Abstract
Cotton linter nano-fibers (CLNFs) were prepared from cotton linters by a refining process. The prepared CLNFs were characterized for morphology, crystallinity and degree of polymerization. CLNF was used as a reinforcing agent in guar gum to improve its performance properties. Guar gum/CLNF nanocomposite films were prepared by a solution-casting process. CLNF was added in concentrations of 0.1, 0.25, 0.5 and 1.0 % (w/w) in guar gum. The prepared guar gum/CLNF nano-composite films were characterized for mechanical, thermal, rheological, crystallinity, water vapor transmission rate (WVTR) and light transparency properties. The enthalpy of melting and melting temperature of guar gum increased with increased concentration of CLNF; but up to 0.25 % (w/w) concentration, above which they started decreasing. Tensile strength and Young’s modulus of guar gum increased by 32 and 35 %, respectively, by 0.25 % (w/w) addition of CLNF; however, it decreased on further increase in the concentration of CLNF. The percentage elongation at break and WVTR decreased by 58 and 57 % for 0.25 % (w/w) CLNF-added guar gum. The observed improvements in the properties were due to better interaction between CLNF and guar gum. CLNF was found to have uniformly dispersed in guar gum on addition up to 0.25 % concentration; however, it started forming aggregates at higher concentration, as evident from scanning electron microscopy. Viscosity increased, whereas transparency decreased with increased concentration of CLNF in guar gum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Attempts to improve the properties of polymers by reinforcing agents, either inorganic or organic, are not new [1]. For years, synthetic polymer-based composites have been developed and applied in various industrial fields, domestic equipment, automotive industry and even in the aerospace industry. However, these synthetic polymers are produced from limited, non-renewable sources, which are not easily decomposed [2, 3] by microorganisms present in nature. In addition, global warming caused by the release of carbon dioxide from the combustion of fossil fuels has become an increasingly important problem. The disposal of items made of petroleum-based plastics such as fast food utensils, packaging containers and carrier bags also creates a serious environmental problem.
A growing environmental awareness all over the world and the pressures to use greener technologies [4] have encouraged researchers and industrialists to consider bio-based plastics (biopolymers) as a potential alternative for petroleum-based plastics [5]. Biopolymers are naturally occurring polymers that are found in all living organisms. Biopolymers are therefore said to originate from renewable resources and are biodegradable. As a result, their use in practical applications will have a very less negative effect on our environment compared to petroleum-based plastics [6]. Biocomposites are composite materials comprising one or more phase(s) derived from biological origin [1, 7].
Cellulose is one of the most abundant and renewable biopolymers from biomass in the biosphere [8–10]. Cellulose is the structural material of the fibrous cells with a high level of strength and stiffness per unit weight and has a straight carbohydrate polymer chain consisting of β-1-4 glucopyranose units and a degree of polymerization of about 10,000 [11]. The molecules form aggregates and are present in the form of microfibrils [12]. Hydroxyl (–OH) groups in the cellulose structure play a major role in governing the reactivity and physical property of cellulose. It has attracted rising interest in composites due to its good mechanical properties, low density and biodegradability. In all native cellulose, the molecules are found in fibrillar form with the microfibrils highlighted as the primary constituent of the supermolecular structure. The cellulose chains are arranged in the microfibrils as stacked sheets separated by molecular layers that are connected by interchain hydrogen bonds [13]. The potential of cellulose fibrils as reinforcement provides a new direction for the development of value-added novel composites [14]. Cotton linters are produced in the mechanical process of separating the cotton from its seeds. The linters are short fibers attached to the seeds and other areas of cotton ball which fall out during the separation process. These fibers are viewed as a lower-value by-product or waste in the production of textile-grade fibers [15]. Cotton linters are cellulose fibers. These cellulose fibers can be mechanically disintegrated to structural micro/nanoscale fibrils [16, 17]. The micro/nanofibrils isolated from the natural fibers have much better mechanical and other functional properties as envisaged by Dufresne et al. [18].
Guar gum is a non-ionic, hydrophilic polygalactomannan with a galactose to mannose ratio of approximately 1:2 in which galactose units are randomly distributed on the polymannose backbone. Guar gum is extracted from the endosperm of cyamopsis tetragonalobus, commercially grown in the Indian subcontinent, North Africa and South America [19].
Many researchers have worked on biodegradable polymeric films such as starch [20–22], soy protein [23], silk fibroin [24], polylactide (PLA) [25] or poly(vinyl alcohol) (PVA) [26] and non-biodegradable polymeric films such as polypropylene [27], poly(vinyl chloride) [28], poly(oxyethylene) [29] and epoxy resin [30] for making nanocomposites containing cellulose nanowhiskers, nanocrystals or nanofibers as reinforcing agents to improve the mechanical and functional properties.
The present work deals with the preparation of cotton linter nano-fibers (CLNFs) from cotton linters. CLNFs were characterized for morphology, crystallinity and degree of polymerization properties and were used as a potential reinforcing agent in guar gum. The prepared guar gum/CLNF nano-composites were characterized for mechanical, thermal, rheological, morphological, water vapor transmission rate, crystallinity and transparency properties. This knowledge of composite films could make beneficial contributions to the food and pharmaceutical packaging applications.
Experimental
Materials
Cotton linters, used as the starting material to prepare CLNF, were obtained from Balaji Cotton Linter, Gujarat, India. Sodium hydroxide (AR), nonyl phenol ethylene oxide (AR), sodium silicate (AR) and hydrogen peroxide (AR) were supplied by S.D. Fine Chem. Pvt. Ltd., Mumbai, India. Guar gum (intrinsic viscosity: 10.0 dL/g, Fig. 1) was procured from Sigma Aldrich, Mumbai, India. Distilled water was obtained from Bio Lab Diagnostics India Pvt. Ltd., Mumbai, India.
Preparation of cotton linter nano-fibers (CLNFs)
Cotton linters were first bleached prior to undergoing a refining process. Cotton linters were soaked in a caustic solution (1 % NaOH) with 1:20 material/liquor ratio. To this was added 0.2 mL of nonyl phenol ethylene oxide (wetting agent) per 100 mL of the soaked solution. The vessel containing cotton linters with caustic solution was autoclaved (kier boiling) for 1.5 h at 0.1 MPa to remove waxy and non-cellulosic materials. Then this was bleached using sodium silicate and hydrogen peroxide. After bleaching, the cotton linters were washed with distilled water and dried in an oven at 50 °C.
The CLNFs were prepared by lab-disc refining process from bleached cotton linters by a top-down approach. The lab-disc refiner (Universal Engineering Ltd, India) was used for nanofibrillation which consisted of two discs—one being stationary (stator) and the other rotating (rotor) which was driven by an electrical motor. The distance between the stator and rotor was maintained at 5 thou (127 μm), while the rotor was operated at 1,500 rpm. The cotton linters were added to the hopper of the refiner at 1 % consistency and fed into the fibrillation zone by a helical screw. The output from the fibrillation zone of refiner was collected in a vessel and the entire process was completed in 2 min, and was considered as one pass. It was found that a greater number of passes were required for complete and uniform nano-fibrillation of cotton linters. The cotton linters were refined up to 30 passes and characterization was done after every 5 passes.
Preparation of guar gum/CLNF composite films
The prepared CLNFs were used in different concentrations (0.1, 0.25, 0.5 and 1 % w/w) in guar gum as the reinforcing agent, to improve its performance properties, using the solution-casting process wherein distilled water was used as solvent. CLNFs were first dispersed into the distilled water (1,000 rpm for 2 h) and then a weighed amount of guar gum was added to the mixture at 60 °C. The mixture was stirred continuously for 2 h to obtain a uniform mixture. The solution was then cooled to 40 °C under continuous agitation (1,000 rpm). The homogeneous mixture was then cast in acrylic sheet trays (22 × 18 cm) and allowed to dry at 40 °C for 24 h in a hot air-circulating oven (Siena Instruments, Mumbai, India). A control film was also prepared by the same procedure without any addition of CLNF. The average thickness of the films prepared was 60 ± 2.3 µm as measured by a thickness gauge.
Characterization of CLNF
Scanning electron microscopy
A JEOL® 6380 LA (Japan) scanning electron microscope which operated at 15 kV was used to take micrographs of the samples before and after refining. A drop of diluted (1 mg refined sample diluted in 100 mL deionized water) sample was taken on a mica sheet and dried at room temperature. Dried samples were then vacuum sputtered with gold–palladium mixture to improve their conductivity.
Atomic force microscopy
A scanning probe microscope (atomic force microscope, AFM, Veeco®, USA) was used to characterize the surface morphology of CLNF. AFM height images of the cotton linter samples after refining were taken in a tapping mode with silicon cantilever at a frequency of 253 Hz.
X-ray diffraction analysis
X-ray diffraction (XRD) measurements were performed on a Rigaku® Miniflex wide angle X-ray diffractometer. The XRD of CLNF was measured in a 2θ range from 4° to 40°. The percent of crystallinity of the samples was measured according to Eq. (1) [31, 32]:
where I c is the peak intensity of the crystal plane at 2θ = 22°, which is also called principal peak, and I a is the peak intensity of the amorphous phase at 2θ = 18°.
Degree of polymerization
The degree of polymerization was measured with the help of an Ubbelohde viscometer. A 50 mg sample was dissolved in 100 mL of cupra-ammonium hydroxide. The mixture was vigorously shaken and kept in a water bath at 25 °C. The viscosity was measured only when the sample was completely dissolved in the solvent. The viscosity was calculated from the efflux time of the sample solution and pure solvent. The degree of polymerization was calculated as per Eqs. (2, 3) [33]:
where η spec is the specific viscosity, η/η 0 is the relative viscosity, c is the concentration (g/L), t 0 is the efflux time of the solvent and t is the efflux time of the sample.
Characterization of the guar gum/CLNF composite films
Mechanical properties
The tensile strength (MPa), Young’s modulus (MPa) and percent elongation at break (%) of the guar gum and guar gum/CLNF composite films were determined with a Universal Testing Machine (LR-50 K, LLOYD Instrument, UK) using a 500 N load cell in accordance with ASTM D 882.
Rheological analysis
Measurements of solution viscosity (η) were made at 30 °C using a cone and plate rheometer (MCR 101, Anton Paar, Austria) employing the plate having 35 mm diameter and 2° cone angle, measured over a shear rate range of 0.01–10,000 s−1.
Thermal properties
A differential scanning calorimeter (DSC) was used to measure the thermal transitions of the guar gum and guar gum/CLNF composite films. The test was performed using a Q100 DSC (TA Instruments, USA) equipment, fitted with an nitrogen-based cooling system. The samples were weighed in aluminum pans, whereas an empty pan was used as the reference pan. All the measurements were performed in the temperature range from −40 to 200 °C at a heating rate of 10 °C/min.
X-ray diffraction analysis
X-ray diffraction (XRD) patterns of the prepared films were obtained using a Rigaku Miniflex X-ray diffractometer using Cu target and having X-ray wavelength of 1.54 A through 4°–40° angle.
Light transmittance property
Light transmittance of the guar gum and guar gum/CLNF composite films was measured using a Shimadzu ultraviolet–visible (UV–Vis) spectroscope (UV-160A, Japan) in a wavelength range from 200 to 800 nm.
Water vapor transmission rate
Water vapor transmission rate (WVTR) of the films was determined gravimetrically in accordance with ASTM E96. The composite films were cut into circles of 90 mm diameter and sealed on the permeation cells, containing calcium chloride, using paraffin wax. The permeation cells were placed in a desiccator in which RH was maintained at 71 %. The water transferred through the film was absorbed by the desiccant and determined from the weight of the permeation cell. Each permeation cell was weighed at an interval of 24 h. The WVTR was expressed in g/h m2 per day.
Morphological properties
The morphology of the composite films was observed under a scanning electron microscope (SEM). SEM analysis was carried out using a JEOL® 6380 LA (Japan) scanning electron microscope. Samples were fractured under liquid nitrogen to avoid any disturbance to the molecular structure. The specimens were then coated with gold and palladium using sputter coating before imaging.
Results and discussion
Characterization of CLNF
Morphological properties
The effect of refining on the morphology of linters before and after fibrillation (refining) is shown in Fig. 1 (a and b: cotton linters at 3,500× and 10,000× magnifications, c: cotton linters after ten passes in the refiner and 10,000×, d: cotton linters after 30 passes in the refiner and 10,000×). It was observed that the initial diameter of cotton linters was about 20 µm and it was reduced to 98 nm after fibrillation in a lab-disc refiner (as determined from the AFM analysis mentioned previously). Fibrillation started after one pass itself, but the fibrils were not of uniform size; as the number of passes increased, more uniformity in the size of the fibrils was achieved. After 30 passes, cotton linters were completely converted into CLNF with uniform size distribution. The significant reduction in the average diameter of the linters was attributed to the action of shear and compressive forces on the linters, which is set about in the disintegration process.
The surface morphology of the fibrillated linters was taken using atomic force microscopy with silicon nitride probe in a tapping mode. AFM height image of the fibrillated cotton linters (Fig. 2), obtained after 30 passes, was taken in a tapping mode at a frequency of 253 Hz using silicon nitride cantilever. It can be observed from the height image that the diameter of CLNF was about 98 nm.
X-ray diffraction analysis
Figure 3 illustrates the X-ray diffractograms of the cotton linters before and after 5, 10, 15, 20, 25 and 30 passes of refining. The diffractograms clearly revealed well-defined peaks, typically of highly crystalline structure. The percent of crystallinity of the control cotton linter was found to be 88.33 %, whereas after 5, 10, 15, 20, 25 and 30 passes in the refiner was found to be 88.12, 88.12, 87.06, 87.14, 90.44 and 89.39 %, respectively. Refining process did not affect the percentage of crystallinity of the fibrils. This may be attributed to a highly crystalline nature of cellulose, in which its structural arrangement is not readily disturbed by mechanical forces.
Degree of polymerization
Degree of polymerization is the number of repeating units present in a polymer. Mechanical stresses generated due to shear and impact have great influence on chain scission and hence on the degree of polymerization. During the refining process, cotton linters were subjected to shearing and impact forces; therefore chain scission as well as fibrillation took place, which resulted in significant reduction in the degree of polymerization and diameter of the cotton linter. From Fig. 4, it was observed that the degree of polymerization was reduced by 21.5, 19.45, 21.59 and 24.33 % after refining for 5, 10, 15 and 20 passes, respectively, and afterward remained almost constant till 30 passes. The reduction in the degree of polymerization may be attributed to the continuous exposure of cotton linters to mechanical forces, such as frictional and impact forces when subjected to the lab-disc refining process.
Characterization of guar gum/CLNF composite films
Mechanical properties
Figure 5 (a, b and c) depicts the tensile strength, Young’s modulus and percentage elongation-at-break values obtained for the control guar gum and guar gum/CLNF composite films. Tensile strength and Young’s modulus increased up to 0.25 % concentration of CLNF in guar gum, however, above which it started decreasing. Tensile strength and Young’s modulus values increased by 45 and 36.79 %, respectively, at 0.25 % concentration of CLNF, but at the same time percent elongation was reduced down to 57.9 %. CLNF and guar gum, both as hydrophilic materials, were compatible with each other. This compatibility helped in inducing hydrogen bonding between them, enabling them to interact with each other. This increased the interaction brought about by better alignment of guar gum polymer chains around CLNF, which helped to increase the crystallinity of guar gum. However, the uniform and each distribution level of nano-sized CLNF tremendously increased the available surface area for interaction with guar gum. These were the reasons for the increase obtained in the tensile strength and Young’s modulus for 0.25 % CLNF-loaded guar gum. However, percentage elongation at break is inversely proportional to crystallinity. Higher the crystallinity, lesser will be the elongational property. Thus, percentage elongation at break decreased for 0.25 % CLNF-loaded guar gum. Above 0.25 % concentration of CLNF, an aggregation might have started as evident by SEM micrographs. The presence of aggregates might have resulted in more number of stress concentration points and reduced the effective surface area for interaction with guar gum, which could have resulted in the decrease of tensile strength and Young’s modulus values above 0.25 % CLNF concentration. This also resulted in the increase in the percentage elongation at break. However, percentage elongation at break, tensile strength and Young’s modulus values obtained for the composite films containing CLNF concentration higher than 0.25 % are still better than those obtained for control guar gum.
Rheological analysis
Figure 6 illustrates the plot of viscosity vs. shear rate obtained for guar gum and guar gum/CLNF composites. Viscosity of all the prepared guar gum composite solutions decreased with increase in shear rate. Thus, they all show shear-thinning behavior. Second, the viscosity of guar gum is continuously increased with increased concentration of CLNF.
Guar gum and CLNF, both being hydrophilic materials, have very good compatibility and hence better molecular interaction. The interaction between guar gum and CLNF is increased with increase in concentration of CLNF, leading to increase in viscosity of the composite. However, this study cannot be directly correlated with the properties of the prepared guar gum and guar gum/CLNF composite films, as this study was performed on 1 % solution of the composite material. Nevertheless, all solutions were prepared with the same concentration. Thus, the increase in the viscosity of guar gum with increase in the concentration of CLNF can be suggested to have been caused by better interaction between CLNF and guar gum.
Thermal properties
Figure 7 illustrates the DSC thermograms obtained for the prepared guar gum and guar gum/CLNF composite films. Melting temperature and enthalpy of melting values determined for control guar gum were 116.43 °C and 131.8 J/g, respectively. These values increased for guar gum films loaded with 0.1 and 0.25 % CLNF; however, the values started decreasing for higher CLNF concentrations, but were still higher than those for the control guar gum. Melting temperature values determined for 0.1, 0.25, 0.5 and 1 % CLNF-loaded guar gum composite films were 120.12, 122.64, 117.65 and 116.85 °C, respectively, whereas the enthalpies of melting were 197.1, 212.5, 191.2 and 186.0 °C, respectively.
Individual level and uniform distribution of CLNF in guar gum up to 0.25 % concentration, and better compatibility induced by the hydrophilic nature of both materials, led to better interaction and subsequent molecular alignment of guar gum polymer chains about CLNF, increasing the crystallinity of the composite films as compared to control guar gum. This increase in crystallinity of guar gum increased molecular packing per unit volume, and so requires higher amount of energy and temperature to convert into molten mass. However, for CLNF concentrations above 0.25 %, the aggregates of CLNF were seen to have formed in the guar gum matrix (confirmed through SEM analysis). This led to the overall decrease in the effective surface area of CLNF that interacted with guar gum, decreasing the level of guar gum molecular alignment around CLNF and thus decreasing the crystallinity and molecular packing per unit volume. So, melting temperature and enthalpy of melting decreased for guar gum films added with CLNF concentration higher than 0.25 %.
X-ray diffraction analysis
Figure 8 indicates the X-ray diffractograms obtained for cotton linter nano-fibers (CLNFs), control guar gum and guar gum/CLNF composite films. The peaks at 2θ = 14.7°, 16.3° and 22.6° correspond to the 101, 101 and 002 crystallographic planes of cellulose, respectively [34, 35]. The increase in the crystalline region increased the rigidity of the composite [36]. It was observed that as the concentration of the CLNF in guar gum increased, the crystallinity of the composite films also increased. The crystallinity of the guar gum was 1.9 %; however, it increased to 2.6, 2.8, 2.2 and 2.0 % after incorporation of 0.1, 0.25, 0.5 and 1 % CLNF, respectively. The crystallinity of the guar gum was found to have increased by 47.4 % on just 0.25 % incorporation of CLNF, which is very significant. Thus, the highest improvement in the crystallinity of guar gum was observed on 0.25 % loading of CLNF. However, the crystallinity values obtained for higher CLNF-loaded guar gum were still higher than those observed for control guar gum. The observed trend in the crystallinity of the prepared control and composite guar gum films is similar to that observed in the mechanical and thermal properties. This increase in crystallinity (whatsoever) is attributed to the better interaction of CLNF with guar gum matrix obtained due to the hydrophilic nature of both guar gum and CLNF, bringing about better alignment of guar gum polymer chains around CLNF.
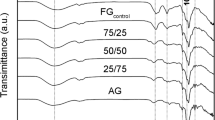
Light transmittance property
The transparency of the films depends on the particle size and degree of dispersion of the fillers in the matrix [37]. It was observed from Fig. 9 that control guar gum films were more transparent and their transparency started decreasing as the concentration of CLNF increased from 0.1 to 1 %. Addition of CLNF to guar gum increased its crystallinity and provided barrier to the transmission of light, thus increasing the haziness of the composite film. However, the highest crystallinity was obtained for 0.25 % CLNF-loaded guar gum. Therefore, transparency should have decreased only up to 0.25 % CLNF-loaded guar gum, but on the contrary transparency decreased even for higher concentrations of CLNF. This was attributed to the CLNF aggregates formed at higher CLNF concentrations.
Water vapor transmission rate (WVTR)
Figure 10 depicts the WVTR of control guar gum and guar gum/CLNF composite films. It was observed that as the concentration of CLNF increased, WVTR decreased up to 0.25 % concentration of CLNF, above 25.5, 56.7, 49.7 and 24.4 % which started increasing. As compared to control guar gum, the reductions in the WVTR were noticed for 0.1, 0.25, 0.5 and 1 % CLNF-loaded guar gum, respectively. The addition of CLNF in guar gum increased the crystallinity of the composite providing barrier to the transmission of water vapor by increasing the tortuosity, and thus increasing the barrier property of the composite film. It has also been discussed by Berglund et al. [38] that moisture uptake decreases with increasing content of cellulose in starch. However, the increase in WVTR for 0.5 and 1 % CLNF-loaded guar gum composite films could have been due to the formation of CLNF aggregates as evident from SEM micrographs.
Morphological properties
Scanning electron microscopy (SEM) analysis was done to understand the correlation between the dispersion behavior of CLNFs into guar gum films and their performance properties. Figure 11 (a: control guar gum, b: 0.25 % CLNF and c: 0.5 % CLNF-loaded guar gum) illustrates the SEM micrographs of the control guar gum and guar gum/CLNF composite films. It was observed that the dispersion of CLNF was uniform in the guar gum matrix at 0.25 % concentration, but as the concentration of the CLNF was increased to 0.5 %, aggregations were observed. Formation of aggregates in 0.5 % CLNF-loaded guar gum corroborates overall with the reduction in the performance properties above 0.5 % CLNF concentration in guar gum.
Conclusion
Cotton linter nano-fibers (CLNFs) were successfully prepared from bleached cotton linters using the lab-disc refining process. SEM micrographs showed that as the number of passes in the refiner was increased, more fibrillation was achieved with uniform fiber dimensions. The degree of polymerization was found to decrease by 24.7 % after 30 passes in the refiner. The crystallinity of CLNF was insignificantly affected even after 30 passes in the refining process. Guar gum/CLNF composite films were prepared by solution-casting process, using distilled water as the solvent. Tensile strength and Young’s modulus of the guar gum/CLNF composite films were improved by 33.3 and 52.3 %, respectively, whereas the percentage elongation at break decreased by 69.6 % for 0.25 % CLNF-loaded guar gum films. The crystallinity of the 0.25 % CLNF-loaded composite film increased from 1.9 % (control guar gum) to about 2.8 %. DSC analysis showed that the melting temperature and enthalpy of melting increased with increase in concentration of CLNF in guar gum. WVTR decreased by 56.75 % for 0.25 % CLNF-loaded guar gum. The SEM micrographs of the composites showed uniform dispersion at 0.25 % concentration of CLNF, but as the concentration increased further CLNF started forming aggregates. The observed improvement in the performance properties was due to the proper interaction between guar gum and CLNF, brought about by the hydrophilic nature of both materials.
References
Lu DR, Xiao CM, Xu SJ (2009) Starch-based completely biodegradable polymer materials. Express Polym Lett 3:366–375
Tokiwa Y, Calabia BP, Ugwu CU, Aiba S (2009) Biodegradable plastics. Int J Mol Sci 10:3722–3742
Bismarck A (2008) Are hierarchical composite structures the way forward to improve the properties of truly green composites? Express Polym Lett 2:687
Thakur VK, Singha AS (2010) Natural fibres-based polymers: part 1-Mechanical analysis of pine needles reinforced biocomposites. Bull Mater Sci 33:257–264
Petersson L, Kvien I, Oksman K (2007) Structure and thermal properties of poly(lactic acid)/cellulose whiskers nanocomposite materials. Compos Sci Technol 67:2535–2544
Hule RA, Pochan DJ (2007) Polymer nanocomposites for biomedical applications. MRS Bull 32:354–358
Zeng X, Gan YX (2011) Advances in composite materials for medicine and nanotechnology, 1st edn. InTech, Croatia 119
Lee SY, Mohan DJ, Kang IA, Doh GH, Lee S, Han SO (2009) Nanocellulose reinforced PVA composite films: effects of acid treatment and filler loading. Fibre Polym 10:77–82
Wang S, Cheng Q, Rials TG and, Lee SH (2006) Cellulose microfibril/nanofibril and its nanocompsites. Proceedings of the 8th Pacific Rim Bio-based Composites Symp, Kuala Lumpur, Malaysia. 301–308
Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110:3479–3500
Kamel S (2007) Nanotechnology and its applications in lingo-cellulosic composites, a mini review. Express Polym Lett 1:546–575
Hult EL, Iversen T, Sugiyama J (2003) Characterization of the supermolecular structure of cellulose in wood pulp fibers. Cellulose 10:103–110
Li Q, Renneckar S (2009) Molecularly thin nanoparticles from cellulose: isolation of sub-microfibrillar structures. Cellulose 16(6):1025–1032
Lee SY, Chun SJ, Kang IA, Park JY (2009) Preparation of cellulose nanofibrils by high-pressure composite films. J Ind Engg Chem 15:50–55
Shi B, Shannon TG, Pelky E (2010) Novel use of waste keratin and cotton linter fibres for prototype tissue papers and their evaluation. Bioresources 5:1425–1435
Ahola S, Salmi J, Johansson LS, Laine J, Osterberg M (2008) Model films from native cellulose nanofibrils. Preparation, swelling, and surface interactions. Biomacromolecues 9:1273–1282
Nakagaito AN, Yano H (2005) Novel high-strength biocomposites based on microfibrillated cellulose having nano-order-unit web-like network structure. Appl Phys A 80:155–159
Siqueira G, Bras J, Dufresne A (2010) Cellulosic bionanocomposites: a review of preparation, properties and applications. Polymer 2:728–765
D’Mello D, Sabnis A, Shenoy MA, Kathalewar M (2012) Preparation of acetylated guar gum—unsaturated polyester composites and effect of water on their properties. Curr Chem Lett 1:147–156
Savadekar NR, Mhaske ST (2012) Synthesis of nano cellulose fibers and effect on thermoplastics starch based films. Carbohydr Polym 89:146–151
Choi Y, Simonsen J (2006) Cellulose nanocrystal-filled carboxymethyl cellulose nanocomposites. J Nanosci Nanotechnol 6:633–639
Lu Y, Weng L, Cao X (2006) Morphological, thermal and mechanical properties of ramie crystallites—reinforced plasticized starch biocomposites. Carbohydr Polym 63:198–204
Lu Y, Weng L, Zhang L (2004) Morphology and properties of soy protein isolate thermoplastics reinforced with chitin whiskers. Biomacromolecules 5:1046–1051
Wongpanit P, Sanchavanakit N, Pavasant P, Bunaprasert T, Tabata Y, Rujiravanit R (2007) Preparation and characterization of chitin whisker-reinforced silk fibroin nanocomposite sponges. Eur Polym J 43:4123–4135
Huang M, Yu J, Ma X (2006) High mechanical performance MMT-urea and formamide-plasticized thermoplastic cornstarch biodegradable nanocomposites. Carbohydr Polym 63:393–399
Zhang J, Elder TJ, Pu Y, Ragauskas AJ (2007) Facile synthesis of spherical cellulose nanoparticles. Carbohydr Polym 69:607–611
Ljungberg N, Bonini C, Bortolussi F, Boisson C, Heux L, Cavaillé JY (2005) New nanocomposite materials reinforced with cellulose whiskers in atactic polypropylene: effect of surface and dispersion characteristics. Biomacromolecules 6:2732–2739
Chazeau L, Cavaill JY, Perez J (2000) Plasticized PVC reinforced with cellulose whiskers II. Plastic behavior. J Polym Sci Part B Polym Phys 38:383–392
Bondeson D, Mathew A, Oksman K (2006) Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 13:171–180
Shimazaki Y, Miyazaki Y, Takezawa Y, Nogi M, Abe K, Ifuku S, Yano H (2007) Excellent thermal conductivity of transparent cellulose nanofiber/epoxy resin nanocomposites. Biomacromolecules 8:2976–2978
Nelson ML, O’Connor RT (1964) Relation of certain infrared bands to cellulose crystallinity and crystal lattice type part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J Appl Polym Sci 8:1325–1341
Terinte N, Ibbett R, Schuster KC (2011) Overview on native cellulose and microcrystalline cellulose I structure studied by X-ray diffraction (WAXD): comparison between measurement techniques. Lenzing Ber 89:118–131
Grobe A, Brandrup J, Immergut EH (1989) Polymer handbook, Chapter 5. Wiley, NY, pp 101–107
Sun Y, Lin L, Pang C, Deng H, Peng H, Li J, He B, Liu S (2007) Hydrolysis of cotton fibre cellulose in formic acid. Energy Fuels 21:2386–2389
Wang L, Kumar R, Zhang L (2009) Investigation into hemp fiber- and whisker- reinforced soy protein composites. Frontiers Chem China 4:313–320
Alemdar A, Sain M (2008) Isolation and characterization of nanofibers from agricultural residues—wheat straw and soy hulls. Bioresour Technol 99:1664–1671
Chen Y, Liu C, Chang PR, Anderson DP, Huneault MA (2009) Pea starch-based composite films with pea hull fibers and pea hull fiber-derived nanowhiskers. Polym Eng Sci 49:369–378
Svagan AJ, Hedenqvist MS, Berglund L (2009) Reduced water vapour sorption in cellulose nanocomposites with starch matrix. Compos Sci Technol 69:500–506
Vilas K, Bharimalla AK, Vighneswaran VN, Mhaske ST (2012) Effect of cellulose nanowhiskers on performance properties of guar gum. ANTEC Mumbai 2012, paper no: 1456451
Acknowledgments
The authors are thankful to the National Agricultural Innovation Project (NAIP), Indian Council of Agricultural Research (ICAR) for providing financial support for this work, through a project entitled “Synthesis and characterization of nanocellulose and its applications as biodegradable polymer composites to enhance their performance properties” (code number 417101). We are also thankful to the Society of Plastic Engineers (SPE) for giving us the opportunity to participate in Antec-Mumbai 2012 [39].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karande, V.S., Bharimalla, A.K., Vigneshwaran, N. et al. Cotton linter nano-fibers as the potential reinforcing agent for guar gum. Iran Polym J 23, 869–879 (2014). https://doi.org/10.1007/s13726-014-0283-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-014-0283-2