121.6, 116.8, 114.0, 91.1, 88.3, 76.7, 67.8, 56.2, 38.9, 36.5; Calcd for C15H16O4S4: C, 46.37; H, 4.15; Found: C, 46.38; H, 4.16].
Synthesis of 2-(dimethylamino methyl) eugenol (5)
Eugenol (1.64 g, 10 mmol), aqueous dimethyl amine (4.1 mL), and ethanol (10 mL) were mixed in a flask. Paraformaldehyde (0.60 g, 20 mmol) in ethanol (10 mL) was added dropwise into the flask. The mixture was refluxed for 6 h, and then cooled to room temperature. Excess ethanol was removed by distillation, and the residue was dissolved in dichloromethane (20 mL). The solution was washed with water and brine, and dried on anhydrous Na2SO4. The crude product was purified on a silica gel column (eluent: petroleum ether/ethyl acetate = 5:1) to give 5 as a light brown oil [2.93 g; 82%; boiling point 151–154 °C (30 mmHg); 1H NMR (400 MHz, CDCl3): δ 6.62–6.60 (m, 1H), 6.41–6.37 (m, 1H), 5.92–5.86 (m, 1H), 5.28 (s, 1H), 5.06–4.98 (m, 2H), 3.84 (s, 3H), 3.59 (s, 2H), 3.28–3.24 (m, 2H), 2.30 (s, 6H); Calcd for C13H19NO2: C, 70.56; H, 8.65; N, 6.33; Found: C, 70.55; H, 8.63; N, 6.32].
Preparation of eugenol-based HNIPU-D
Dicyclic carbonate (4) (162 mg, 0.5 mmol) and 4,4′-diaminodiphenylmethane (0.1 g, 0.5 mmol) were dissolved in dimethyl sulfoxide (2 mL). Under nitrogen atmosphere, the solution was stirred at 100 °C for 48 h. The reaction mixture was cooled to room temperature, followed by adding excess cold methanol to form a precipitate. After filtration, the filtrate cake was collected and dried to give the product [0.2 g; 80%; IR (KBr, cm−1): ν 3594, 3286, 1802, 1594, 1118, 1049; and Mn 3700 Da (GPC relative to PMMA standards)].
Preparation of eugenol-based HNIPU-H
In a similar manner, polymerization of dicyclic carbonate (4) (162 mg, 0.5 mmol) and 1,6-hexanediamine (58 mg, 0.5 mmol) gave the polymer HNIPU-H [0.18 g; 83%; IR (KBr, cm−1): ν 3350 (br), 2943, 1796, 1693; and Mn 15,970 Da (GPC relative to PMMA standards)].
Preparation of eugenol-based HNIPU-X
In a similar manner, polymerization of dicyclic carbonate (4) (162 mg, 0.5 mmol) and p-xylene diamine (68 mg, 0.5 mmol) produced the polymer HNIPU-X [0.20 g; 87%; IR (KBr, cm−1): ν 3389 (br), 1769, 1632; and Mn 84,930 Da (GPC relative to PMMA standards)].
Preparation of eugenol-based PTU
The eugenol-derived dithiocarbonate (4) (0.202 g, 0.5 mmol) and 4,4′-diaminodiphenylmethane (0.1 g, 0.5 mmol) were dissolved in tetrahydrofuran (2 mL). The solution was stirred at ambient temperature for 48 h followed by addition of excess cold methanol. The precipitate was collected by suction filtration and then dried. A light brown solid (0.24 g; 80%) was obtained [IR (KBr, cm−1): ν 1035, 1100, 1164, 1295, 1404, 1455, 1519, 1606; and Mn 2800 g/mol (GPC relative to PMMA standards)].
Preparation of UV-crosslinked resin of eugenol-based PTU
The eugenol-based PTU (1.0 g), benzophenone (0.02 g), 2-(dimethylamino methyl) eugenol (5) (0.05 g), and 1,6-hexanediol diacrylate (0.05 g) were mixed and dissolved in tetrahydrofuran (5 mL). The solution was coated uniformly on a glass substrate, followed by UV (365 nm) irradiation for a certain period of time.
Preparation of UV-crosslinked resin of eugenol-based PTU and cardanol
In a similar manner as previous procedure, eugenol-based PTU and cardanol, together with other additives, were irradiated under UV (365 nm) for a certain period of time. UV-crosslinked PTU and cardanol were prepared similarly.
Chemical resistance of UV-crosslinked resins
A sheet of UV-crosslinked resin (ca. 0.1 g) was immersed in 2 mL of THF and kept for one week. The dried sample, and the mass changes were determined before and after immersion.
Results and discussion
Synthesis of eugenol-derived diepoxide 2
There is an allylic group in the molecule of eugenol, which allows the synthesis of diepoxide compound through epoxidation reactions (Scheme 1). We have tried to design a two-step route to compound 2, i.e., substitution with epichlorohydrin followed by epoxidation using m-CPBA as the oxidant. However, the complex brown mixtures obtained were difficult to purify in the epoxidation reaction step. Thus, a three-step route was used to synthesize 2 with a 43.9% total yield. 1H NMR spectra of compounds from eugenol to compound 2 are indicated in Fig. 1. Disappearance of peaks at 5–6 ppm indicates that the carbon–carbon double bond in the side chain of eugenol has been converted to epoxide group. Disappearance of the single peak at about 2.3 ppm and appearance of peaks at 4.0–4.3 ppm demonstrate the removal of acetyl protecting group and formation of an ether between eugenol and epichlorohydrin.
Synthesis of eugenol-derived dicyclic carbonate 3 and dicyclic thiocarbonate 4
There are several routes to the synthesis of cyclic carbonates, but the most promising one is the way from selective addition between epoxides and CO2 [5, 36,37,38,39]. Various catalysts from inorganic, organic to complex structures are designed and studied for preparation of these compounds [40,41,42]. According to literature, we think that the most convenient method for preparation of cyclic carbonates is to use metallic halides such as LiBr under atmospheric pressure of CO2 [43]. Thereafter, the diepoxide 2 under CO2 atmospheric pressure together with the catalyst LiBr were heated to 100 °C for hours to afford the corresponding dicyclic carbonate 3 in moderate yield (Scheme 2). 1H NMR spectrum of the dicyclic carbonate 3 is shown in Fig. 2. The singlet peak (a) at 3.8 ppm is the signal of methoxy group. Two multiplet peaks (b) at 6.5–7.0 ppm represent three protons of aryl ring. Two multiplet peaks (c and g) at 4.8 and 5.0 ppm mean two protons (2CH-O) of cyclic carbonates. A doublet-like peak (d) at 4.65 ppm represents two protons of methylene of the ether. The multiplet peaks (f) at 4.0–4.5 ppm may correspond to methylene protons (2CH 2O) of cyclic carbonates. The multiplet peak (e) at 3.0 ppm is methylene protons (ArCH 2-) of benzyl group. Note that weak peaks at 0.5–2 ppm may be solvent and water residues which have no negative effects on the subsequent polymerization.
In addition to CO2, the molecule CS2 can also undergo addition reactions with epoxide groups under certain catalytic conditions, leading to cyclic dithiocarbonates. The mixture of substrate 2, CS2 and LiBr in THF was stirred at room temperature for 24 h, the desired cyclic dithiocarbonate 4 was easily obtained as a viscous yellow solid (Scheme 2). 1H NMR spectrum of 4 is shown in Fig. 3. Main peaks of this spectrum are similar to those of carbonate 3. The chemical shift of peaks (f) is at 3.3–4.0 ppm, shifting to higher field compared to 4.0–4.5 ppm for carbonate 3. This high-field shift for thiocarbonate 4 can be explained as follows: the methylene group in carbonate 3 is bonded to oxygen (i.e., CH 2-O), while that in 4 it is bonded to sulfur (i.e., CH 2-S). It is known that oxygen is more negative than sulfur. Thus, the weak inductive effect of sulfur causes a higher electron density around protons. A peak at 212 ppm in the 13C NMR spectrum of 4 is a powerful proof of the existence of carbon sulfur double bond (C=S) (refers to supporting information).
Preparation of eugenol-based HNIPUs and PTU
Generally, esters can be aminolyzed to form the corresponding amides due to strong nucleophilicity of amino groups. Thus, carbamate groups may be obtained by aminolysis of cyclic carbonates. As a matter of fact, this strategy has been widely studied and used to prepare polyurethanes in recent years. In our reaction system, dicyclic carbonate 3 and 4,4′-diamino diphenylmethane were polymerized to form eugenol-based HNIPU (labeled as HNIPU-D to differentiate it from those made by aliphatic amines) (Scheme 3). Number-average molecular weight of the HNIPU-D is 3700 Da (measured by GPC relative to a PMMA standard). Structures of the HNIPU-D as well as dicyclic carbonate 3 were characterized by IR spectra (Fig. 4). In spectrum (a), a peak at 1802 cm−1 corresponds to the stretching vibration of carbonyl group of carbonate, while this peak almost disappears in spectrum (b), indicating that cyclic carbonate groups are transformed into other groups. The fact that a new peak at 1594 cm−1 appears in spectrum (b) may imply the formation of carbamate groups. Furthermore, a broad peak at 3200–3500 cm−1 in spectrum (b) suggests the existence of hydroxyl (-OH) and/or amino groups in the HNIPU-D.
In addition, aliphatic diamines 1,6-hexamethylene diamine and p-xylene diamine were reacted with dicyclic carbonate 3, and the corresponding polymers HNIPU-H and HNIPU-X were characterized by FTIR (Figs. 5 and 6). The appearance of broad peaks at 3200–3600 cm−1 indicated that hydroxyl (-OH) group was formed by the corresponding reactions. The number-average molecular weights (Mn) of HNIPU-H and HNIPU-X were found as 15,970 and 84,930 Da, respectively (measured by GPC relative to a PMMA standard). By contrast, the order of Mn for three HNIPUs is Mn, HNIPU-X > Mn, HNIPU-H > Mn, HNIPU-D. These results may suggest that aliphatic amines are more reactive than aryl amines in the ring-opening polymerization reactions.
Similarly, cyclic dithiocarbonates can undergo ring-opening reactions under nucleophilic attack of amines (Scheme 4). When the cyclic dithiocarbonate 4 and 4,4′-diamino diphenylmethane were mixed in THF, the reaction proceeded under very mild conditions, and nearly 85% of monomer conversion was obtained after 72 h. Number-average molecular weight of the prepared PTU was Mn 2800 Da (measured by GPC relative to a PMMA standard). According to the reaction conditions, ring-opening of the cyclic thiocarbonate 4 was much easier than that of cyclic carbonate 3. This may be attributed to that carbon oxygen single bond (C–O, bond energy 351 kJ.mol−1) which was much stronger than carbon sulfur single bond (C–S, bond energy 272 kJ.mol−1). Note that when we try to measure T g of the prepared PTU, no inflexion point was found.
Preparation and characterization of UV-crosslinked eugenol-based PTU
Number-average molecular weights (Mn) of our eugenol-based HNIPU-D and PTU are 2500–4000 Da, which are more like oligomers rather than polymers. These oligomers cannot be used straight as polymer materials due to their small Mn values. Photo-curing technology is extensively researched and applied in producing photo-cured thermoset resins [44,45,46]. Thiol-ene click reaction has become a powerful tool to prepare various polymers as well as small molecules in recent years [47,48,49]. In eugenol-based PTU, there is a mercapto group in each unit. Thus, if a proper diene or polyene compound is added, the two components may be crosslinked under thiol-ene reaction conditions. Thus, as a preliminary experiment, the eugenol-based PTU, dilution promoter 1,6-hexanediol diacrylate, photoinitiator benzophenone, and co-photoinitiator 2-(dimethylamino methyl)eugenol (5) are dissolved in THF. The solution is then coated on a glass substrate to form a liquid-like membrane that is subsequently irradiated by UV light (365 nm). After 10 min irradiation, the resulting yellow film becomes finger-touch dry and has the pencile hardness of H (Entry 3, Table 1). Furthermore, it is difficult for the film sample to dissolve in THF. These changes prove that thiol-ene crosslinking reactions do occur under UV irradiation. Note that the film of sole eugenol-based PTU without any additive is bright yellow, fully soluble in THF, and has hardness of 2B (Entry 1, Table 1). Even if no dilution promoter is used, the properties of eugenol-based PTU also change after UV irradiation (Entry 2, Table 1). These changes may be ascribed to possible mercapto group oxidation and/or other radical reactions under UV light. Cardanol, a main component of natural cashew nut shell liquid (CNSL), is a liquid phenol derivative with a C15 linear carbon chain at meta-site [50]. This renewable compound can also be used as a dilution promoter in preparing UV-cured resins [26]. When cardanol is used as the dilution promoter instead of 1,6-hexanediol diacrylate, the UV-induced crosslinking reactions can occur, but reaction rate is much slower than with 1,6-hexanediol diacrylate. This phenomenon can be explained as follows: 1,6-hexanediol diacrylate is a good commercial dilution promoter, whose carbon–carbon double bonds are very reactive towards thiol-ene reactions; while in cardanol, carbon–carbon double bonds in side chains are common and not so reactive. Therefore, cardanol and 1,6-hexanediol diacrylate are combined together as dilution promoters, and 10 min of irradiation affords a film with 2H of hardness (Entry 5, Table 1). The UV-crosslinking reactions are illustrated in Scheme 5. When the irradiation time is extended to 30 min, hardness of the film rises to 4H. Further extending irradiation time to 60 min cannot improve hardness, which means that 30 min is enough for full crosslinking. Unfavorably, long irradiation time such as 60 min will make film color become deep brown or even black.
Conclusion
Hydroxyl non-isocyanate polyurethanes (HNIPUs) and mercapto non-isocyanate polythiourethane (PTU) are successfully prepared by using renewable eugenol as the starting material. When the eugenol-derived diepoxide intermediate reacts with atmospheric pressure CO2, the corresponding dicyclic carbonate is obtained, which is subsequently converted to HNIPUs by ring-opening reactions with aryl and aliphatic diamines. Number-average molecular weights (Mn) of the HNIPUs are Mn HNIPU-D 3700 Da, Mn,HNIPU-H 15,970 Da, and MnHNIPU-X 849,300 Da. If CO2 is replaced by CS2 in the cyclic carbonate-forming reactions, the corresponding dicyclic dithiocarbonate is obtained. The dithiocarbonate intermediate further reacts with diamine to afford mercapto-containing non-isocyanate polythiourethane (PTU). Mn value of the PTU is 2800 Da. To improve its potential application as a material, the oligomer-like PTU further undergoes UV light-promoted thiol-ene crosslinking reactions with cardanol. The optimized conditions for UV-crosslinking are mass ratio of PTU/cardanol/1,6-hexanediol acrylate = 1:1:0.05, and UV irradiation time 30 min. The brown crosslinked polymer prepared under optimized conditions shows certain resistance to THF and has pencil hardness of 4H.
References
Engels HW, Pirkl HG, Albers R, Albach RW, Krause J, Hoffmann A, Casselmann H, Dormish J (2013) Polyurethanes: versatile materials and sustainable problem solvers for today’s challenges. Angew Chem Int Ed 52:9422–9441
Zhou S, Lu HD, Song L, Wang ZZ, Hu Y, Ni JX, Xing WY (2008) Microencapsulated ammonium polyphosphate with polyurethane shell: application to flame retarded polypropylene/ethylene-propylene diene terpolymer blends. J Macromol Sci A 46:136–144
Kamaci M, Kaya I (2014) Photophysical, electrochemical, thermal and morphological properties of polyurethanes containing azomethine bonding. J Macromol Sci A 51:805–819
Nohra B, Candy L, Blanco JF, Guerin C, Raoul Y, Mouloungui Z (2013) From petrochemical polyurethanes to biobased polyhydroxyurethanes. Macromolecules 46:3771–3792
Maisonneuve L, Lamarzelle O, Rix E, Grau E, Cramail H (2015) Isocyanate-free routes to polyurethanes and poly(hydroxyl urethane)s. Chem Rev 115:12407–12439
Delebecq E, Pascault JP, Boutevin B, Ganachaud F (2013) On the versatility of urethane/urea bonds: reversibility, blocked isocyanate, and non-isocyanate polyurethane. Chem Rev 113:80–118
Guan J, Song YH, Lin Y, Yin XZ, Zuo M, Zhao YH, Tao XL, Zheng Q (2011) Progress in study of non-isocyanate polyurethane. Ind Eng Chem Res 50:6517–6527
Ochiai B, Utsuno T (2013) Non-isocyanate synthesis and application of telechelic polyurethanes via polycondensation of diurethanes obtained from ethylene carbonate and diamines. J Polym Sci A 51:525–533
More AS, Gadenne B, Alfos C, Cramail H (2012) AB type polyaddition route to thermoplastic polyurethanes from fatty acid derivatives. Polym Chem 3:1594–1605
Duval C, Kébir N, Charvet A, Martin A, Burel F (2015) Synthesis and properties of renewable nonisocyanate polyurethanes (NIPUs) from dimethylcarbonate. J Polym Sci A 53:1351–1359
Deepa P, Jayakannan M (2008) Solvent-free and nonisocyanate melt transurethane reaction for aliphatic polyurethanes and mechanistic aspects. J Polym Sci A 46:2445–2458
Leitsch EK, Beniah G, Liu K, Lan T, Heath WH, Scheidt KA, Torkelson JM (2016) Nonisocyanate thermoplastic polyhydroxyurethane elastomers via cyclic carbonate aminolysis: critical role of hydroxyl groups in controlling nanophase separation. ACS Macro Lett 5:424–429
Inoue Y, Matsumoto K, Endo T (2015) Synthesis and properties of poly(thiourethane)s having soft oligoether segments. J Polym Sci A 53:1076–1081
Ma XX, Xie H, Shi WF (2013) A highly branched polythiourethane acrylate used for UV-curable high antireflection coatings. Prog Org Coat 76:870–875
Moriguchi T, Endo T (1995) Polyaddition of bifunctional dithiocarbonates derived from epoxides and carbon disulfide: synthesis of novel poly(thiourethanes). Macromolecules 28:5386–5387
Li Q, Zhou H, Wicks DA, Hoyle CE (2007) Thiourethane-based thiol-ene high Tg networks: preparation, thermal, mechanical, and physical properties. J Polym Sci A 45:5103–5111
Kultys A, Rogulska M, Pikus S (2008) The synthesis and characterization of new thermoplastic poly(thiourethane-urethane)s. J Polym Sci A 46:1770–1782
Tyagi M, Suri G, Chhlabra P, Seshadri G, Malik A, Aggarwal S, Khandal RK (2013) Novel way of making high refractive index plastics; metal containing polymers for optical applications. e-Polymers 9:1197–1214
Ranganathan T, Ramesh C, Kumar A (2004) Synthesis of new thermotropic liquid crystalline polyurethanes containing biphenyl mesogens using a novel AB-type self-polycondensation. Chem Commun 2:154–155
Palaskar DV, Boyer A, Cloutet E, Alfos C, Cramail H (2010) Synthesis of bio-based polyurethane from oleic and ricinoleic acids as the renewable resources via the AB-type self-condensation approach. Biomacromol 11:1202–1211
Cheng CJ, Zhang X, Huang QH, Dou XQ, Li J, Cao XX, Tu YM (2015) Preparation of fully bio-based UV-cured non-isocyanate polyurethanes from ricinoleic acid. J Macromol Sci A 52:485–491
Iwata T (2015) Biodegradable and bio-based polymers: future prospects of eco-friendly plastics. Angew Chem Int Ed 54:3210–3215
Yao KJ, Tang CB (2013) Controlled polymerization of next-generation renewable monomers and beyond. Macromolecules 46:1689–1712
Wilbon PA, Chu FX, Tang CB (2013) Progress in renewable polymers from natural terpenes, terpenoids, and rosin. Macromol Rapid Commun 34:8–37
Stemmelen M, Pessel F, Lapinte V, Caillol S, Habas JP, Robin JJ (2011) A fully biobased epoxy resin from vegetable oils: from the synthesis of the precursors by thiol-ene reaction to the study of the final material. J Polym Sci A 49:2434–2444
Cheng CJ, Bai XX, Liu SJ, Huang QH, Tu YM, Wu HM, Wang XJ (2013) UV cured polymer based on a renewable cardanol derived RAFT agent. J Polym Res 20(7):197
Wang R, Luo YN, Cheng CJ, Huang QH, Huang HS, Qin SL, Tu YM (2016) Syntheses of cardanol-based cationic surfactants and their use in emulsion polymerization. Chem Papers 70:1218–1227
Chen GQ, Patel MK (2012) Plastics derived from biological sources: present and future: a technical and environmental review. Chem Rev 112:2082–2099
Kamatou GP, Vermaak I, Viljoen AM (2012) Eugenol-from the remote maluku islands to the international market place: a review of a remarkable and versatile molecule. Molecules 17:6953–6981
Hu KL, Zhao DP, Wu GL, Ma JB (2015) Synthesis and properties of polyesters derived from renewable eugenol and α, ω-diols via a continuous overheating method. Polym Chem 6:7138–7148
Dai JY, Jiang YH, Liu XQ, Wang JG, Zhu J (2016) Synthesis of eugenol-based multifunctional monomers via a thiol–ene reaction and preparation of UV curable resins together with soybean oil derivatives. RSC Adv 6:17857–17866
Qin JL, Liu HZ, Zhang P, Wolcott M, Zhang JW (2014) Use of eugenol and rosin as feedstocks for biobased epoxy resins and study of curing and performance properties. Polym Int 63:760–765
Rahim EA, Sanda F, Masuda T (2006) Synthesis and properties of optically active amino acid based polyacetylenes bearing eugenol and fluorine moieties. J Polym Sci A 44:810–819
Deng JP, Yang B, Chen C, Liang JY (2015) Renewable eugenol-based polymeric oil-absorbent microspheres: preparation and oil absorption ability. ACS Sustainable Chem Eng 3:599–605
Cheng CJ, Zhang X, Chen XH, Li J, Huang QH, Hu ZY, Tu YM (2016) Self-healing polymers based on eugenol via combination of thiol–ene and thiol oxidation reactions. J Polym Res 23(6):110
Huang YC, Liang LY, Ren X, Tan BE (2011) Nonisocyanate polyurethanes and their applications. Prog Chem 23:1181–1188 (in Chinese)
Rokicki G, Parzuchowski PG, Mazurek M (2015) Non-isocyanate polyurethanes: synthesis, properties, and applications. Polym Adv Technol 26:707–761
Guo LP, Wang CM, Luo XY, Cui GK, Li HR (2010) Probing catalytic activity of halide salts by electrical conductivity in the coupling reaction of CO2 and propylene oxide. Chem Commun 46:5960–5962
Sun J, Wang JQ, Cheng WG, Zhang JX, Li XH, Zhang SJ, She YB (2012) Chitosan functionalized ionic liquid as a recyclable biopolymer-supported catalyst for cycloaddition of CO2. Green Chem 14:654–660
Whiteoak CJ, Kielland N, Laserna V, Escudero-Adán EC, Martin E, Kleij AW (2013) A powerful aluminum catalyst for the synthesis of highly functional organic carbonates. J Am Chem Soc 135:1228–1231
Aoyagi N, Furusho Y, Endo T (2013) Convenient synthesis of cyclic carbonates from CO2 and epoxides by simple secondary and primary ammonium iodides as metal-free catalysts under mild conditions and its application to synthesis of polymer bearing cyclic carbonate moiety. J Polym Sci A 51:1230–1242
Sheng XF, Ren GJ, Qin YS, Chen XS, Wang XH, Wang FS (2015) Quantitative synthesis of bis(cyclic carbonate)s by iron catalyst for non-isocyanate polyurethane synthesis. Green Chem 17:373–379
Zhou X, Zhang Y, Yang XG, Yao J, Wang GY (2010) Hydrated alkali metal halides as efficient catalysts for the synthesis of cyclic carbonates from CO2 and epoxides. Chin J Catal 31:765–768
Lee SK, Yoon SH, Chung I, Hartwig A, Kim BK (2011) Waterborne polyurethane nanocomposites having shape memory effects. J Polym Sci A 49:634–641
Cheng CJ, Zhang X, Bai XX, Li J, Cao XX, Wang JL (2015) Synthesis of cardanol-based photo-active SET-LRP initiator and its application to preparation of UV-cured resin. Chem Papers 69:1608–1616
Wu GL, Liu GP, Zang YL, Lu YB, Xiong YQ, Xu WJ (2010) Preparation and characteration of UV-cured EA/MMT nanocomposites via in situ polymerization. J Macromol Sci A 47:647–654
Hoyle CE, Loweb AB, Bowman CN (2010) Thiol-click chemistry: a multifaceted toolbox for small molecule and polymer synthesis. Chem Soc Rev 39:1355–1387
Roth PJ, Boyer C, Lowe AB, Davis TP (2011) RAFT polymerization and thiol chemistry: a complementary pairing for implementing modern macromolecular design. Macromol Rapid Commun 32:1123–1143
Yu L, Wang LH, Hu ZT, You YZ, Wu DC, Hong CY (2015) Sequential Michael addition thiol-ene and radicalmediated thiol–ene reactions in one-pot produced sequence-ordered polymers. Polym Chem 6:1527–1532
Eksik O, Maiorana A, Spinella S, Krishnamurthy A, Weiss S, Gross RA, Koratkar N (2016) Nanocomposites of a cashew nut shell derived epoxy resin and graphene platelets: from flexible to tough. ACS Sustain Chem Eng 4:1715–1721
Acknowledgements
The work was financially supported by the Natural Science Foundations of China (NO. 21,564,004 and 21,264,008) and the Natural Science Foundations of Jiangxi Province (No. 2009GZH0035).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, C., Li, Y., Zhang, X. et al. Eugenol-based non-isocyanate polyurethane and polythiourethane. Iran Polym J 26, 821–831 (2017). https://doi.org/10.1007/s13726-017-0567-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-017-0567-4