Abstract
The corrosion protection of aluminum flake pigments has been extended by means of an encapsulating inorganic/organic silica/polystyrene hybrid nanolayer. A silica nanolayer encapsulated the surface of aluminum flakes (Al) by hydrolysis and polycondensation of tetraethylorthosilicate via sol–gel process to yield Al/Si flakes. Then, 3-methacryloxypropyltrimethoxysilane (MPS) was used as surface modifier which has polymerizable groups to participate in polymerization reaction (Al/Si/MPS). A polystyrene (PS) coating layer was applied on Al/Si/MPS flakes by free radical polymerization of styrene initiating with Azobisisobutyronitrile at 60 °C and subsequent washing of free chains with solvent yielded Al/Si/PS flakes. Fourier transform infrared spectroscopy, energy-dispersive X-ray spectroscopy and scanning electron microscopy showed that silica and PS nanolayers were formed on the aluminum flakes. The attached PS chains on the surface were detached by hydrofluoric acid aqueous solution and analyzed by gel permeation chromatography (GPC). Also, a transmission electron microscopy image showed clearly that the encapsulating layers are in the scale of nano. Good results were obtained in terms of corrosion protection in acidic and alkaline solutions, indicating that the silica/polymer hybrid nanolayer coating acts as an efficient protective film. After encapsulating the flakes, the evolved hydrogen volume was dropped and hybrid nanolayer resulted in no evolved hydrogen volume.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminum pigments have been widely used in paints, inks and plastic industries for many years due to their special metallic appearance [1, 2]. These flakes are usually produced by grinding aluminum foil in ball mill with white spirit and fatty acid as solvent and lubricant, respectively. They can then be dried to form powder or be held often in form of paste with organic compounds [3, 4]. However, when they contact with water, acid and alkali media, corrosion causes evolution of hydrogen as follows [5–10]:
To improve the chemical corrosive inhibition of aluminum flakes, extensive research has been performed on their surface modification including surface treatment with corrosion inhibitors and encapsulation of the pigments by organic or inorganic materials. Early works in this field involved harmful chromium (VI) complexes or other heavy metal-based compounds [11, 12] and now, the work is developed on less poisonous alternatives. However, encapsulation of pigments is an efficient way to protect them from corrosive environments [13, 14]. This can be made using inorganic silica layer by sol–gel method [15–17]. Although silica-coated aluminum pigments have good corrosion protection characteristics, some gassing is observed due to brittle structure of the silica layer. To overcome this limitation, a combination of inorganic layer with a second organic layer can be used. Also, organic polymer layers resulted from in situ polymerization can be coated on surface of pigments to protect them from corrosion [18–20]. Nowadays, different organic layers are used in this field such as hydroxyl-terminated polybutadiene [14], poly(methylmethacrylate) [18, 21], poly(trimethylolpropane triacrylate) [4] and copolymer of styrene and maleic acid [22]. In regard to organic coatings, the poor adhesion of the coating material to the aluminum surface greatly limits their application. The combination of inorganic and organic coatings for the encapsulation of the aluminum pigments may be more effective and promising, but until now few reports have been found in this field [15, 23]. Li et al. [23] used tetraethoxysilane (TEOS) and vinyltriethoxysilane (VTES) as precursors to produce a smooth coating layer containing unsaturated double bond groups via sol/gel reaction. Then, outside coating by styrene, divinylbenzene and maleic anhydride copolymer was used to encapsulate the particles.
However, silica-based layers can absorb water molecules due to their hydrophilic hydroxyl groups [24] while an organic hydrophobic nanolayer such as polystyrene (PS) could prevent this phenomenon. Therefore, we decided to use inorganic silica layer to encapsulate aluminum flakes and organic polystyrene nanolayer to make the surface hydrophobic. To this end, the sol–gel process, involving the hydrolysis and condensation of TEOS, has been used to make a silica layer which was modified using 3-methacryloxypropyltrimethoxysilane (MPS) and finally an in situ polymerization of styrene was performed to prepare organic layer. The structure and morphology of the nanolayers were characterized by means of Fourier transform infrared spectroscopy (FTIR), energy-dispersive X-ray spectroscopy (EDX), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). The molecular weight distribution of attached polymer chains was obtained via gel permeation chromatography (GPC). Also, the hydrophilicity and hydrophobicity of the flakes were examined by contact angle measurement. Furthermore, the stability of the coated aluminum pigments in acidic and alkaline aqueous media was measured.
Experimental
Materials
Blitz aluminum paste (Benda Lutz, Austria) was washed with dimethylformamide several times to remove the organic compounds used in milling process and then it was dried in vacuum at 100 °C for 24 h. Styrene (Tabriz Petrochemical Company, Iran) was passed through a basic alumina-filled column to remove the anti-polymerization agent before use. Azobisisobutyronitrile (AIBN) as an initiator was recrystallized from methanol prior to use. Tetraethylorthosilicate (TEOS, Merck, 99 %), 3-methacryloxypropyltrimetoxysilane (MPS, Merck, 98 %), maleic anhydride (Merck, 99.9 %), toluene (Merck, 99 %), acetone (Merck, 99.8 %), ethanol (Merck, 99.9 %) and ammonia solution (Merck, 25 %) were used as received without further purification.
Silica coating of the aluminum pigments
Into a 250-mL flask, aluminum flakes (5.000 g) were dispersed in ethanol (125 mL) with a magnetic stirrer and then 1 mL of TEOS solution in ethanol and 3 mL of aqueous ammonia solution were added. The reactants were refluxed at 45 °C for 6 h under stirring at nitrogen atmosphere. After the reaction, the resulting product (Al/Si) was filtered, and then washed with ethanol several times. The residue was dried for 12 h at 40 °C in vacuum oven.
Modification of Al/Si powder with MPS
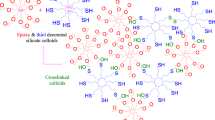
Al/Si powder (3.000 g), MPS (3 mL) and acetone (100 mL) were added into a three-necked flask equipped with a magnetic stirrer and a reflux condenser. The mixture was boiled up to 56 °C for 2 h under a nitrogen atmosphere. Then, maleic anhydride (0.030 g) diluted in distilled water (0.2 mL) and acetone (30 mL) was added to the reaction media. The mixture was further refluxed at 56 °C for 3 h under nitrogen atmosphere. At the end, the resulting product (Al/Si/MPS) was filtered and washed several times with acetone, and finally dried at 40 °C in vacuum oven for 12 h. The modification process of aluminum flakes is shown in Scheme 1 [18, 25].
In situ polymerization of styrene
To attach polystyrene chains onto the surface of MPS-grafted Al/Si flakes, the flakes (1.000 g) were first dispersed in toluene (22 mL), followed by addition of styrene (22 mL). After the dissolution of all reactants under stirring, AIBN (0.063 g) was added to start the polymerization at 60 °C. The mixture was refluxed and stirred for 24 h under nitrogen atmosphere. After polymerization, the mixture was filtered and the residue was washed with toluene to remove unreacted monomer and free polymer chains. The resulting pigments (Al/Si/PS) were dried under vacuum at 40 °C for 12 h.
Cleavage of attached chains from silica layer
To cleave the attached chains from the surface of silica-coated Al surface, Al/Si/PS flakes were dispersed in toluene (5 mL) and then methyltrioctylammonium chloride (0.300 g) was added. After addition of 5 % aqueous HF (4 mL), the mixture was stirred at room temperature for 2 h. The organic layer was separated by 10,000 rpm centrifugation during 30 min and filtration via nitrocellulose filters.
Instrumentation
FTIR spectra were recorded on a Bruker Tensor 27 FTIR-spectrophotometer, within a range of 500–4,000 cm−1 using a resolution of 4 cm−1. An average of 24 scans has been carried out on each sample. The samples were prepared on a KBr pellet in vacuum desiccators under a pressure of 0.01 torr. Average molecular weights and molecular weight distributions were measured by GPC technique. A Waters 2,000 ALLIANCE with a RI detector and a set of three series columns of pore sizes of 10,000, 1,000, and 100 Å was utilized to determine polymer average molecular weight and polydispersity index (PDI). Tetrahydrofuran was used as the eluent at a flow rate of 1.0 mL min−1, and the calibration was carried out using low polydispersity PS standards. EDX was carried out on a JSM-6360LV instrument. Surface morphology of powder samples was examined by scanning electron microscope (SEM, Philips XL30, Netherlands) with acceleration voltage of 15 kV. Transmission electron microscope (TEM, Tescan Mira) with an accelerating voltage of 100 kV was used by two methods to study the morphology of the nanocomposites. In the first method, the samples of 70 nm thickness were prepared by Reichert-ultramicrotome (type OMU 3, Germany). In the second method, the sample was prepared by dispersion in ethanol (0.2 mg mL−1) and subsequent deposition on the lacey carbon mesh grids. Hydrogen volume was used to evaluate corrosion resistance of samples, and the evolved hydrogen detection was carried out as described in the literature [26]. To evaluate the effect of the encapsulation described here, the stability test was carried out. An amount of 0.3 g of the encapsulated or pristine aluminum flakes was dispersed in HCl solutions (120 mL) of pH 1 for stability test in acid media and in NaOH solutions (120 mL) of pH 12 for stability test in alkaline media, respectively. The suspension was put in glass bottles and stored at 30 °C. The hydrogen evolved was collected to estimate the effect of the encapsulation. To measure contact angles, aluminum flakes with different surface modifications were compressed to build a disk of 1.4 mm diameter by a hydraulic press (Atlas Series Laboratory Press, Specac). Then, a 2-μL water drop was used to measure contact angles with a TZM-2 microscope (BEL).
Results and discussion
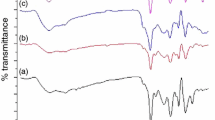
The FTIR spectra of Al, Al/Si, Al/Si/MPS and Al/Si/PS are shown in Fig. 1. Hydroxyl absorption peak at 3,430 cm−1 in the spectrum of Al attests to the existence of –OH groups on the surface of aluminum flake [18, 25]. The peaks at 500–600 cm−1 are attributed to the stretching vibration of Al–O [27]. The peak at 1,105 cm−1 in FTIR spectrum of Al/Si sample is ascribed to the bending vibration of Si–O–Si which means that the silica layer exists on the Al surface [16]. In sample Al/Si/MPS, the characteristic absorption of C–H stretching for CH2 and CH3 at 2,854 cm−1 indicates that MPS has been successfully coupled with flakes by means of chemical bonding [18]. The C = C stretching bonds of aromatic rings usually appear between 1,450 and 1,600 cm−1 as shown in Al/Si/PS spectrum.
To confirm the formation of composite particles, EDX analysis was employed to investigate the surface relative elemental percentage. As shown in Table 1, there was no Si observed in Al samples, but after the sol–gel process it reached 6.31 wt.%. An amount of 3.68 wt.% of carbon in aluminum powder flake was due to the existence of some organic additives on the surface of aluminum during the manufacturing process. We tried to wash these additives completely, but there were still some left. It was clearly observed that after surface modification with MPS, the content of carbon increased from 3.03 to 8.12 wt.% and this trend continued after polymerization to 36.26 wt.%, which indicated that PS chains had been grafted onto the surface of the flakes. Also, to determine the molecular properties of the attached chains, they were cleaved from the surface and were analyzed by GPC. As shown in Fig. 2, the molecular weight (M n) and the PDI were obtained at about 79,000 and 2.11, respectively.
Figure 3 shows the SEM images of Al, Al/Si, Al/Si/MPS and Al/Si/PS. The difference in SEM images indicates that a polymeric layer is continuously coated on particle surface. The surface of the Al flakes is smooth except for some tiny fragments of the aluminum pigments. After encapsulation by silica layer, the surface becomes rougher than Al surface. Also, many granules, which are congeries of attached MPS molecules, appear on the surface of the aluminum pigments after modification with MPS and the attached MPS molecules result in formation of rougher surface with some flocculations which enlarge after polymerization of styrene and polymeric molecules attachment on the surface.
The morphology of the encapsulated aluminum is particles shown in TEM image (Fig. 4). It can be seen that the hybrid layers are continuously coated on the particles surface, and the thickness values of silica and PS layers are estimated to be 10–15 and 25–30 nm, respectively.
Figure 5a, b shows the extent of evolved hydrogen volume for samples (0.300 g) dispersed in NaOH aqueous solution (120 mL) at pH 12 and in HCl aqueous solution (120 mL) at pH 1, respectively, at 30 °C. As it is seen, the hydrogen evolution starts after a certain time from the immersion of the aluminum in the test solution. This time corresponds to the period/time which is needed by the media to destroy the protective film and is known as incubation period [26]. Incubation period in acid media increases after encapsulation of flakes with silica layer and it remains constant after further modification with MPS. No incubation time is observed for Al/Si/PS flakes as no hydrogen is evolved. Within corrosion reaction, both Al/Si and Al/Si/MPS pigments evolved less hydrogen than the untreated pigments. Similar results were obtained by Li et al. [17] and Schubert et al. [28], while in their work each TEOS modified sample evolved some hydrogen. It can be seen that the evolved hydrogen volume of encapsulated particles decreased to 0 from 43 mL in basic media and to 0 from 314 mL in acid media (Table 2). Although some research works have been carried out on organic coatings, but many have reported on hydrogen evolution of the pigments [4, 18, 25]. Therefore, it could be concluded that the combination of inorganic and hydrophobic organic layers could be the best method of encapsulation to prevent corrosion phenomenon.
Contact angle measurements with different probe liquids with known surface tension form the basis for the calculation of the surface free energy of solids [29]. Owens and Wendt equation (two-liquid method) is widely used for the calculation of surface free energy of solids:
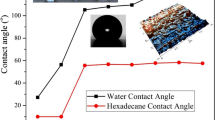
where, θ is the contact angle and γ d L and γ p L are the dispersion and polar components of the surface tension of the probe liquid. Similarly, γ d s and γ p s are the dispersion and polar components of the surface energy of the solid. Since there are two unknown quantities, we need to measure the contact angle with two probe liquids to have two independent equations. Finally, the sum of these two quantities eventually gives the total surface energy of the solid. For this purpose, one polar liquid and one non-polar liquid are used as test liquids [29–31]. In this study, we used diiodomethane and formamide for the calculation of surface energies, as their surface tension data are listed in Table 3. Table 4 shows the contact angle values and the shape of droplets is depicted in Fig. 6. The contact angles for Al and Al/Si/PS are 80.92 and 96.94, respectively. These results show that encapsulated particles are more hydrophobic.
The contact angle measurements can be used for characterization of the sample surface in terms of its surface free energy components. As it is seen in Table 4, compared to Al, the surface free energy of Al/Si/PS is decreased.
Conclusion
A combination of sol–gel method with in situ polymerization of styrene is adopted to form a hybrid silica/polystyrene nanolayer for the corrosion protection of aluminum pigments. The FTIR spectra of the samples proved that all steps comprising sol–gel process and polymerization reaction were carried out successfully. Also, SEM images showed that the surface of the pristine aluminum pigments is smooth, but the rougher surface with some flocculations was achieved after encapsulation of aluminum flakes. TEM image of encapsulated aluminum flakes has elucidated a hybrid nanolayer in which the thickness of silica and PS layers is estimated to be 10–15 and 25–30 nm, respectively. Evolved hydrogen volume tests showed that after modification of aluminum pigments, their corrosion resistance is improved and no hydrogen volume is observed for pigments encapsulated by hybrid nanolayer. According to contact angles data, Al shows a hydrophobic surface, while encapsulated flakes are more hydrophobic than bare aluminum flakes.
References
Pi PH, Chen J, Chen K, Cai ZQ, Zheng DF, Wen XF, Cheng J, Yang ZR (2012) Effects of acid treatment on adhesive performance of encapsulated aluminium pigments on plastic sheets. Can J Chem Eng 90:1224–1230
Müller B, Franze K, Mebarek D (1995) Corrosion inhibition of aluminum pigments in aqueous alkaline media at different pH values. Corrosion 51:625–630
Jadhav N, Vetter CA, Gelling VJ (2013) The effect of polymer morphology on the performance of a corrosion inhibiting polypyrrole/aluminum flake composite pigment. Electrochim Acta 102:28–43
Liu H, Ye H, Zhang Y, Tang X (2008) Preparation and characterization of poly(trimethylolpropane triacrylate)/flaky aluminum composite particle by in situ polymerization. Dyes Pigment 79:236–241
Razavi-Tousi SS, Szpunar JA (2014) Mechanism of corrosion of activated aluminum particles by hot water. Electrochim Acta 127:95–105
Karlsson PM, Esbjörnsson NB, Holmberg K (2009) Admicellar polymerization of methyl methacrylate on aluminum pigments. J Colloid Interf Sci 337:364–368
Sabagh S, Bahramian AR, Kokabi M (2012) SiAlON nanoparticles effect on the corrosion and chemical resistance of epoxy coating. Iran Polym J 21:837–844
Ershad-Langroudi A, Rahimi A (2014) Effect of ceria and zirconia nanoparticles on corrosion protection and viscoelastic behavior of hybrid coatings. Iran Polym J 23:267–276
Sabagh S, Bahramian AR, Kokabi M (2012) SiAlON nanoparticles effect on the behaviour of epoxy coating. Iran Polym J 21:229–237
Karlsson P, Palmqvist AEC, Holmberg K (2006) Surface modification for aluminium pigment inhibition. Adv Colloid Interf Sci 128–130:121–134
Supplit R, Schubert U (2007) Corrosion protection of aluminum pigments by sol-gel coatings. Corros Sci 49:3325–3332
Popoola PAI, Omotayo S, Loto CA, Popoola OM (2013) Inhibitive action of ferrous gluconate on aluminum alloy in saline environment. Adv Mater Sci Eng 2013:639071
Yan M, Vetter CA, Gelling VJ (2010) Electrochemical investigations of polypyrrole aluminum flake coupling. Electrochim Acta 55:5576–5583
Guo L, Song W, Hu M, Xie C, Chen X (2008) Preparation and reactivity of aluminum nanopowders coated by hydroxyl-terminated polybutadiene (HTPB). Appl Surf Sci 254:2413–2417
Liang B, Wang GD, Zhang BY, Zhang XM (2014) Optimization of sol-gel coating on the surface of aluminum alloy powder for corrosion protection in the condition of ultrasonic radiation. Mater Corros. doi:10.1002/maco.201307270
Zhang Y, Ye H, Liu H, Han K (2011) Preparation and characterisation of aluminium pigments coated with silica for corrosion protection. Corros Sci 53:1694–1699
Li L, Pi P, Wen X, Cheng J, Yang Z (2008) Optimization of sol-gel coatings on the surface of aluminum pigments for corrosion protection. Corros Sci 50:795–803
Liu H, Ye H, Zhang Y (2008) Preparation and characterization of PMMA/flaky aluminum composite particle in the presence of MPS. Colloid Surf A Physicochem Eng Asp 315:1–6
Yan M, Vetter CA, Gelling VJ (2013) Corrosion inhibition performance of polypyrrole Al flake composite coatings for Al alloys. Corros Sci 70:37–45
Joubert M, Save M, Mornet S, Lavaud F, Pellerin V, Morvan F, Tranchant J-F, Duguet E, Billon L (2014) Surface patterning of micron-sized aluminum flakes by seeded dispersion polymerization: towards waterborne colored pigments by gold nanoparticles adsorption. Polymer 55:762–771
Liu H, Ye H, Zhang Y (2007) Preparation of PMMA grafted aluminum powder by surface-initiated in situ polymerization. Appl Surf Sci 253:7219–7224
Liu H, Ye H, Tang X (2007) Aluminum pigment encapsulated by in situ copolymerization of styrene and maleic acid. Appl Surf Sci 254:616–620
Li LJ, Pi PH, Wen XF (2008) Aluminum pigments encapsulated by inorganic-organic hybrid coatings and their stability in alkaline aqueous media. J Coat Technol Res 5:77–83
Sarsabili M, Parvini M, Salami-Kalajahi M, Asfadeh A (2013) Effect of MCM-41 nanoparticles on the kinetics of free radical and RAFT polymerization of styrene. Iran Polym J 22:155–163
Liu H, Ye H (2008) Synthesis and property of poly(trimethylolpropane triacrylate)/Al nanocomposite particle by in situ solution polymerization. Appl Surf Sci 254:4432–4438
Umoren SA, Obot IB, Ebenso EE, Okafor PC, Ogbobe O, Oguzie EE (2006) Gum arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics. Anti-Corros Methods Mater 53:277–282
Diaconu G, Micusık M, Bonnefond A, Paulis M, Leiza JR (2009) Macroinitiator and macromonomer modified montmorillonite for the synthesis of acrylic/MMT nanocomposite latexes. Macromolecules 42:3316–3325
Supplit R, Schubert US (2007) Corrosion protection of aluminum pigments by sol-gel coatings. Corros Sci 49:3325–3332
Carre A (2007) Polar interactions at liquid/polymer interfaces. Adhes Sci Technol 21:961–981
Żenkiewicz M (2007) Methods for the calculation of surface free energy of solids. J Achieve Mater Manuf Eng 24:137–145
Subedi DP (2011) Contact angle measurement for the surface characterization of solids. Himalayan Phys 2:1–4
Acknowledgments
We are grateful for the financial support of the Iran National Science Foundation (INSF) (grant no. 91002479).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amirshaqaqi, N., Salami-Kalajahi, M. & Mahdavian, M. Corrosion behavior of aluminum/silica/polystyrene nanostructured hybrid flakes. Iran Polym J 23, 699–706 (2014). https://doi.org/10.1007/s13726-014-0264-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-014-0264-5