Abstract
In this paper, PLA/RSF with different mass ratios and aspirin-loaded PLA/RSF composite nanofiber membranes were prepared via electrospinning. Polylactic acid (PLA) and regenerated silk fibroin (RSF) were dissolved in trifluoroacetic acid and dichloromethane at a volume ratio of 70/30. The structure analysis made by Fourier transform infrared spectroscopy (FTIR) suggested that PLA and RSF blended very well in the composite membranes. In addition, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) revealed that the average diameters of composite nanofibers were between 80 and 210 nm. Furthermore, the composite nanofibers had better uniformity in the range of the experiment, and the average diameter of composite nanofibers decreased with the increase of aspirin content. Wettability performance was investigated via contact water angle meter; the hydrophilic property of composite membranes had been improved with the existence of SF. Drug release property was tested by detecting the absorbency of drug in PBS solution via UV–visible spectroscopy, and the results showed that drug release rate reached the maximum when the mass ratio of PLA/RSF was 8/3. With the raise of aspirin content, the drug release rate increased. The in vitro anticoagulation behavior was studied by static platelet adhesion test. The results revealed that the anticoagulation property of composite membranes was superior to that of pure PLA nanofiber membranes. The anticoagulation property significantly improved in the presence of aspirin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrospinning is a versatile and effective technique to prepare continuous fibers with large surface area to volume ratio, small pores and diameters in the range from several micrometers to several ten nanometers, and many polymers have been successfully electrospun as nanofibers for several years. In recent years, the electrospinning process has received much attention in manufacturing composite nanofibers for various technological applications.
Actually, polylactic acid (PLA) has been widely studied due to its good film-forming ability, biocompatibility, biodegradability and excellent mechanical properties [1, 2]. Furthermore, PLA is also a potential matrix for controlled release in various forms including fibroin scaffolds, electrospun non-woven mats, microspheres and films [3, 4]. However, the biological activity of PLA is low, and cell adhesion is more difficult.
Regenerated silk fibroin (RSF) from Bombyx mori was another component used in the preparation of electrospun PLA/RSF composite nanofibers in our work. Silk fibroin (SF) is a fibrous protein consisting 17 amino acids, and its main components are nonpolar such as glycine, alanine and serine. In addition, SF can exist in two general conformations, i.e., random coil and β-sheet form [5, 6]. Silk fibroin exhibits favorable tunable degradation rates ranging from weeks to months in vivo due to control of crystallinity, excellent biocompatibility with low inflammatory and immunogenic response, diverse processing windows and all aqueous material processing options to form films, fibers, gels, sponges and microspheres [7]. It is currently being investigated for several technological biomedical applications [8–10]. An important application is in drug delivery system.
However, normally used in biomaterials, regenerated silk fibroin fiber is known to have low mechanical property, mainly due to structure changes and disorientation of proteins at the molecular level [11]. Therefore, we take the advantages from PLA and SF by blending them into composite nanofibers to form a composite material with desirable properties. In contrast with other PLA or RSF composite nanofibers reported in previous literatures [4, 5, 7], the advantages of aspirin-loaded electrospun PLA/RSF composite nanofibers are listed as follows: improving the wettability property of PLA since it is very essential for biomedical application, while exploring a potential composite material as a novel drug delivery system. The prepared biodegradable and non-cytotoxic composite material showed enormous potential in possessing a stable release rate and was expected to solve the “brust release”. In view of this, the study aims to prepare a matrix of composite polymer incorporating a drug for drug delivery application and investigates the drug activity released from the electrospun composite nanofibers simultaneously.
Micro- and nano-particulate systems have been widely studied in the drug delivery system. For a drug delivery system, the rate of drug delivery is controlled by the equilibrium among drug diffusion across a concentration gradient, the polymer relaxation occurring as the cross-linked polymer imbibes water and the osmotic pressure occurring during the swelling process [5].
Aspirin, which was attained by the acetylation of salicylic acid, has a long history of being antipyretic analgesic drug. It is applied in the prevention and treatment of ischemic heart disease, angina and cerebral thrombosis formation. It is the first drug for clinical anticoagulant-platelet aggregation. Aspirin prohibits platelet cyclooxygenase from changing arachidonic acid to prostaglandin intermediates. Thus, it prevents the generation of thromboxane A2 (TXA2, TXA2 may promote platelet aggregation) and thereby avoids platelet aggregation, making it difficult to release clotting factors. Thus, aspirin has certain anti-clotting effect [12].
At present, the preparation of electrospun PLA/RSF composite nanofibers has been reported [13], but the preparation of drug-loaded PLA/RSF composite nanofibers via electrospinning and the study of its controlled-release and blood compatibility properties have been seldom described.
In this work, we described the preparation and characterization of aspirin-loaded PLA/RSF composite nanofibers via electrospinning. The morphology and mixing situation of the composite nanofibers were revealed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The chemical composition of PLA/RSF composite membranes was investigated by Fourier transform infrared spectroscopy (FTIR). The wettability of composite membranes was characterized with static water contact angle (WCA) test. The drug controlled-release property in vitro was evaluated, as well. Drug activity released from membrane was analyzed by platelet adhesion test.
Experimental
Materials
Raw silk from Bombyx mori was purchased from Zhejiang (China). Aspirin (purity of 99 wt%) was supplied by Xi’an Shanlian Biotechnology Pharmaceutical Co., Ltd., China. PLA (Mw = 100,000) was obtained from Shenzhen Brightchina Industrial Co., Ltd., China. Trifluoroacetic acid (TFA), dichloromethane (DCM) and trisodium citrate dehydrate were purchased from Sinopharm Chemical Reagent Co., Ltd., China. Glutaraldehyde solution (25 % concentration) was bought from biochemical reagent, Sinopharm Chemical Reagent Co., Ltd., China. Physiological saline was supplied by Jiangsu Sihuan Bioengineering Co., Ltd., China.
Instruments
The experiments involved the following instruments: electrospinning instrument (self-constructed electrospinning equipment in laboratory), the SEM and TEM (SEM, SU-1510, TEM, H-800-1, Hitachi Co., Japan), FTIR (Nicolet Nexus 470, Thermo Electron Corporation Co., USA), contact angle meter (SL200, Kino Industry Co., Ltd., USA), ultraviolet–visible spectrophotometer (UV-9600, Beijing north points Rayleigh analysis instrument Co., Ltd., China), vacuum freeze-drying machine (FreeZone 2.5–7670570, Labconco Co., USA).
Preparation of RSF
Raw silk was degummed three times with 0.5 % (w/w) Na2CO3 solution at 100 °C for 30 min and then washed with deionized water three times. Degummed silk was dissolved in a ternary solvent system of CaCl2/H2O/EtOH solution (1/8/2 in mole ratio) at 75 °C for 90 min with a liquor ratio at 1:50. After dialysis by a dialysis bag with a cutoff molecular weight of 12 kD in deionized water for 3 day at room temperature (the water was renewed every 2 h), the silk fibroin solution was filtered and lyophilized to obtain the RSF sponges [14, 15].
Preparation of spinning solution
Calculated PLA, RSF and aspirin were dissolved in binary solvent (TFA/DCM, 70/30, v/v) and stirred with a magnetic stirrer for 12 h at room temperature. Then, pure PLA, PLA/RSF (8/1, 8/3 and 8/5, wt/wt), aspirin-loaded (0.7 wt%) PLA/RSF (8/1, 8/3 and 8/5, wt/wt) and PLA/RSF (8/3) containing aspirin (0.7, 0.9 and 1.1 wt%) solutions were prepared.
Electrospinning process
The solutions were filled into a plastic syringe (20 mL) with a blunt-ended metal needle (inner diameter of 0.7 mm) as spinneret, which was connected with a high-voltage power supply, and mounted in a micro-infusion pump. A piece of grounded aluminum foil was placed opposite the syringe as a collector at a distance of 18 cm. A voltage of 22 kV was applied across the needle and collector. The flow rate of syringe pump was maintained at 0.5 mL/h. The solvent evaporated during electrospinning, leaving nanofibers attached on the aluminum foil, forming randomly oriented nanofiber membranes [16].
Characterization of electrospinning membranes
The surface morphology of the nanofibers was observed with an SEM operating at 5 kV after coating with gold. Afterward, the average diameters of the nanofibers were measured using Image Tool software statistically and calculated by selecting 120–150 fibers in the SEM micrographs randomly. The combination of PLA and RSF was observed by TEM.
Chemical analysis of pure PLA and PLA/SF (8/1, 8/3 and 8/5, wt/wt) fiber films was performed using the Nicolet Nexus 470 IR (USA) spectrometer in the range of 4000–700 cm−1 wavenumbers.
The hydrophilicity of the obtained nanofibrous mats was measured with a static water contact angle instrument. 0.25 µL of deionized water was dropped onto the sample and WCA was calculated automatically with the associated software [17]. Each sample was measured at five different locations and the readings were averaged.
The drug-loaded composite nanofiber films were accurately cut off into pieces of 2 × 2 cm samples. The specimens were placed in conical flasks which contained 10 mL phosphate buffer solution (PBS) aqua (pH = 7.4). Finally, the specimens was incubated at 37 °C in the full temperature flask cabinet with a shake speed of 150 rpm. At intervals, 1 mL of PBS aqua was taken out and an equal amount of fresh buffer solution was put into the conical flask to keep the volume of PBS aqua constant. The aspirin released from the composite nanofibers was monitored using the UV spectrophotometer at a wavelength of 270 nm. The UV absorbance of aspirin in the PBS was detected and converted to aspirin concentration according to the calibration curve of aspirin in the same PBS aqua. After that, the cumulative amount of the released aspirin was calculated as a function of incubation time on the basis of the following formula. The time of 72 h was chosen as the maximum drug release duration.
Each specimen was accurately cut off into pieces of 2 × 2 cm samples, laid flat in a 96-well plate and equilibrated with a definite amount of physiological saline for 1 h. Simultaneously, anticoagulant fresh human blood (mixed trisodium citrate dehydrate and human blood at the rate of 1:9 in volume) was centrifuged at 800 rpm for 10 min to obtain platelet-rich plasma (PRP). After that, 0.2 mL of PRP was poured on each specimen and laid aside for 1 h at 37 °C in a constant temperature incubator, and then the samples were washed three times with physiological saline to remove non-adherent platelets. Further, the samples were fixed in 2 mL of 2.5 % glutaraldehyde solution for 1 h. After washing two times with PBS for 10 min, the platelets adhering to samples were dehydrated in an ethanol grade series (60, 80, 90, 95 and 100 %) for 15 min. These samples were permuted with tertiary butanol and allowed to stay in a refrigerator at 4 °C for 24 h. Finally, the platelets attached to the surfaces were examined via SEM.
Results and discussion
Morphology of nanofibers
Figure 1 shows the SEM micrographs of the obtained composite nanofibers under the same electrospinning process. These fibers were smooth and uniform without any beads on the surface. The average diameters and coefficient of variance (CV) values are shown in Tables 1 and 2, respectively. The data increased with the increase in the mass ratio of PLA/RSF. The survey showed that when the proportion of PLA and SF was about the same in the combination, there might be two crystalline structures of PLA and SF [18]. When the content of SF increased, the degree of compatibility in PLA and SF reduced, resulting in an increase in the diameter and CV value. When the aspirin content increased from 0 to 1.1 wt% in the spinning solution, the average diameter decreased from 210 to 80 nm.
These results were mainly attributed to aspirin powders increasing the charge density on the surface of the jet flow. Thus, the charges could suffer from drafting force in overcoming the surface tension of the solution. In this case, on one hand, the fiber diameter obtained might be smaller, on the other hand, if more electric charges were distributed unevenly, the conglutination and branch would appear in the fibers [19]. The average diameter of the sample containing 1.1 wt% aspirin was the least owing to the highest aspirin content; thus, slight conglutination and branch appeared due to the non-uniform distribution of the charges. TEM images present further information for nanofibers morphology study. As shown in Fig. 2, it is observed that PLA and PLA/SF composite fibers were similar in shape and smooth in surface without holes. There was no phase separation in PLA/SF composite fibers, indicating that PLA and RSF blended well in the fibers.
Structure of nanofibers
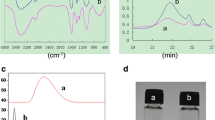
Figure 3 displays the FTIR spectra of the electrospun pure PLA nanofibers, PLA/RSF (8/3) nanofibers and pure RSF. PLA fibers presented characteristic absorption bands at 1,752, 1,460, 1,183 and 1,090 cm−1 which are attributed to C=O stretching vibration, C–H deformation vibration and C–O stretching vibration in PLA [3]. These absorption peak positions did not move or disappear obviously in the composite membranes. The peaks observed at 3,370, 1,637 and 1,515 cm−1 indicated the presence of O–H stretching vibration, amides I and II β-sheet structure in the RSF structure [20]. Visibly, with the content of RSF increase, the relative strength of these peaks increased. An analysis of the characteristic peaks proved that PLA and SF blended well.
Surface wettability of eletrospun nanofibrous mats
Wettability is an important parameter for materials used in cell response and drug release. As shown in Fig. 4, all the WCAs were >90o, showing that the electrospun PLA and PLA/RSF nanofibrous mats were hydrophobic. The WCAs of PLA/RSF nanofibrous mats with a mass ratio of 8/3, 8/5, and 8/1 were 103.27o, 114.75o, 121.15o, respectively, while the WCA of pure PLA nanofibrous mats was 131.31o. The composite membranes had smaller WCAs. These results indicated that the hydrophilic property improved with the addition of SF.
In vitro aspirin release studies
Effect of PLA/RSF mass ratio on drug release
It is well known that silk fibroin is a hydrophilic material which can be combined with more water molecules. The aforementioned FTIR results revealed that PLA and RSF blended well and PLA diminished the hydrophilicity of SF. The effect of the blend composition on drug release is shown in Fig. 5. It was found that the blend film with 8/3 mass ratio of PLA/RSF showed the maximum drug release rate. This could be explained by the swelling of composite nanofibers. It is known that the release of the drug is controlled by the swelling behavior of the material of the delivery system.
Risbud et al. [21] indicated that the release of amoxicillin from air-dried and freeze-dried chitosan/poly(vinyl pyrrolidone) hydrogels was related to the swelling degree of the hydrogels. Peng et al. [22] and Yao et al. [23] studied the release of chlorhexidine diacetate and cimetidine from a chiosan/polyether semi-interpenetrating hydrogel. They found that the higher the degree of swelling, the higher was the amount of drug released. In this study, the degree of swelling of composite nanofibers with a mass ratio of PLA/RSF in 8/1-8/3 was higher than that in 8/3-8/5. Therefore, PLA/RSF (8/3) composite nanofibers would be more easily swollen and corroded when they were dipped in the buffer and had a higher drug release rate as shown in Fig. 5.
Effect of aspirin content on drug release
The release profiles of aspirin from PLA/RSF composite nanofibers during 72 h in PBS are illustrated in Fig. 6. The content of aspirin had an influence on the controlled release of PLA/RSF nanofibers. The more aspirin, the greater the drug release rate. This was because that the more aspirin, the smaller the diameter of the nanofibers. This would result in the larger specific surface area, so more aspirin could be finally loaded in the fiber surface of higher drug loading amount. Simultaneously the diffusion concentration gradient of drug powders were higher, as a result, more amount of aspirin released in PBS [24]. Within the first 5 h, aspirin was released rapidly and then the drug release rate slowed down. In 72 h, the cumulative amount of released aspirin of all the samples was more than 10 %, and drug release amount from samples increased with extension of time.
SEM images of electrospun PLA/RSF nanofibrous mats with 0.7 wt% aspirin before and after drug release for 72 h are shown in Fig. 7. Before aspirin release, the surface morphology of electrospun fibers was clear and uniform. Aspirin powders were not observed apparently, since drug powders were embedded well in the interior part of the composite nanofibers. After drug release for 72 h, electrospun nanofibers swelled to a certain extent, which might result from the absence of the insolubilizing process [25]. The appearance of the composite membrane became rough and somewhat ruptured. Furthermore, well-distributed small drug particles could be observed clearly on the surface. We can also conclude that the drug released from biodegradable polymer occurred by diffusion and matrix erosion following swelling and degradation [26]. Given the truth that both PLA and SF are biodegradable, the waste section of the membranes were eventually completely degraded and converted into carbon dioxide and water due to hydrolysis and biological metabolism after the aspirin was released. Therefore, the aspirin-loaded electrospun PLA/SF composite membranes are suitable as drug carriers.
Platelet adhesion experiment
Platelet spreading and aggregation are marks of platelet activation and are considered to be a major mechanism of thrombosis. Platelet adhesion is one of the intuitive methods to measure the blood compatibility of biomaterials [17]. The extent of platelet adhesion and morphology of adherent platelets are considered as an early indicator of thrombogenicity of blood-contacting biomaterials. When a foreign material comes into contact with blood, the initial blood response is the adsorption of blood proteins, followed by platelet adhesion and activation of coagulation pathways, leading finally to thrombus formation [27]. Activation of platelets initiates the deformation of the cells with pseudopod formation and ends with blood coagulation or thrombus formation [28].
Figure 8 shows the SEM images of platelet adhesion to pure PLA, PLA/RSF (8/3), and PLA/RSF with 0.9 wt% aspirin samples. From Fig. 8, we can see that numerous adherent platelets are observed on pure PLA nanofiber membrane. The platelets aggregated, adhered and even covered the nanofibers; what is more, the platelets became deformed and produced distinct pseudopodia. This test phenomenon indicated the poor blood compatibility of pure PLA nanofiber film. The number of platelet adhering on the surface of PLA/RSF (8/3) was less than that of pure PLA and there were less platelets deformed and activated.
An important reason that affected platelet adhesion was the balance between hydrophilic region and hydrophobic region and micro-phase separation structure of the material. In this experiment, PLA nanofiber had the strongest hydrophobicity and PLA/RSF composite nanofibers possessed excellent hydrophilicity/hydrophobicity balance and good micro-phase separation structure. Therefore, PLA/RSF had better blood compatibility. As can be seen in Fig. 8, few platelets adhered and retained their original discoid shape on the membrane surface of PLA/RSF sample with 0.9 wt% aspirin, indicating an inactivated state. From the above description, we could draw the conclusions that the introduction of aspirin inhibited the activation and adhesion of platelet, reduced platelet adhesion and improved the anticoagulant property of the PLA/RSF composite nanofibers.
Conclusion
In the present study, the pure PLA nanofibers, PLA/RSF and aspirin-loaded PLA/RSF composite nanofibers were successfully prepared via electrospinning. Good spinnability was also demonstrated by SEM and TEM tests. The average nanofiber diameters were between 80 nm and 210 nm. The FTIR results proved that PLA and RSF blended well together. Wettability experiment showed that the hydrophilic property of the composite membranes had been improved by adding SF, which is important for cell response and drug release. Their properties of drug release and blood compatibility were investigated in detail. PLA/RSF (8/3, wt/wt) composite membrane showed the maximum release compared with PLA/RSF (8/1) and PLA/RSF (8/5) composite nanofiber films. The drug release rate increased with the increase in aspirin content; thus, electrospun PLA/RSF composite nanofibers are expected to be used as a controlled drug release material. As was discussed in the experimental results, aspirin enhanced the anticoagulation effect of the PLA/RSF nanofibers, which possesses both biodegradable and biocompatible properties and is useful as a potential candidate for biomedical applications, especially in fabricating drug-eluting stents.
References
Fei YN, Chen Y, Wang HB, Gao WD, Yang RH, Wan YQ (2011) Preparation, characterization of antibacterial PLA/TP nanofibers. Fiber Polym 12:340–344
Kim EN, Kim SH, Lee CH (2010) Electrospinning of polylactide fibers containing silver nanoparticles. Macromol Res 18:215–221
Nagarwal RC, Kumar R, Dhanawat M, Pandit JK (2011) Modified PLA nano in situ gel: A potential ophthalmic drug delivery system. Colloids Surf B 86:28–34
Dev A, Binulal NS, Anitha A, Nair SV, Furuike T, Tamura H, Jayakumar R (2010) Preparation of poly(lactic acid)/chitosan nanoparticles for anti-HIV drug delivery applications. Carbohydr Polym 80:833–838
Rujiravanit R, Kruaykitanon S, Jamieson AM, Tokura S (2003) Preparation of crosslinked chitosan/silk fibroin blend films for drug delivery system. Macromol Biosci 3:604–611
Tsukada M, Goto Y, Freddi G, Shiozaki H (1992) Chemical modification of silk with aromatic acid anhydrides. J Appl Polym Sci 45:1189–1194
Wang XQ, Yucel T, Lu Q, Hu X, Kaplan DL (2010) Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials 31:1025–1035
Wenk E, Wandrey AJ, Merkle HP, Meinel L (2008) Silk fibroin spheres as a platform for controlled drug delivery. J Control Release 132:26–34
Meinel L, Betz O, Fajardo R, Hofmann S, Nazarian A, Cory E, Hilbe M, McCool J, Langer R, Vunjak-Novakovic G, Merkle HP, Rechenberg B, Kaplan DL, Kirer-Head C (2006) Silk based biomaterials to heal critical sized femur defects. Bone 39:922–931
Hofmann S, Knecht S, Langer R, Kaplan DL, Vunjak-Novakovic G, Merkle HP, Meinel L (2006) Cartilage-like tissue engineering using silk scaffolds and mesenchymal stem cells. Tissue Eng 12:2729–2738
Li LH, Li HB, Qian YN, Li X, Singh GK, Zhong L, Liu WQ, Lv YG, Cai KY, Yang L (2011) Electrospun poly (ε-caprolactone)/silk fibroin core-sheath nanofibers and their potential applications in tissue engineering and drug release. Int J Biol Macromol 49:223–232
Galloway CF, Stevenson JC (2011) Aspirin in the primary prevention of cardiovascular disease. Maturitas 68:3–4
Wang SD, Zhang YZ, Yin GB, Wang HW, Dong ZH (2009) Electrospun polylactide/silk fibroin–gelatin composite tubular scaffolds for small-diameter tissue engineering blood vessels. J Appl Polym Sci 113:2675–2682
Zhang KH, Yin AL, Huang C, Wang CY, Mo XM, Al-Deyab SS, El-Newehy M (2011) Degradation of electrospun SF/P(LLA-CL) blended nanofibrous scaffolds in vitro. Polym Degrad Stab 96:2266–2275
Zhou WT, He JX, Du S, Cui SZ, Gao WD (2011) Electrospun silk fibroin/cellulose acetate blend nanofibres: structure and properties. Iran Polym J 20:389–397
Chen Y, Lin J, Fei YN, Wang HB, Gao WD (2010) Preparation and characterization of electrospinning PLA/curcumin composite membranes. Fiber Polym 11:1128–1131
He CL, Wang M, Cai XM, Huang XB, Li L, Zhu HM, Shen J, Yuan J (2011) Chemically induced graft copolymerization of 2-hydroxyethyl methacrylate onto polyurethane surface for improving blood compatibility. Appl Surf Sci 258:755–760
Cheung H-Y, Lau K-T, Tao X-M, Hui D (2008) A potential material for tissue engineering: silkworm silk/PLA biocomposite. Compos Part B Eng 39:1026–1033
Zhao YY, Yang QB, Lu XF, Wang C, Wei Y (2005) Study on correlation of morphology of electrospun products of polyacrylamide with ultrahigh molecular weight. J Polym Sci, Part B: Polym Phys 43:2190–2195
Chen X, Knight DP, Shao ZZ, Vollrath F (2001) Regenerated bombyx silk solutions studied with rheometry and FTIR. Polym 42:09969–09974
Risbud MV, Bhat SV, Bhonde RR, Hardika AA (2000) pH-sensitive freeze-dried chitosan–polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J Control Release 68:23–30
Peng T, Yao KD, Yuan C, Goosen MFA (1994) Structural changes of pH-sensitive chitosan/polyether hydrogels in different pH solution. J Polym Sci A Polym Chem 32:591–596
Yao KD, Peng T, Feng HB, He YY (1994) Swelling kinetics and release characteristic of crosslinked chitosan: polyether polymer network (semi-IPN) hydrogels. J Polym Sci A Polym Chem 32:1213–1223
Almeria B, Deng WW, Fahmy TM, Gomez A (2010) Controlling the morphology of electrospray-generated PLGA microparticles for drug delivery. J Colloid Interface Sci 343:125–133
Muthumanickkam A, Subramanian S, Goweri M, Beaula WS, Ganesh V (2013) Comparative study on eri silk and mulberry silk fibroin scaffolds for biomedical applications. Iran Polym J 22:143–154
Song XF, Gao ZT, Ling FG, Chen XS (2012) Controlled release of drug via tuning electrospun polymer carrier. J Polym Sci B Polym Phys 50:221–227
Huang X-J, Guduru D, Xu Z-K, Vienken J, Groth T (2011) Blood compatibility and permeability of heparin-modified polysulfone as potential membrane for simultaneous hemodialysis and LDL removal. Macromol Biosci 11:131–140
Kumar KP, Paul W, Sharma CP (2011) Green synthesis of gold nanoparticles with Zingiber officinale extract: characterization and blood compatibility. Process Biochem 46:2007–2013
Acknowledgments
The authors wish to acknowledge the financial support from (1) Jiangnan University scientific researches fund for doctoral students, (2) the Jiangsu Provincial Natural Science Foundation of China (No. BK2012112) and (3) the National Natural Science Foundation of China under Grant No. 31201134.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, J., Jiang, Y., Fu, J. et al. Evaluation of drug release property and blood compatibility of aspirin-loaded electrospun PLA/RSF composite nanofibers. Iran Polym J 22, 729–737 (2013). https://doi.org/10.1007/s13726-013-0171-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-013-0171-1