Abstract
In this study, glass flakes were incorporated into the spherical nanosilica component of the dental composites and its effect on the mechanical properties of these composites was investigated. To achieve a good interfacial adhesion between matrix resin and fillers, the particles were surface treated with a silane coupling agent (γ-MPS). Composites with different plate-like and spherical nanoparticle contents were prepared and their mechanical properties, including flexural strength, flexural modulus and fracture toughness were measured. The morphology of the particles and fracture surface of the composites were studied by SEM. The distribution of the flakes in the composite was also monitored using EDXA. Statistical analysis of the data was performed with ANOVA and the Tukey’s post hoc test was at a significance level of 0.05. The results showed that the flexural modulus and fracture toughness of specimens were improved with increasing the glass flake content up to 15 vol % which then declined upon further increase due to the stacking of the flakes on each other. A good interfacial adhesion was observed between matrix resin and particles in the SEM micrographs. The results of this study suggest that incorporation of glass flakes into the dental composites containing spherical nanosilica particles may enhance their mechanical properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
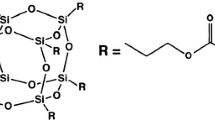
Resin composites are widely used in dental applications because of their good esthetic characteristics. These materials commonly consisted of 2,2 bis[4-(2-hydroxy-3-methacryloxypropoxy)-phenyl]propane (bis-GMA) and triethyleneglycol dimethacrylate (TEGDMA) as organic phases, inorganic fillers for polymer reinforcement, coupling agent and initiator/co-initiator [1, 2]. Silane coupling agents provide a strong link between matrix and filler, improving the performance of the composites where, the most commonly used silane in dental resin composite is 3-methacryloxypropyltrimethoxysilane (γ-MPS) [3, 4].
Fillers play an important role in the mechanical behavior of dental composites depending on filler content, type, shape, size, and morphology [5]. The failure of dental composites has been attributed to surface and/or bulk cracks, degradation of matrix and fillers, water uptake, and lack of sufficient mechanical properties. In this regard, it is expected to increase the dental materials performance by improvement in fracture toughness, which describes as the ability of materials to resist fracture [6].
A significant correlation between the modulus of elasticity and filler volume fraction (vol %) has been reported [7, 8]. Silica particles are the most commonly used fillers for dental composites [9, 10]. Glass flakes have recently received attention as reinforcing fillers in polymeric matrixes [11, 12]. The incorporation of the glass flakes with appropriate coupling agents have shown to enhance the mechanical properties of glass-flake-reinforced polypropylene [11]. Significant improvement in the hardness and compressive strength has been reported for the glass-flake-reinforced dental composites [12]. It has also been reported that fillers with elongated shape particles have more resistance against crack growth compared with the ones with round-shape particles [13].
This study, therefore, with the aim of investigating of the effect of plate-like fillers on the properties of dental composites, evaluates the effect of incorporation of the glass flakes on the flexural properties and fracture toughness of an experimental dental composite containing spherical nanosilica as filler.
Experimental
Materials
2,2-Bis-[4-(methacryloxypropoxy)-phenyl]-propane (Bis-GMA), triethyleneglycol dimethacrylate (TEGDMA) as matrix resins, 3-(Trimethoxysilyl)propyl methacrylate (γ-MPS, Dynasylan® MEMO), and Aerosil® OX50 with a primary particle size of 40 nm and true density of 2.2 g cm−3 were obtained from Evonik (Germany). The photoinitiator system, camphorquinone (CQ) and N–N’-dimethyl aminoethyl methacrylate (DMAEMA) were purchased from Sigma-Aldrich (Germany). Nano glass flakes (GF100 nm) with an average specific surface area of 9.78 m2 g−1 (measured using BET method, N2 adsorption, ChemBET 3000, Quantachrome, USA) and true density of 2.6 g cm−3 were obtained from Glassflake Ltd. (UK). All the solvents were of analytical grade and purchased from Merck (Germany).
Silanization of nanoparticles
To enhance interfacial adhesion between resin and inorganic particles, the surface of the filler particles was treated with γ-MPS. First, glass flake powder was washed by HCl solution for 3 h to remove any impurities and then dried. Both nanoparticles, i.e., OX50 and GF were silanized by γ-MPS. In all cases, the amount of silane was kept constant at 5 wt% with respect to the nanoparticles.
-
The particles (OX50 and GF, separately), the silane, cyclohexane as solvent and n-propylamine as catalyst (2 wt%) were stirred in a rotary evaporator at 60 °C for 2 h. The solvent and volatile by-products were then removed at an elevated temperature using an evaporator [14]. The particles were then washed several times with cyclohexane, to remove unreacted silane, and then were dried. The silanized nanoparticles were identified by FTIR spectroscopy (Equinox 55, Bruker, Germany) using KBr pellet method. Thermogravimetric analysis of the silanized nanoparticles was also performed by TGA thermal analyzer (Mettler TG 50, Switzerland) from room temperature to 700 °C at a heating rate of 10 °C min−1 under N2 atmosphere.
Preparation of nanocomposites
The resin matrix consisting of Bis-GMA/TEGDMA mixture (70:30 wt%) was prepared which contained CQ (0.5 wt%) and DMAEMA (0.5 wt%) as photoinitiator system. The silanized nanoparticles with different volume fractions (according to Table 1) were mixed with the resin by hand spatulation. The total volume fraction was considered constant at 33 vol %. The pastes were then inserted into the stainless steel test molds and cured using a light curing unit (Optilux 501, Kerr, USA) at an intensity of ca. 550 mW cm−2.
Mechanical properties
The mechanical properties of the composites were measured by a universal testing machine (STM-20, Santam, Iran). Having inserted the composite paste into the test molds, the specimens were irradiated on both sides for 40 s using the light curing unit. All specimens were then stored in distilled water at 37 °C for 24 h prior to mechanical testing.
Five rectangular specimens (25 mm × 2 mm × 2 mm) were prepared for three-point bending test according to ISO 4049. The span distance of 20 mm and crosshead speed of 1 mm min−1 were considered. The flexural strength (FS) was calculated using Eq. (1):
where P represents the load in Newtons exerted on the specimen, L is the span length in mm, h is the height and b is the width of the specimen in mm measured before testing.
To determine the fracture toughness, single-edge notch beam (SENB) specimens were prepared according to ASTM E399-05. The specimens were fabricated in steel molds with dimensions of 5 × 2 × 25 mm with a razor blade providing a 2.5 mm notch in the middle of the specimens. A crosshead speed of 0.1 mm min−1 and a load cell of 60 N were considered for the fracture test. The fracture toughness was calculated according to the Eq. (2):
where P is the load at fracture (N), L, W, B, and a are length, width, thickness and notch length (mm), respectively. Span length was 20 mm [15]. The test results are the averages of five repeats.
Scanning electron microscopy
To study the fracture mode and interface between resin and nanoparticles, the fracture surfaces of the specimens were observed by SEM (TESCAN, VEGAII, XMU, Czech Republic). The specimens were gold coated by a sputter coater before the electron microscopy. Energy dispersive X-ray analysis (EDXA) was used to detect the distribution map of the fillers through the composites.
Statistical analysis
The results (five repeats for the mechanical properties) were analyzed and compared using one-way ANOVA and the Tukey’s HSD test at a significance level of 0.05.
Results and discussion
Characterization of silanized nanoparticles
To improve the interfacial adhesion between the nanoparticles and resin matrix, coupling agents are usually used. In this study, both nanoparticles were silanized using direct condensation of γ-MPS in the presence of cyclohexane as solvent and n-propylamine as catalyst [14]. The method suggests that silane would chemically bond to nanoparticles via direct condensation of –CH3 of the silane with the surface hydroxyl groups of the filler to form a covalent band [16].
The silanized nanoparticles were identified by FTIR spectroscopy. Figures 1 and 2 exhibit the FTIR spectra of the silanized and non-silanized OX50 and GF particles. The γ-MPS-grafted nanoparticles showed absorbing bands at ≈1720 cm−1 which is attributed to the carbonyl groups of γ-MPS confirming the grafting reaction [3, 17–19]. The TGA curves of the silanized OX50 and GF particles (Fig. 3) reveal the degree of grafting of 2.2 wt% for GF and 2 wt% for OX50, respectively. The weight loss between 200 and 600 °C was due to the decomposition of γ-MPS bound onto the particles, which represents silane grafting degree.
Flexural properties
The flexural modulus and strength are shown in Figs. 4 and 5. An increasing trend in the flexural modulus was observed with increasing filler content, up to 15 vol % GF (P < 0.05). The increase was attributed to the appropriate orientation of the flakes between the spherical particles. The properties were declined with further increase in the GF content (P < 0.05) due to the stacking of the GF plates on each other providing a weak path for crack growth. As Fig. 5 shows, no significant increase in the flexural strength of the GF containing specimens is observed (P > 0.05). Flexural strength is not only dependent on the strength of the composite ingredients, but also is strongly influenced by the quality of filler–matrix interface [20].
Fracture toughness
The ability of materials to resist fracture and crack propagation is defined as fracture toughness. As shown in Fig. 6, the fracture toughness of the specimens was enhanced with increasing the GF content up to 15 vol % (P < 0.05). The presence of GF between the spherical silica particles caused deviation of crack direction, which increased the energy required for fracture of the composites [13]. However, with increasing the filler fraction, a decrease in the fracture energy absorption was observed which was attributed to the stacking of the GF plates on each other providing a weak path for the propagating crack. The SEM micrographs (Fig. 7) of specimen containing 25 vol % GF shows the stacking of the GF plates.
SEM analysis
SEM was employed to observe the cross section of the specimens. The fracture surfaces of the composite specimens reinforced with 15 and 25 vol % GF are shown in Fig. 7a, b, respectively. The micrographs demonstrate that there is a good adhesion between the resin matrix and the fillers at 15 vol % GF content and the flakes are evenly distributed between the silica nanoparticles. The random distribution of the flakes throughout the specimen thickness may prevent the propagation of the growing crack by providing a tortuous path resulting in higher fracture toughness (Fig. 6). However, at higher GF contents, the stacking of GF plates on each other along with a poor matrix–filler interface adhesion is observed in the Fig. 7b.
The parallel aligned flakes provide a straight path for the cracks to propagate through the specimen leading to a drop in the fracture toughness of the composite (Fig. 6). SEM micrographs also indicate a brittle fracture for the composites. The distribution of the GF in the composites was also monitored using Na map of EDXA (Fig. 8) which also confirmed the stacking of the flakes at higher concentrations.
Conclusion
Glass flakes were utilized to improve the mechanical properties of dental composites. The evenly distributed flakes among the spherical filler particles of the dental composites significantly improved the fracture toughness of the composite. The highest mechanical properties were observed for a dental composite sample with 15 vol % of GF. Further increase in the GF content resulted in a drop in the mechanical properties of the dental composite due to the stacking of the flakes on each other.
References
Foroutan F, Javadpour J, Khavandi A, Atai M, Rezaie H (2011) Mechanical properties of dental composite materials reinforced with micro and nano-size Al2O3 filler particles. Iran J Mater Sci Eng 8:25–33
Valente LL, Peralta SL, Ogliari FA, Cavalcante LM, Moraes RR (2013) Comparative evaluation of dental resin composites based on micron-and submicron-sized monomodal glass filler particles. Dent Mater 29:1182–1187
Karabela MM, Sideridou ID (2011) Synthesis and study of properties of dental resin composites with different nanosilica particles size. Dent Mater 27:825–835
Alsharif SO, Akil HBM, El-Aziz NAA, Ahmad ZAB (2014) Effect of alumina particles loading on the mechanical properties of light-cured dental resin composites. Mater Des 54:430–435
Samuel SP, Li S, Mukherjee I, Guo Y, Patel AC, Baran G, Wei Y (2009) Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent Mater 25:296–301
Beigi S, Yeganeh H, Atai M (2013) Evaluation of fracture toughness and mechanical properties of ternary thiol–ene–methacrylate systems as resin matrix for dental restorative composites. Dent Mater 29:777–787
Braem M, Lambrechts P, Van Doren V, Vanherle G (1986) The impact of composite structure on its elastic response. J Dent Res 65:648–653
Masouras K, Silikas N, Watts DC (2008) Correlation of filler content and elastic properties of resin-composites. Dent Mater 24:932–939
Atai M, Pahlavan A, Moin N (2012) Nano-porous thermally sintered nano silica as novel fillers for dental composites. Dent Mater 28:133–145
Wang H, Zhu M, Li Y, Zhang Q, Wang H (2011) Mechanical properties of dental resin composites by co-filling diatomite and nanosized silica particles. Mater Sci Eng C 31:600–605
Broughton WR, Lodeiro MJ, Pilkington GD (2010) Influence of coupling agents on material behaviour of glass flake reinforced polypropylene. Compos Part A Appl S 41:506–514
Uo M, Sasaki A, Masuda J, Ino J, Watari F (2010) Application of flake shaped glass (Glass Flake) filler for dental composite resin. J Ceram Soc Jpn 118:425–427
Houshyar A, Khavandi AR, Javadpour J, Samani S, Naimi-Jamal MR, Atai M (2013) Enhancement of mechanical properties of experimental composite by Fuller’s earth nanofibers for cervical restoration. J Biomed Mater Res B Appl Biomater 101:911–918
Wilson KS, Zhang K, Antonucci JM (2005) Systematic variation of interfacial phase reactivity in dental nanocomposites. Biomaterials 26:5095–5103
Sakai M, Bradt RC (1993) Fracture toughness testing of brittle materials. Int Mater Rev 38:53–78
Strle M, Kurbus B, Pejovnik S (1993) Silane treatment of silicate fillers—I. Adv Powder Technol 4:49–58
Kango S, Kalia S, Celli A, Njuguna J, Habibi Y, Kumar R (2013) Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites-a review. Prog Polym Sci 38:1232–1261
Karmaker A, Prasad A, Sarkar NK (2007) Characterization of adsorbed silane on fillers used in dental composite restoratives and its effect on composite properties. J Mater Sci Mater Med 18:1157–1162
Herrera NN, Letoffe JM, Reymond JP, Bourgeat-Lami E (2005) Silylation of laponite clay particles with monofunctional and trifunctional vinyl alkoxysilanes. J Mater Chem 15:863–871
Danusso F, Tieghi G (1986) Strength versus composition of rigid matrix particulate composites. Polymer 27:1385–1390
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohseni, M., Atai, M., Sabet, A. et al. Effect of plate-like glass fillers on the mechanical properties of dental nanocomposites. Iran Polym J 25, 129–134 (2016). https://doi.org/10.1007/s13726-015-0407-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-015-0407-3