Abstract
In this study, chitin was nanofibrillated, cationized, and then used as a reinforcing agent for xanthan gum hydrogels. Amidinated chitin nanofibers (CNFs), which were prepared by partial deacetylation of the nanofibrillated chitin and the subsequent reaction of the generated amino groups with N,N-dimethylacetamide dimethyl acetal, were converted into an amidinium chitin bicarbonate with nanofiber morphology by CO2 gas bubbling and ultrasonic treatments in water. Xanthan gum hydrogels, which were prepared by exchange of disperse media from xantham gum ion gels with 1-butyl-3-methylimidazolium chloride, were then soaked in the resulting cationic CNF aqueous dispersions with different degrees of substitution (DSs) of amidinium groups to progress composition, giving rise to the CNF-reinforced xanthan gum hydrogels. The presence of CNFs in the hydrogels was confirmed by SEM measurement of the lyophilized samples. The amounts of CNFs in the hydrogels increased with increasing the DS values. The compression testing of the hydrogels suggested the reinforcing effect of CNFs, which were induced by electrostatic interaction owing to anionic nature of xanthan gum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polysaccharides are widely distributed in nature, and accordingly, regarded as representative biomass resources [1, 2]. For example, natural polysaccharides composed of β(1 → 4)-linked monosaccharide repeating units act as important structural materials and can be expected as the components in bio-based functional materials alternative to petroleum-based materials because of their strength, biodegradable, low-toxic, and eco-friendly properties [3, 4]. Representatively, cellulose and chitin (Fig. 1) are composed of β(1 → 4)-linked d-glucose and N-acetyl-d-glucosamine (GlcNAc) repeating units, which are the most abundant organic resources in nature, mainly present in the cell walls of plants and the exoskeletons of crustaceans, shellfishes, and insects as structural materials, respectively [5,6,7,8]. Xanthan gum is a polysaccharide, also comprising a β(1 → 4)-linked d-glucose main-chain as same as cellulose, but which further shows a anionic nature owing to the presence of trisaccharide side chains with carboxylate groups (mannose-β(1 → 4)-glucuronic acid-β(1 → 2)-mannose-α(1 → 3)-), attached to alternate glucose units (Fig. 1) [9]. Such β(1 → 4)-linked polysaccharides often show solubility problem in common organic solvents owing to numerous intra- and intermolecular hydrogen bonds and stiff molecular chain packing, leading to poor in feasibility and processability in material application [10]. Therefore, the research concerning efficient incorporation of such polysaccharides into functional materials has attracted much attention even in recent years. One of the efficient approaches to their materialization is nanofibrillation, such as nanofibers and nanowhiskers [11,12,13,14,15,16,17]. For example, breaking down the starting bulk fibril materials from native cellulose and chitin sources by appropriate treatments in water has been conducted to induce nanofibrillation, such as by acid hydrolysis, grinding mechanical technique, and electrostatic repulsion after the introduction of carboxylate groups [18,19,20,21,22]. We also reported facile disentanglement of a native fibril chitin powder by N2 gas bubbling and ultrasonic treatments in water upon top-down approach to obtain an aqueous dispersion of chitin nanofibers (CNFs). Amino groups were then generated by partial deacetylation of CNFs (partially deacetylated (PDA)-CNFs), which were potentially converted into cationic amidinium groups through amidination and subsequent cationization with CO2 to fabricate cationic CNFs (Fig. 2) [23]. Because of exceptional properties of such polysaccharide nanomaterials as their lightweight character and high tensile strength, they have been used as reinforcing agents by composition for enhancement of mechanical properties of polymeric materials. The abovementioned cationic CNFs have also been used for composition with anionic polymers by electrostatic interaction to fabricate composite materials [24, 25].
On the other hand, we have reported the efficient formation of a xanthan gum hydrogel through exchange of disperse media from an ion gel with an ionic liquid, 1-butyl-3-methylimidazolium chloride (BMIMCl) [26, 27], based on the fact that ionic liquids are identified as good media for materialization of polysaccharides [28,29,30,31,32,33,34,35,36]. Furthermore, the mechanical property of the xanthan gum hydrogel was enhanced by the formation of ionic cross-linking of carboxylates in xanthan gum with multivalent metal cations, such as Ca2+. In the present study, we investigated the use of the cationic amidinium CNFs as a reinforcing agent for the xanthan gum hydrogel to enhance the mechanical properties. After exchange of disperse media from BMIMCl in the xanthan gum ion gels to water, the resulting hydrogels were soaked in aqueous dispersions of the cationic CNFs to fabricate the composite materials with multipoint ionic cross-linking by electrostatic interaction.
Experimental
Materials
Chitin powder from crab shell was purchased from Wako Pure Chemicals, Tokyo, Japan. An ionic liquid, 1-ally-3-methylimidazolium bromide (AMIMBr), was prepared by the reaction of 1-methylimidazole with 3-bromo-1-propene according to a method adapted from the literature [37]. N2 and CO2 gases were directly introduced into experimental media by bubbling through a glass pipette from gas cylinders. Other reagents and solvents were available commercially and used without further purification.
Preparation of Amidinated Chitin
A mixture of chitin (0.252 g, 1.23 mmol) with water (40 mL) was subjected to N2 gas bubbling and ultrasonic treatments for 8 h to give a dispersion, which was lyophilized to obtain the nanofibrillated chitin (0.172 g). The product was then treated with 30 wt% NaOH aq. (30 g) at 80 °C for 5 h. After the mixture was immersed in a mixed solution of methanol (200 mL) and water (100 mL) overnight, the N-deacetylated product was isolated by filtration, washed with methanol and water, and lyophilized (0.135 g). The degree of deacetylation of the product was estimated by the integrated ratio of the CH3 signal to the H-1 signal in the 1H NMR spectrum of the sample solubilized by acidic hydrolysis of the produced PDA-chitin in DCl/D2O to be 28% for the total repeating units.
A mixture of the resulting PDA-chitin (0.12 g, 0.18 unit mmol) and N,N-dimethylacetamide dimethylacetal (10 equiv. with amino group, 0.24 g, 1.8 mmol) with AMIMBr (2.0 g, 9.80 mmol) was heated at 80 °C for 2 h. After the mixture was immersed in methanol (300 mL) for 24 h, the product was isolated by filtration, washed with methanol and water, and lyophilized to give the amidinated chitin (0.106 g). 1H NMR spectrum of the sample solubilized by acidic hydrolysis of the product in DCl/D2O: δ 2.4–2.5 (m, CH3C=O), 2.92, 3.08 (br, CH3C–N(–N)), 3.5–4.4 (br, H2–H6, CH3–N), 4.80 (br s, H1 of β(1 → 4)-linked GlcNAc chain), 4.9–5.1 (br, H1β of GlcNAc and D-glucosamine (GlcN), H1 of β(1 → 4)-linked GlcN (hydrochloride) and amidinated GlcN (hydrochloride)), 5.37 (br s, H1α of GlcNAc), 5.56 (br s, H1α of GlcN). From the integrated ratio of the CH3 signals of acetamidine to the H1 signals (δ 4.80–5.56), the degree of substitution (DS) value was calculated to be ca. 6% for the total repeating units.
Similarly, the reactions of PDA-chitin with 25, 50, and 100 equivs. of N,N-dimethylacetamide dimethylacetal gave amidinated chitins with the DS values of 14, 17 and 21%, respectively.
Preparation of CNF-Reinforced Xanthan Gum Hydrogel
A typical experimental procedure was as follows (run 1, Table 1). Xanthan gum (0.2 g, 0.21 mmol) was added to BMIMCl (1.1 g, 6.3 mmol) and stirred for 3 min at 100 °C to be a homogeneous solution (15 wt%). After the solution was continuously heated at 100 °C for 12 h without stirring, it was kept at room temperature for 30 min to give a xanthan ion gel. The resulting gel was soaked in water (100 mL) for 24 h to give a hydrogel, which was taken out from the water solution. The resulting hydrogel was lyophilized to estimate the water content, which was calculated to be 94 wt%. A mixture of the amidinated chitin (DS = 6%, 0.10 g, 0.51 mmol) with water (50 mL) was subjected to CO2 gas bubbling and ultrasonic treatments for 8 h to give an amidinium CNF dispersion. The xanthan hydrogel was then soaked in the resulting dispersion to give CNF-reinforced hydrogel (3.0 g). The resulting hydrogel was lyophilized to estimate the water content, which was calculated to be 95 wt%.
The abovementioned nanofibrillated chitin was used as a reinforcing agent in a contrast experiment, in which the same procedure as above was conducted to obtain a reference sample.
Water-Treatment of CNF-Reinforced Xanthan Gum Hydrogel
A mixture of the lyophilized sample (0.02 g) from the CNF-reinforced xanthan gum hydrogel of run 4 with water (40 mL) was gently stirred at 50 °C for 6 h and subsequently ultrasonicated for 1 h. The residual material was isolated by filtration and dried under reduced pressure at 60 °C for 3 h (0.016 g). From the xanthan gum hydrogel, the same procedure was conducted as a contrast experiment.
Measurements
SEM images were obtained using a Hitachi S-4100H electron microscope (Hitachi High-Technologies Corporation, Tokyo, Japan). Stress–strain curves were measured using a tensile tester (Little Senstar LSC-1/30, Tokyo Testing Machine, Tokyo Japan). The 1H NMR spectra were recorded using ECX400 and ECA600 spectrometers (JEOL, Akishima, Tokyo, Japan).
Results and Discussion
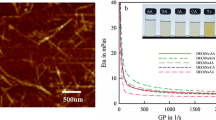
To prepare the cationic CNFs, the amidinated chitin was prepared by the reaction of nanofibrillated PDA-chitin (degree of deacetylation = 28%), which was obtained by nanofibrillation of a commercial chitin powder by N2 gas bubbling/ultrasonic treatments in water and subsequent deacetylation under alkaline conditions, with N,N-dimethylacetamide dimethyl acetal in an ionic liquid, AMIMBr, according to a procedure previously reported by us (Fig. 2) [23]. The structure of the resulting amidinated chitin was established by the 1H NMR analysis of the sample solubilized by acidic hydrolysis of the product in DCl/D2O because of its insolubility in common NMR solvents. By changing the ratios of N,N-dimethylacetamide dimethyl acetal to amino groups (10, 25, 50, and 100 equivs.), amidinated chitins with DS values of 6, 14, 17 and 21% were obtained. The amidine groups in the products were then converted into amidinium bicarbonates by protonation, along with simultaneous additional nanofibrillation by CO2 gas bubbling and ultrasonic treatment of the resulting amidinated chitins (0.10 g) in water (50 mL), giving rise to aqueous dispersions of the cationic CNFs. The nanofiber morphology was seen in the SEM image of the aqueous dispersion (DS = 14%, Fig. 3a).
SEM images of a amidinium CNF aqueous dispersion (DS = 14%), b surface area of lyophilized sample from xanthan gum hydrogel, c–g) surface areas of lyophilized samples from CNF-reinforced xanthan gum hydrogels of runs 1–5, and h–j cross-sectional areas (10, 50, and 100 µm depths) of lyophilized sample from CNF-reinforced xanthan gum hydrogel of run 4
A xanthan gum hydrogel was prepared by soaking a xanthan gum ion gel (xanthan gum content = 15 wt%), which was prepared by a heating–cooling process of a xanthan gum/BMIMCl mixture, in water for 24 h [26, 27]. A water content was calculated by the weight difference of the lyophilized sample from the hydrogel to be 94 wt%. The obtained hydrogel (xanthan gum content = 0.2 g) was then soaked in the abovementioned amidinium CNF dispersions (CNF content = 0.10 g, xanthan gum:CNF = 2:1 (w/w)) with different DS values for 24 h to obtain CNF-reinforced xanthan gum hydrogels (Fig. 4). The products were turbid because of the presence of CNFs, while color unevenness was not largely observed, indicating fairly dispersion of CNFs at the surface areas of the products. As a contrast experiment, the nanofibrillated chitin without amidinium cations was used as a reinforcing agent in the same procedure to produce the reference hydrogel sample. Water contents of the resulting hydrogels, which were calculated by the weight differences of the lyophilized samples from the hydrogels, were around 93–95 wt% regardless of the DS values of amidinium groups. The amounts of CNFs composited into the hydrogels were calculated by weights of unincorporated CNFs. As shown in Table 1, the amounts of CNFs in the hydrogels increased in accordance with increase of the DS values (runs 1–4), suggesting the controllable introduction of CNFs as a reinforcing agent by the present method. This is probably owing to the formation of more electrostatic interactions between amidinium cations and carboxylate anions on CNF surfaces with increasing the DS values. Indeed, the amount of CNFs in the reference sample without electrostatic interaction, because of the absence of amidinium cations, was much smaller (run 5) than the other products. Figure 3c–f shows the SEM images of surface areas of the lyophilized samples from the hydrogels of runs 1–4 in comparison of those of the lyophilized xanthan gum hydrogel (Fig. 3b). All the images from the different DS values observe well-dispersed nanofiber morphologies, strongly supporting composition of CNFs with xanthan gum. On the other hand, the SEM image of a surface area of the lyophilized sample from the reference hydrogel (run 5) observes much less amounts of nanofibers (Fig. 3g), that is in good agreement with the lower weight ratio of CNFs listed in Table 1. The SEM images of cross-sectional areas of the lyophilized sample (run 4) exhibit to decrease the amounts of nanofibers depending on depths from the surface (10, 50, and 100 µm, Fig. 3h–j), suggesting that CNFs have been mostly present at the surface layer in the hydrogel.
The composition of xanthan gum with the cationic CNFs by electrostatic interaction was evaluated by water-treatment of the lyophilized sample (run 4) at 50 °C for 6 h, followed by untrasonication. Consequently, the sample was mostly remained as shown in Fig. 5a, in which its weight after isolation by filtration and drying was comparable to that before the treatment (0.016 and 0.020 g, respectively). In contrast, the lyophilized sample from the pure xanthan gum hydrogel was solubilized by the same treatment with water (Fig. 5b) and no residue was obtained by filtration. These results strongly supported the composition between the two components by multipoint ionic cross-linking owing to electrostatic interaction.
The mechanical properties of the hydrogels were evaluated by compression testing. The stress–strain curves in Fig. 6a–e indicate increase of fracture stress values and decrease of fracture strain values with increasing the amounts of CNFs, compared with those of the xanthan gum hydrogel. These results suggest the hydrogels to be strengthened in accordance with the amounts of CNFs, strongly supporting the reinforcing effect of CNFs probably by electrostatic interactions with xanthan gum in the hydrogels. Because of the much smaller amount of CNFs in the reference sample of run 5, the reinforcing effect has not obviously been observed compared with the other samples, as its stress–strain curve exhibits the similar profile to that of the xanthan gum hydrogel (Fig. 6a, f).
Conclusions
In this study, we performed the preparation of amidinium CNF-reinforced xanthan gum hydrogels. The cationic CNFs were prepared according to the previously reported method by us. The xanthan gum hydrogel, which was prepared from the xanthan gum ion gel with BMIMCl, was soaked in the CNF aqueous dispersion for the progress of composition by electrostatic interaction between the cationic CNFs and anionic xanthan gum. The DS values of amidinium groups affected the composition amounts of CNFs and also the mechanical properties of the resulting hydrogels under compression mode. The present materials resulted from natural polysaccharide sources have a potential for practical applications as new functional bio-based materials, such as drug carriers, biocompatible substrates, and tissue matrixes, in biomedical and tissue engineering fields in the future, because of their biodegradable, eco-friendly, and non-toxic properties.
References
Schuerch C (1986) Polysaccharides. In: Mark HF, Bilkales N, Overberger CG (eds) Encyclopedia of polymer science and engineering, vol 13, 2nd edn. Wiley, New York, pp 87–162
Berg JM, Tymoczko JL, Stryer L (2012) Biochemistry, 7th edn. W.H. Freeman, New York
Rouilly A, Rigal L (2002) Agro-materials: a bibliographic review. J Macromol Sci Polym Rev C42:441–479
Mohanty AK, Misra M, Drzal LT (2002) Sustainable bio-composites from renewable resources: opportunities and challenges in the green materials world. J Polym Environ 10:19–26
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Kurita K (2006) Chitin and chitosan: functional biopolymers from marine crustaceans. Marine Biotechnol 8:203–226
Pillai CKS, Paul W, Sharma CP (2009) Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Polym Sci 34:641–678
Muzzarelli RAA (2011) Biomedical exploitation of chitin and chitosan via mechano-chemical disassembly, electrospinning, dissolution in imidazolium ionic liquids, and supercritical drying. Mar Drugs 9:1510–1533
Melton LD, Mindt L, Rees DA (1976) Covalent structure of the extracellular polysaccharide from Xanthomonas campestris: evidence from partial hydrolysis studies. Carbohydr Res 46:245–257
Younes I, Rinaudo M (2015) Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 13:1133–1174
Kadokawa J, Takegawa A, Mine S, Prasad K (2011) Preparation of chitin nanowhiskers using an ionic liquid and their composite materials with poly(vinyl alcohol). Carbohydr Polym 84:1408–1412
Zeng JB, He YS, Li SL, Wang YZ (2012) Chitin whiskers: an overview. Biomacromolecules 13:1–11
Ifuku S (2012) Preparation of chitin nanofibers from crab shell and their applications. Kobunshi Ronbunshu 69:460–467
Tajiri R, Setoguchi T, Wakizono S, Yamamoto K, Kadokawa J (2013) Preparation of self-assembled chitin nanofibers by regeneration from ion gels using calcium halide · dihydrate/methanol solutions. J Biobased Mater Bioener 7:655–659
Kadokawa J (2013) Ionic liquid as useful media for dissolution, derivatization, and nanomaterial processing of chitin. Green Sustain Chem 03:19–25
Muzzarelli RAA, El Mehtedi M, Mattioli-Belmonte M (2014) Emerging biomedical applications of nano-chitins and nano-chitosans obtained via advanced eco-friendly technologies from marine resources. Mar Drugs 12:5468–5502
Kadokawa J (2015) Fabrication of nanostructured and microstructured chitin materials through gelation with suitable dispersion media. RSC Adv 5:12736–12746
Fan Y, Saito T, Isogai A (2008) Chitin nanocrystals prepared by TEMPO-mediated oxidation of α-chitin. Biomacromolecules 9:192–198
Fan YM, Saito T, Isogai A (2009) TEMPO-Mediated oxidation of β-chitin to prepare individual nanofibrils. Carbohydr Polym 77:832–838
Ifuku S, Nogi M, Abe K, Yoshioka M, Morimoto M, Saimoto H, Yano H (2009) Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 10:1584–1588
Ifuku S, Nogi M, Yoshioka M, Morimoto M, Yano H, Saimoto H (2010) Fibrillation of dried chitin into 10–20 nm nanofibers by a simple grinding method under acidic conditions. Carbohydr Polym 81:134–139
Ifuku S (2014) Chitin and chitosan nanofibers: preparation and chemical modifications. Molecules 19:18367–18380
Tanaka K, Yamamoto K, Kadokawa J (2014) Facile nanofibrillation of chitin derivatives by gas bubbling and ultrasonic treatments in water. Carbohydr Res 398:25–30
Sato K, Tanaka K, Takata Y, Yamamoto K, Kadokawa J (2016) Fabrication of cationic chitin nanofiber/alginate composite materials. Int J Biol Macromol 91:724–729
Sato K, Yamamoto K, Kadokawa J (2018) Preparation of cationic/anionic chitin nanofiber composite materials. J Polym Environ 26:3540–3549
Izawa H, Kaneko Y, Kadokawa J (2009) Unique gel of xanthan gum with ionic liquid and its conversion into high performance hydrogel. J Mater Chem 19:6969–6972
Izawa H, Kadokawa J (2010) Preparation and characterizations of functional ionic liquid-gel and hydrogel materials of xanthan gum. J Mater Chem 20:5235–5241
El Seoud OA, Koschella A, Fidale LC, Dorn S, Heinze T (2007) Applications of ionic liquids in carbohydrate chemistry: a window of opportunities. Biomacromol 8:2629–2647
Liebert T, Heinze T (2008) Interaction of ionic liquids with polysaccharides 5. Solvents and reaction media for the modification of cellulose. Bioresources 3:576–601
Feng L, Chen ZI (2008) Research progress on dissolution and functional modification of cellulose in ionic liquids. J Mol Liq 142:1–5
Pinkert A, Marsh KN, Pang SS, Staiger MP (2009) Ionic liquids and their interaction with cellulose. Chem Rev 109:6712–6728
Zakrzewska ME, Bogel-Łukasik E, Bogel-Łukasik R (2010) Solubility of carbohydrates in ionic liquids. Energy Fuels 24:737–745
Gericke M, Fardim P, Heinze T (2012) Ionic liquids - promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 17:7458–7502
Isik M, Sardon H, Mecerreyes D (2014) Ionic liquids and cellulose: dissolution, chemical modification and preparation of new cellulosic materials. Int J Mol Sci 15:11922–11940
Wang WT, Zhu J, Wang XL, Huang Y, Wang YZ (2010) Dissolution behavior of chitin in ionic liquids. J Macromol Sci Phys 49:528–541
Jaworska MM, Kozlecki T, Gorak A (2012) Review of the application of ionic liquids as solvents for chitin. J Polym Eng 32:67–69
Zhao DB, Fei ZF, Geldbach TJ, Scopelliti R, Laurenczy G, Dyson PJ (2005) Allyl-functionalised ionic liquids: synthesis, characterisation, and reactivity. Helv Chim Acta 88:665–675
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawano, A., Sato, K., Yamamoto, K. et al. Preparation of Chitin Nanofiber-Reinforced Xanthan Gum Hydrogels. J Polym Environ 27, 671–677 (2019). https://doi.org/10.1007/s10924-019-01380-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01380-8