Abstract
Encapsulated aluminum pigments were prepared by sol–gel derived inorganic–organic hybrid coatings. Aluminum pigments were first coated with sol–gel film by using tetraethoxysilane (TEOS) and vinyltriethoxysilane (VTES) as the precursor, followed by free radical copolymerization of styrene (St), divinylbenzene (DVB) and maleic acid anhydride (MAA) with the vinyl group of the VTES. The as-prepared encapsulated aluminum pigment was characterized by Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS). Subsequently the stability of the aluminum pigments in alkaline aqueous media was examined. It was found that both the TEOS-and-VTES-coated (TV-coated) and the TEOS–VTES–St–DVB–MAA-coated (TVSDM-coated) aluminum pigments were superior in the stability test over the uncoated aluminum pigments. Furthermore, the corrosion protection efficiency of the TVSDM-coated aluminum pigments reaches 99.8%, indicating that the inorganic–organic hybrid composite layer on the surface of the aluminum pigments can protect them well.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lamellar aluminum pigments (‘‘aluminum flakes’’) have been used in solventborne metallic paints or inks for many years1 due to their metallic appearance and “flop-effect.”2 Recently, growing importance of environmental considerations have led the paint and coatings industry to the development of coating systems with a reduced content of volatile organic compounds (VOC).2,3 Waterborne coatings are the preferred way to solve the problem. However, since the waterborne coatings are mostly prepared in an alkaline medium, the aluminum pigments react with water:
resulting in severe deterioration of metallic luster and dangerous pressure build up in the storage vessels.1–7
As mentioned above, aluminum flakes, as a kind of metallic pigments, possess a bright, metallic luster. Therefore, corrosion inhibition of aluminum pigments must take care not to damage their appearance or shield their luster. In regard to aluminum sheets, focus is given to how to inhibit their corrosion in various conditions regardless of the luster. Considering the difference between corrosion inhibition of aluminum pigments and sheets, the method adopted for aluminum pigments stabilization in aqueous media can be divided into two principal categories8: the adsorption of corrosion inhibitors on the pigment surface1,3–7,9–18 and the encapsulation of the pigment with a protective coating.2,8,19–22 The encapsulation method is more promising since the protective layer can insulate the aluminum pigments from the corrosion medium. Inorganic coatings, such as SiO2, show excellent mechanical strength, but poor compatibility with resins and other organic compounds contained in waterborne coatings. With regard to organic coatings, the poor adhesion of the coating material to the aluminum surface greatly limits their application. The combination of inorganic and organic compounds for the encapsulation of the aluminum pigments may be more effective and promising, but few reports have been found in this field until now.
The sol–gel process, involving the hydrolysis and condensation of the metal alkoxide (typically tetraethoxysilane (TEOS)), has been used for corrosion protection of aluminum8 by condensation of the sol–gel film as a barrier layer on the aluminum surface. Silane coupling agents can be incorporated into the sol–gel network to enhance the adhesion between the inorganic surface and the organic compound. One of the well-known silane coupling agents is vinyltriethoxysilane (VTES), which can readily react with themselves and other metal alkoxides to form hybrid inorganic/organic polymers.23 In addition, organic functional group of the silane coupling agent can further polymerize with the organic monomers to form a polymeric layer.
In this article, aluminum pigments encapsulated by novel inorganic–organic hybrid coatings were reported. The structure and morphology of the encapsulation was characterized by means of FTIR, SEM, and XPS. Furthermore, the stability of the coated aluminum pigments in alkaline aqueous media was also measured.
Experimental
Materials
Raw materials used are listed in Table 1. Aluminum pigments (median particle size of 30 μm) were washed with ethanol and distilled water before encapsulation, then dried under vacuum at 50°C. TEOS and VTES were purified by distillation prior to use. Styrene (St) monomer was washed with 100 mol m−3 sodium hydroxide solution to remove inhibitor and purified by reduced pressure distillation. 2,2′-azobis (isobutyronitrile) (AIBN), used as an initiator, was recrystallized prior to use. Absolute ethanol, ammonia solution, maleic acid anhydride (MAA), divinylbenzene (DVB), and N-methyl-2-pyrrolidone (NMP) were used as received without further purification.
Encapsulation
Two grams of aluminum pigments and 50 mL of ethanol were put into a four neck-round bottom flask connected to a condenser, thermometer, and nitrogen gas inlet/outlet. The solution was stirred at room temperature for 1 h and then heated to 40°C. About 3 mL ammonia and 5 mL distilled water diluted by 30 mL ethanol, TEOS and VTES also diluted by 30 mL ethanol, were added drop-to-drop over a period of 1 h to the solution simultaneously (the quantities of TEOS and VTES used in the experiment are listed in Table 2). The mixing solution was further stirred for 6 h. Then the solution was heated to 80°C. The monomer mixture of St, DVB, and MAA (dissolved in 10 mL ethanol) was dropped into the above-mixed solution over a 1-h period. (The quantities of the monomers used in the experiment are also listed in Table 2.) Simultaneously, a solution of 1% (on monomers, dissolved in NMP and diluted by 10 mL of ethanol) AIBN was added. The post-reaction time was 24 h. The solution was subsequently filtered and washed with ethanol. The resulting pigments were dried under vacuum at 60°C for 5 h.
Characterization
Fourier transform infrared (FTIR) measurements were carried out by using a Bruker Vector 33 spectrometer to characterize the functional groups of the pigments. The samples were ground with dried potassium bromide (KBr) powder, and compressed into a disk. The KBr disk was subjected to analysis by an IR spectrophotometer. The scanning electron microscopy (SEM) investigations were performed with a Philips FEI XL-30 ESEM. X-ray photoelectron spectroscopy (XPS) is a surface sensitive analysis technique. The XPS spectra were obtained on a Kratos Axis Ultra (DLD) photoelectron spectrophotometer with a magnesium anode (Mg Kα 1253.6 eV). The pigments were irradiated with photons from a soft x-ray source with a well-defined energy. The survey scan was from 0 to 1100 eV to find atoms of surface.
To evaluate the effect of the encapsulation described in this article, the stability test was carried out. About 0.6 gram of the encapsulated or unencapsulated aluminum pigments were dispersed in sodium hydroxide solutions at pH 11 in 60 mL glass bottles and stored at room temperature for 720 h. The hydrogen evolved was collected to estimate the effect of the encapsulation. The pH was adjusted to 11 if necessary. The corrosion protection efficiency (P, %) was calculated using the following equation:
where the V unenc and V enc are the evolved hydrogen volume of the unencapsulated and encapsulated aluminum pigments in the stability tests, respectively.
Results and discussion
Encapsulation process
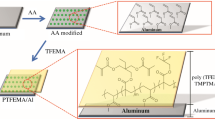
The flowchart for preparation of the hybrid inorganic and organic coatings is given in Fig. 1. The ethoxy group of the TEOS and VTES hydrolyze and condense in the ethanol/water media under catalysis of the ammonia solution. On the other hand, there is a layer of aluminum oxide on the surface of the aluminum pigments due to its exposure to the air. The surface of the aluminum oxide layer in a humid or moist environment has a significant population of hydroxyl groups.24 These surface hydroxyls can participate in the sol–gel condensation reaction of the TEOS and VTES to form a chemical linkage, Si–O–Al, between the aluminum and the silicon sol–gel film. This chemical bond formation produces the strong interaction of the sol–gel layer with the aluminum surface. Moreover, the vinyl radical of the VTES can further polymerize with the St, DVB, and MAA. Herein, the DVB was used as crosslinker.
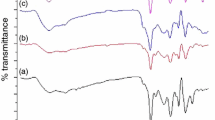
The FTIR spectra of pure VTES, St–DVB–MAA copolymer, untreated aluminum pigments, TEOS- and VTES-coated (TV-coated) pigments and TEOS–VTES–St–DVB–MAA coated (TVSDM-coated) pigments are shown in Fig. 2. In the spectrum of TV-coated pigments, the new characteristic peaks near 1125 and 1082 cm−1 appeared, which are assigned to the asymmetric stretch of the Si–O group.25,26 This indicates that TEOS and VTES deposited on the surface of aluminum pigments. The shift of the band position near 1105 cm−1 (pure VTES) to 1125 cm−1 (TV-coated pigments) suggests the reaction of TEOS and VTES in the sol–gel process. The band near 3434 cm−1 is assigned to the vibration modes of the Si–OH group,27 which demonstrates that ethoxy group of TEOS and VTES hydrolyzed in the sol–gel reaction. In the spectrum of TVSDM-coated pigments, the new bands near 1608, 1496, 760, and 698 cm−1 are assigned to the vibration modes of St and DVB,28,29 while the bands near 1712 cm−1 refer to the vibration modes of MAA.30 To further demonstrate the copolymerization of St with VTES, the FTIR spectra of pure VTES, St–MAA–DVB copolymer and TVSDM-coated aluminum pigments are shown in Fig. 3. The absorption peak near 1600 cm−1 is attributed to stretch of C=C group.25,27 As seen from Fig. 3c, it is obvious that the peak of C=C bond disappeared, indicating further polymerization of St–MAA–DVB copolymer with vinyl group of VTES.
The SEM micrographs of the aluminum pigments are shown in Fig. 4. The surface of the uncoated aluminum pigments is basically smooth except for some granules, which are tiny fragments of the aluminum pigments. Comparing the surface of the TV-coated aluminum pigments with that of uncoated aluminum pigments, it is clearly seen that many granules, which are congeries of the hydrolysate of TEOS and VTES, appear on the surface of the aluminum pigments. As seen from Fig. 4c, the polymeric layer is obviously observed for the TVSDM-coated aluminum pigments.
Figure 5 presents the results of XPS measurements for further quantitative analysis of the elements and components on the surface of aluminum flakes. The spectrum of the bare Al flakes (Fig. 5a) shows signals due to the presence of oxygen (531.32 eV, O 1s); carbon (284.32 eV, C 1s) and aluminum atoms (73.32 eV, Al 2p). The characteristic peaks of Si 2s and Si 2p in Fig. 5b reveal that the presence of Si element on the surface of the particles is evident, which suggests that TEOS and VTES have been successfully bonded onto the Al flakes. In Fig. 5c, the characteristic peaks of Si 2s and Si 2p become more obvious compared with those in Fig. 5b; at the same time, the peak corresponding to Al element cannot be detected. Furthermore, the specification of elemental contents on the surface of the Al flakes is shown in Table 2. It is found that on the surface of the TVSDM-coated Al, the content of Al is zero, which also further demonstrates that the encapsulation of aluminum pigments was successfully prepared in our experiment.
Stability tests
Figure 6 shows the time dependence of the hydrogen evolution for TV-coated and TVSDM-coated aluminum pigments. For comparison, the time dependence of the hydrogen evolution for the untreated aluminum pigments is also shown. Within about 100 h, the untreated aluminum pigments evolve hydrogen slowly. However beyond 100 h, the corrosion reaction accelerates and more hydrogen evolved. Within 120 h, the untreated pigments react completely. Within 720 h, both the TV-coated and TVSDM-coated pigments evolve much less hydrogen than the untreated pigments do, which reveals that the encapsulation layer formed on the surface of the aluminum pigments can protect them effectively. The comparison of the time dependence of the hydrogen evolution of the TV-coated pigments with that of the TVSDM-coated pigments shows the superiority of the latter.
Comparison of the hydrogen volumes evolved within 720 h for both TV-coated and TVSDM-coated aluminum pigments is shown in Fig. 7. There is a relationship between the hydrogen volume evolved within 720 h and the content of the TEOS or MAA in the polymeric network: the evolved hydrogen volume decreases with the increase of the content of the TEOS or MAA. The #5 and #8 (see Table 3) encapsulated aluminum pigments show best stability in the alkaline aqueous media of pH 11, the corrosion protection efficiency of which is both 99.8%.
Conclusions
The investigation shows that the sol–gel method can be used to encapsulate aluminum pigments. However, specific conditions are necessary to achieve good stability in alkaline aqueous media. The most important factor is to form a protecting layer on the surface of the aluminum pigments. It was found that in the sol–gel process, the TEOS and VTES could condense on the aluminum pigments surface as a barrier layer and further copolymerize with St, DVB, and MAA monomers to form a compact protecting layer. The stability test shows that the sol–gel derived inorganic–organic hybrid coatings can give the aluminum pigments significantly improved storage stability in alkaline aqueous media.
References
Müller B, Paulus A, Lettmann B, et al (1998) Amphiphilic Maleic Acid Copolymers as Corrosion Inhibitors for Aluminum Pigment. J. Appl. Polym. Sci. 69:2169–2174
Batzilla Th., Tulke A. (1998) Preparation of Encapsulated Aluminum Pigments by Emulsion Polymerization and their Characterization. J. Coat. Technol. 70:77–83
Iriyama Y, Ihara T, Kiboku M (1996) Plasma Polymerization of Tetraethoxysilane on Aluminum Granules for Corrosion Protection. Thin Solid Films 287:169–173
Müller B, Schmelich T (1995) High-Molecular Weight Styrene-Maleic Acid Copolymers as Corrosion Inhibitors for Aluminium Pigments. Corros. Sci. 37:877–883
Müller B (2004) Citric Acid as Corrosion Inhibitor for Aluminium Pigment. Corros. Sci. 46:159–167
Müller B, Shahid M, Kinet G (1999) Nitro- and Aminopenols as Corrosion Inhibitors for Aluminium and Zinc Pigments. Corros. Sci. 41:1323–1331
Müller B (2001) Corrosion Inhibition of Different Metal Pigments in Aqueous Alkaline Media. Corros. Sci. 43:1155–1164
Kiehl A, Greiwe K (1999) Encapsulated Aluminium Pigments. Prog. Organic Coat. 37:179–183
AK Maayta, NAF Al-Rawashdeh (2004) Inhibition of Acidic Corrosion of Pure Aluminum by some Organic Compounds. Corros. Sci. 46:1129–1140
Müller B (2002) Corrosion Inhibition of Aluminium and Zinc Pigments by Saccharides. Corros. Sci. 44:1583–1591
Foad El-Sherbini EE, Abd-El-Wahab SM, Deyab MA (2003) Studies on Corrosion Inhibition of Aluminum in 1.0 M HCl and 1.0 M H2SO4 Solutions by Ethoxylated Fatty Acids. Mater. Chem. Phys. 82:631–637
Abd El Rehim SS, Hassan HH, Amin MA (2001) Corrosion Inhibition of Aluminum by 1,1(lauryl amido)propyl ammonium chloride in HCl Solution. Mater. Chem. Phys. 70:64–72
Ashassi-Sorkhabi H, Ghasemi Z, Seifzadeh D (2005) The Inhibition Effect of some Amino Acids Towards the Corrosion of Aluminum in 1 M HCl + 1 M H2SO4 Solution. Appl. Surf. Sci. 249:408–418
El-Etre AY (2001) Inhibition of Acid Corrosion of Aluminum Using Vanillin. Corros. Sci. 43:1031–1039
Müller B (1998) Aromatic 2-Hydroxy-Oximes as Corrosion Inhibitors for Aluminum and Zinc Pigments. Corros. Sci. 39:1469–1477
Müller B (1999) Polymeric Corrosion Inhibitors for Aluminum Pigment. React. Funct. Polym. 39:165–177
Müller B (1995) Stabilization of Aluminum Pigments in Aqueous Alkaline Media by Styrene Copolymers. J. Coat. Technol. 67(846):59–62
Carpenter, et al, “Nitro-Substituted Polymeric Corrosion Inhibitors for Aluminum Flake Pigment.” [P]. U. S. 5,389,139, 1995-2-14
Zabel KH, Boomgaard RE, Thompson GE, et al (1998) Properties of Ketimine/Acetoacetate Coated Aluminum Substrates. Prog. Organic Coat. 34:236–244
Takashi, K, Kenji, Y, “Polymer Coated Aluminum Pigment having Water Dispersible Property and Method for Producing the Same.” [P]. JP2004292690, 2004-10-21
Shuichi, N, Kazuo, K, “Resin-Coated Aluminum Pigment.” [P]. JP2005146111A, 2005-6-9
Shuichi, N, “Silica-Coated Aluminum Pigment and its Manufacturing Method.” [P]. JP2002088274, 2002-3-27
Tanoglu M, McKnight SH, Palmese GR, et al (1998) Use of Silane Coupling Agents to Enhance the Performance of Adhesively Bonded Alumina to Resin Hybrid Composites. Int. J. Adhesion Adhesives 18:431–434
Joshua Du Y, Damron M, Tang G et al (2001) Inorganic/Organic Hybrid Coatings for Aircraft Aluminum Alloy Substrates. Prog. Oragnic Coat. 41:226–232
Li Y-S, Wright PB, Puritt R, et al (2004) Vibrational Spectroscopic Studies of Vinyltriethoxysilane Sol–Gel and its Coating. Spectrochim. Acta Part A 60:2759–2766
Yeh J-M, Weng C-J, Liao W-J, et al (2006) Anticorrosively Enhanced PMMA-SiO2 Hybrid Coatings Prepared from the Sol–Gel Approach with MSMA as the Coupling Agent. Surf. Coat. Technol. 201:1788–1795
Flis J, Kanoza M (2006) Electrochemical and Surface Analytical Study of Vinyl-Triethoxy Silane Films on Iron after Exposure to Air. Electrochim. Acta 51:2338–2345
Rong Yu, Chen H-Z, Li H-Y, et al (2005) Encapsulation of Titanium Dioxide Particles by Polystyrene via Radical Polymerization. Colloids Surf. A: Physicochem. Eng. Aspects 253:193–197
Deng J, Yang W (2005) Grafting Copolymerization of Styrene and Maleic Anhydride Binary Monomer Systems Induced by UV Irradiation. Eur. Polym. J. 41:2685–2692
Świtała-Żeliazkow M (2006) Thermal Degradation of Copolymers of Styrene with Dicarboxylic Acids—II: Copolymers Obtained by Radical Copolymerisation of Styrene with Maleic Acid or Fumaric Acid. Polym. Degrad. Stability 91:1233–1239
Acknowledgments
We are grateful for the support of Miss Shu-Yi Zhu, Mr. Yang (for SEM), Mr. Jiang (for FTIR) and Mr. Yin (for XPS) for their experimental and measurement contributions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, LJ., Pi, PH., Wen, XF. et al. Aluminum pigments encapsulated by inorganic–organic hybrid coatings and their stability in alkaline aqueous media. J Coat Technol Res 5, 77–83 (2008). https://doi.org/10.1007/s11998-007-9053-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-007-9053-9