Abstract
The hydrophobic nature of Poly L–Lactide (PLLA) limits its application in tissue engineering and drug delivery. In the present study, three methods were used to modify the hydrophobic properties of PLLA. In one method, hydrophilic PLLA fibers with open porous structure were produced by electrospinning method using water bath collector. In the second method, PLLA was made hydrophilic by the addition of hydrophilic polymers such as cellulose acetate (CA) and in the third method PLLA was blended with hydrophilic silk fibroin (SF) from Bombyx mori silk. The surface morphology of the electrospun PLLA based fibers was studied by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The pore size distribution and average fiber diameter of the PLLA based fibrous scaffolds were studied by capillary flow porometry. The contact angle measurements and water uptake test showed remarkable increase in hydrophilicity of the prepared PLLA based fibrous scaffolds. The herbal rich anti-tumor properties of turmeric in the form of curcumin were incorporated into PLLA based scaffolds and the presence of curcumin was identified by FT-Raman. The biocompatibility and anti-cancer activity of the PLLA based scaffolds and curcumin loaded PLLA based scaffolds were studied using mouse embryonic fibroblasts (NIH 3 T3) and human breast cancer (MCF 7) cell lines over a period of 24, 48 and 72 h by {3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium} (MTT) assay. These results confirmed the prepared electrospun fibrous scaffolds as a promising carrier for biomedical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prevention of cancer by intensive and harmless long-term care is one of the major challenges faced today. Drug loaded electrospun scaffolds in the form of fibers can be fabricated and placed as dressing on the surface of tumours to control the growth of cancer cells. The hydrophobic nature in polymers generally does not promote good cell adhesion and exhibits poor cellular affinity [1, 2]. Hence, hydrophobic polymers are blended with hydrophilic matrices to enhance good endothelial cell adhesion and proliferation [3, 4]. The combination of biocompatible natural and synthetic fibrous scaffolds is gaining wide acceptance to improve cell adhesion and proliferation rate. Recently, gelatin was incorporated into poly (έ-caprolactone)-forsterite (PCL-F) and its hydrophilicity was improved by sequential electrospinning method [4]. These hydrophilic fibers are reported to possess same mechanical properties but better cellular infiltration compared to the individual gelatin and PCL-F scaffolds. Electrospun nanofibrous scaffolds are widely used as drug carriers for biomedical applications due to their highly porous structure similar to human extracellular matrix [5–8]. In these electrospun mats, the temperature of collector during electrospinning is reported to greatly influence the surface morphology and porosity (i.e., pore size, depth, shape and distribution) of the fibrous mats [9]. Nanofibrous scaffolds, which have open porous structure, are highly favored due to increase in cell attachment and tissue compatibility. However, low vapor pressure, high boiling point, miscibility of solvent with water and quick solidification of polymers lead to limitations in fabricating porous electrospun fibers [10]. Simple porous membranes or film templates with tunable pore sizes are also successfully fabricated using breath figure mechanism and phase separation methods and used in tissue engineering [11]. However, the advantages of electrospun scaffolds are that they are tailor made based on the needed porosity in fibers. A typical example is a case where two different polymers were electrospun simultaneously; later one type of polymer fiber was selectively removed thereby inducing pores in the other which provided space for cell infiltration [12]. In addition, these electrospun fibers can also be collected over water bath to produce highly porous fibers with large surface area to volume ratio [13], useful in tissue engineering, wound dressings and drug delivery. Highly open porous nanofibrous structure achieves sufficient cell seeding density within the scaffold and also facilitates free transport of nutrients and oxygen thereby leading to effective cell proliferation and differentiation [14]. The prepared porous scaffolds could be utilized for incorporating drugs during electrospinning for drug delivery. In the present study, curcumin (derived from Curcuma Longa L), which is one of the most widely exploited herbal drugs to treat cancer cells [15–17] has been incorporated into PLLA based solution. It was chosen because it is reported to induce apoptosis in cancerous cells [18] without affecting the healthy cells. Moreover, the presence of phenolic group in curcumin is essential for free-radical scavenging activity and the methoxy group present enhances the anticancer activity [19].
The significance of this investigation lies in the usage of three types of methods to modify the hydrophobic nature of PLLA for curcumin delivery. In the first method, a water bath collector was attached to the electrospinning machine to produce PLLA microfibers with an open porous structure. In the second method, natural polymer such as cellulose acetate (CA) was used to increase the hydrophilicity of PLLA and electrospun to obtain PLLA-CA nanofibrous scaffold. In the third method, PLLA was blended with silk fibroin from naturally available Bombyx mori silk and electrospun to produce super hydrophilic PLLA-SF nanofibrous scaffold. Similarly, curcumin was loaded into PLLA based solution and electrospun to produce curcumin dispersed PLLA based micro and nano fibrous scaffold as observed by transmission electron microscopy (TEM) imaging. The hydrophilic properties of the prepared PLLA based fibrous scaffold was studied by contact angle measurements and water uptake test. While the biocompatibility of the prepared hydrophilic PLLA based fibrous scaffold and curcumin loaded PLLA based fibrous scaffold were assessed using mouse embryonic fibroblasts (NIH 3 T3) cell lines, the anticancer activity of curcumin loaded hydrophilic PLLA based fibrous scaffold were assessed using human breast cancer (MCF 7) cell lines. The FT-Raman, capillary flow porometry and in-vitro curcumin release for the prepared PLLA based fibrous scaffold were studied and reported.
Experimental
Materials
Poly L – lactide (Mw = 100,000–150,000), Cellulose acetate (Mn ~ 50,000 by GPC) and Curcumin derived from Curcuma longa L were purchased from Sigma – Aldrich, India. Bombyx mori silk was purchased from Sericulture Lab, Coimbatore, India. Chloroform, 1, 2-dichloroethane (DCE), Ethanol and Tri-fluoro acetic acid (TFA) were purchased from Sisco Research Laboratories Pvt. Ltd., India.
Preparation of electrospun PLLA based fibrous scaffolds
Electrospinning technique requires a high voltage source and solution or melts with sufficient viscoelasticity to be able to spin in the form of nanofibrous or microfibrous scaffolds. The detailed procedure of electrospinning set-up is described [20] earlier. Curcumin (0.5, 1 and 1.5 wt%) was loaded to optimized concentration of PLLA in chloroform at 12 wt% by gentle stirring for 4 h. PLLA (10 wt%) and CA (0.5, 1 and 2 wt%) were co-dissolved in the mixture of DCE and ethanol in the ratio of 4:1 by continuous stirring for 24 h. Silk fibroin was isolated from Bombyx mori silk cocoons by degumming process [21] after removal of sericin. An equal amount of PLLA pellets and SF were co-dissolved in TFA to a concentration of 10 wt%. Then curcumin was added to the homogeneous PLLA-CA and PLLA-SF solution and stirred continuously for 4 h. It was then electrospun at a distance of 12–17 cm from the needle tip of 0.56 mm inner diameter and the electric voltage of 25–30 kV with flow rate of 0.5 mL/h.
Measurements and characterization
The morphology of electrospun fibers was studied by scanning electron microscopy (SEM-Hitachi, Model: S-3400 N) at an accelerating voltage of 0–15 kV. The average diameters of the electrospun fibers were analyzed using Image J software. Transmission electron microscopy (TEM-TF 20: Tecnai) operating at 200 kV was used to study the dispersion of curcumin in PLLA based fibrous scaffold using a carbon-coated grid. The porosity of PLLA based fibrous scaffold was measured using a capillary flow porometer (CST-1200-ASL). The sample thickness, sample diameter and surface tension used in the capillary flow porometer are 0.04 cm, 20 mm and 1.5 dyn/cm respectively. The interaction of curcumin with PLLA was studied by FT-Raman measurements using BRUKER RFS 27 in the range of 500–4000 cm−1 respectively. The percentage of water uptake test was studied using deionised water (DW) [20]. Euromex optical microscope equipped with a CCD camera was used to measure the contact angle of electrospun fibers [20]. In–vitro cytotoxicity test was performed by MTT {3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium} assay [20]. NIH 3 T3 cells were used to assess the biocompatibility of the hydrophilic porous PLLA and PLLA hybrid fibers and MCF 7 cell lines were used to assess the anticancer activity of curcumin loaded hydrophilic porous PLLA and PLLA based fibrous scaffolds. The in-vitro curcumin release from the porous PLLA and PLLA based fibers was studied in phosphate buffer solution (PBS) with 10 % DMSO solution using HPLC with UV detector [20].
Results and discussion
SEM was used to analyze the surface morphology of electrospun PLLA fibers collected under water bath, PLLA–CA fibers using plate collector and PLLA–SF fibers using drum collector. Figure 1 shows large variation on the morphology of the fibrous surface depending upon the composition of the blend, solvent and collector used. PLLA was dissolved in chloroform at 12 wt% concentration and electrospun in a water bath collector to produce the open porous structure in the PLLA microfibers. The average diameters of the fibers were found to be in the range of 2 to 2.5 μm. A highly porous single PLLA microfiber at 1 μm and 500 nm magnifications are shown in Fig. 1a. It is observed that the porous structure in the PLLA microfibers was formed while chloroform was used as solvent, which may be attributed to its immiscibility with water and also could be due to its low boiling point. Moreover, during electrospinning, the residual chloroform molecules that remain on the fibers are encapsulated by the water molecules, that provide additional space in the form of deep holed [14] open porous structure during drying. In the case of curcumin incorporated PLLA, the dispersion of curcumin within the deep holes or pockets was observed in the SEM and even more clearly in the TEM images as shown in Fig. 1b and m. Smooth and continuous PLLA-CA nanofibrous scaffold were prepared using DCE-ethanol mixture in the ratio of 4:1 at a total concentration of 10 wt%, since a total solution concentration of 8 wt% during electrospinning produced fibers with beads. Thus the total concentration of solutions was found to play a vital role in altering the surface morphology of prepared nanofibrous scaffold as reported in literature [22]. When CA (0.5 wt%) was incorporated into PLLA (10 wt%) solution, the electrospun fibers were observed to have a beaded structure. However, when CA (1 wt%) was incorporated into PLLA (10 wt%) solution, smooth rod shaped, continuous and beadless ultrathin fibers were obtained in the range of 110 ± 40 nm. Further on increasing the CA (2 wt%) concentration to PLLA (10 wt%) solution, the average fiber diameter also increased to 190 ± 40 nm. Curcumin (0.5 wt%) in DCE:ethanol mixture, was then added to PLLA-CA (10–1 wt%) solution and electrospun to obtain smooth beadless curcumin loaded PLLA-CA nanofibrous scaffold (i.e., C-PLLA-CA nanofibers) in the average diameter range of 150 ± 40 nm. TEM images show clear dispersion of curcumin in the C-PLLA-CA fibers as shown in Fig. 1n. When PLLA was dissolved in TFA at a concentration of 10 wt% and electrospun it resulted only in electrospraying. Electrospinning was then carried out by increasing the concentration of TFA up to 40 wt%. Smooth beadless morphology was observed at a PLLA concentration of 40 wt% and the average diameter of the PLLA fibers were found to be in the range of 100 ± 20 nm. Electrospinning of PLLA solution at 38 wt% showed a beaded structure as shown in Fig. 3a. The cylindrically shaped PLLA-SF fibers were obtained with an average fiber diameter of 150 ± 30 nm. After incorporation of curcumin (1 wt%) into PLLA-SF, the average fiber diameter of PLLA-SF increased to 180 ± 30 nm due to the dispersion of curcumin as shown in Fig. 3d.
SEM images of a PLLA microfibers b C-PLLA microfibers c PLLA-DCE-ethanol (8 wt%), d PLLA-DCE-ethanol (10 wt%), e PLLA-CA (10–0.5 wt%), f PLLA-CA (10–1 wt%), g PLLA-CA (10–2 wt%) h C-PLLA-CA (0.5–10–1 wt%), i PLLA-TFA (38 wt%), j PLLA-TFA (40 wt%), k PLLA-SF l C-PLLA-SF and TEM images of electrospun m C-PLLA, n C-PLLA-CA and o C-PLLA-SF fibers
The pore size distribution and average diameter of the PLLA based fibers are presented in Table 1. The largest and smallest detected pore diameters of PLLA nanofibrous scaffold are higher than PLLA-CA and PLLA-SF nanofibrous scaffold. The open porous structure present in PLLA microfibers along with the presence of interconnected pores are the reason for higher pore diameter of PLLA microfibrous scaffold compared to PLLA-CA and PLLA-SF nanofibrous scaffold. The larger pores have greater overall fraction of the pore volume within the scaffold [23]. The average diameters in microns versus pore size distribution graphically are shown in Fig. 2. PLLA-CA nanofibrous scaffold has large pore size distribution and less average diameter compared to PLLA and PLLA-SF nanofibrous scaffold due to the addition of less concentration of CA to PLLA.
The hydrophilicity of the electrospun polymer fibers highly induces cell adhesion for biomedical applications [24]. Of the various methods adopted to improve the hydrophilic behavior of PLLA, the addition of natural polymers, such as SF was used as a blend to PLLA which greatly enhanced the hydrophilicity of PLLA compared to PLLA-CA nanofibrous scaffold and PLLA fibers collected over water bath as shown in Fig. 3 (i) and (ii). The contact angle of PLLA microfibrous scaffold, PLLA-CA and PLLA-SF nanofibrous scaffold were found to be 72 ± 3°, 67 ± 3° and 0° respectively. The present result shows the ease in the conversion of super hydrophobic PLLA to super hydrophilic PLLA-SF nanofibrous scaffold upon the addition of SF which has improved diffusion pathways [25], suitable for hydrophobic curcumin delivery. Similar results were also observed from the water up-take test, which supports the data of contact angle measurements. The water uptake test % of PLLA microfibers, PLLA-CA and PLLA-SF nanofibrous scaffold were found to be 60 % (4 days), 80 % (10 days) and 130 % (15 days) respectively.
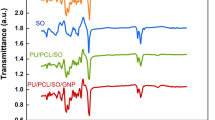
The FT-Raman spectroscopic studies of neat curcumin and electrospun PLLA based fibrous scaffold are shown in Fig. 4. The characteristic peaks of curcumin were observed at 1629 cm−1, 1600 cm−1, 1530 cm−1, 1498 cm−1, 1462 cm−1, 1429 cm−1, 1319 cm−1, 1250 cm−1, 1183 cm−1, 1154 cm−1 and 963 cm−1 as seen in Fig. 4(vi). The most intense characteristic bands of curcumin were observed at 1629 cm−1, 1600 cm−1, 1185 cm−1 and 965 cm−1 which can be attributed to the aromatic ring and C-O-C and C-O-H vibrations. In the case of curcumin loaded PLLA-CA based fibrous scaffold, the peak at 1770 cm−1 disappeared and the presence of curcumin peak appeared at 1634 cm−1 and 1585 cm−1, whereas in the case of curcumin loaded PLLA-SF based fibrous scaffold, the characteristic peaks of silk fibroin that were observed at 1769 cm−1, 1669 cm−1 and 1607 cm−1 were found to shift towards 1767 cm−1, 1631 cm−1 and 1597 cm−1 respectively. These results confirm the presence of curcumin in the curcumin loaded PLLA based fibrous scaffold.
Biocompatibility studies of the electrospun PLLA based fibrous scaffolds were evaluated by MTT assay, using NIH 3 T3 fibroblast cell lines for a period of 24, 48 and 72 h respectively. The SEM images of PLLA and PLLA based nanofibers scaffolds after incubation with NIH 3 T3 cells are shown in Fig. 5. MTT assay results after 72 h of incubation using all the three PLLA based scaffolds, (i.e.,) PLLA, PLLA-SF, PLLA-CA show more than 100 % cell viability. Thus, these results show good cell attachment and proliferation rate in all the prepared scaffolds. Curcumin loaded PLLA and PLLA based CA and SF fibers were found to be non-toxic as proven by their good cell viability (Fig. 5(i)). 1 wt% of curcumin loaded in the C-PLLA, C-PLLA-SF and C-PLLA-CA fibers show anti-proliferative effects in MCF 7 cell lines as shown in Fig. 5 (ii). Curcumin induces reduction in the MCF 7 cell viability which is mediated via caspase-dependent apoptosis [26]. Treatment of MCF 7 cells with curcumin induces an increase in p53 levels which eventually result in cell cycle arrest [27]. In addition, curcumin regulates the expression of many oncogenes involved in proliferation and apoptosis in breast cancer cells [28]. Even though there is no considerable difference in the uptake of curcumin between normal and cancer cells, more apoptosis in cancer cells indicates the affinity of curcumin towards cancer cells. This can be due to the fact that curcumin targets the signaling molecules that are highly expressed in cancer cells. In normal cells, these pathways will be regulated and curcumin will arrest the cell division in G0 phase without inducing apoptosis [29]. These results prove the beneficial effect of using curcumin to treat cancer cells compared to the use of highly toxic anticancer drugs that can result in the death of normal cells at a higher rate.
MTT assay for PLLA based fibers cell viability graph of (i) NIH 3 T3 and (ii) MCF 7 cell lines a control b PLLA, c PLLA-CA, d PLLA-SF, e C-PLLA, f C-PLLA-CA and g C-PLLA-SF and (iii) SEM images of NIH 3 T3 cell lines seeded on PLLA based fibers after 72 h a PLLA, b C-PLLA, c PLLA-CA, d C-PLLA-CA, e PLLA-SF and f C-PLLA-SF
In-vitro curcumin release studies were carried out in PBS (pH 7.4) using direct dispersion method. Different weight percentages of curcumin namely, 0.5, 1 and 1.5 wt% were loaded into PLLA solution to form curcumin loaded PLLA microfibers collected over water bath, C-PLLA-CA and C-PLLA-SF nanofibers collected over drum collector. The percentage cumulative curcumin release from C-PLLA microfibers, C-PLLA-CA and C-PLLA-SF nanofibers are shown in Fig. 6. It is observed that the ‘in-vitro’ release of curcumin at 0.5, 1 and 1.5 wt% showed no significant changes in release profile and moreover, no burst release of curcumin was observed. This could be due to the presence of highly interconnected porous structure in PLLA fibers due to electrospinning [30]. Slow, controlled and sustained release of curcumin was observed over a period of 4 days for C-PLLA, 11 days for C-PLLA-CA and 18 days for C-PLLA-SF fibers respectively. The curcumin release percentage over a period of 11 and 18 days for curcumin loaded electrospun PLLA-CA and PLLA-SF nanofibers were found to be ~ 85 and 86 % respectively. These results conclude that no significant changes in the release percentage occurred although the number of days varied. The time taken for curcumin release from C-PLLA-CA nanofiber was higher (i.e., 18 days) compared to C-PLLA-SF nanofiber (i.e., 11 days); the reason may be due to the high hydrophilicity of PLLA-SF nanofiber. Hence, these results suggest that the hydrophilic CA and SF could be used as a filler or blend to hydrophobic PLLA which is good nanocarrier to deliver poor water soluble hydrophobic curcumin delivery.
Conclusion
This present work describes the enhancement of hydrophilic properties of PLLA for cancer treatment and to investigate curcumin release pattern by three methods using electrospinning. The contact angle measurements and water uptake test results confirmed that the used three methods were effective and increased the hydrophilic properties of PLLA. The cell culture (NIH 3 T3 cell lines) results showed that the prepared PLLA based fibrous scaffolds had an improved hydrophilicity thereby resulting in excellent cell adhesion and growth. The ‘in-vitro’ release of curcumin from the PLLA based fibrous scaffold confirmed the suitability of the prepared fibrous scaffolds for drug delivery and the pore size greatly influenced the releasing time of curcumin.
References
Diba M, Kharaziha M, Fathi MH, Gholipourmalekabadi M, Samadikuchaksaraei A (2012) Preparation and characterization of polycaprolactone/forsterite nanocomposite porous scaffolds designed for bone tissue regeneration. Compos Sci Technol 72:716–723
Wang S, Zhang Y, Wang H, Yin G, Dong Z (2009) Fabrication and properties of the electrospun polylactide/silk fibroin-gelatin composite tubular scaffold. Biomacromolecules 10:2240–2244
Cui W, Cheng L, Li H, Zhou Y, Zhang Y, Chang J (2012) Preparation of hydrophilic poly (L-lactide) electrospun fibrous scaffolds modified with chitosan for enhanced cell biocompatibility. Polymer 53:2298–2305
Venugopal J, Ma LL, Yong T, Ramakrishna S (2005) In vitro study of smooth muscle cells on polycaprolactone and collagen nanofibrous matrices. Cell Biol Int 29:861–867
Kharaziha M, Fathi MH, Edris H (2003) Tunable cellular interactions and physical properties of nanofibrous PCL-forsterite:gelatin scaffold through sequential electrospinning. Compos Sci Technol 87:182–188
Agarwal S, Wendorff JH, Greiner A (2008) Use of electrospinning technique for biomedical applications. Polymer 49:5603–5621
Sohrabi A, Shaibani PM, Etayash H, Kaur K, Thundat T (2013) Sustained drug release and antibacterial activity of ampicillin incorporated poly (methyl methacrylate) – nylon6 core/shell nanofibers. Polymer 54:2699–2705
Goh Y, Shakir I, Hussain R (2013) Electrospun fibers for tissue engineering, drug delivery, and wound dressing. J Mater Sci 48:3027–3054
Supaphol P, Suwantong O, Sangsanoh P, Sowmya S, Jayakumar R, Nair SV (2012) Electrospinning of biocompatible polymers and their potentials in biomedical applications. Adv Polym Sci 246:213–240
Kim CH, Jung YH, Kim HY, Lee DR, Dharmaraj N, Choi KE (2006) Effect of collector temperature on the porous structure of electrospun fibers. Macromol Res 14:59–65
Casper CL, Stephens JS, Tassi NG, Chase DB, Rabolt JF (2004) Controlling surface morphology of electrospun polystyrene fibers: Effect of humidity and molecular weight in the electrospinning process. Macromolecules 37:573–578
Megelski S, Stephens JS, Chase DB, Rabolt JF (2002) Micro- and nanostructured surface morphology on electrospun polymer fibres. Macromolecules 35:8456–8466
Kidoaki S, Kwon IIK, Matsuda T (2005) Mesoscopic spatial designs of nano- and microfiber meshes for tissue-engineering matrix and scaffold based on newly devised multilayering and mixing electrospinning techniques. Biomaterials 26:37–46
Seo YA, Pant HR, Nirmala R, Lee JH, Song KG, Kim HY (2012) Fabrication of highly porous poly (έ-caprolactone) microfibers via electrospinning. J Porous Mater 19:217–223
Norman JJ, Desai TA (2006) Methods for fabrication of nanoscale topography for tissue engineering scaffolds. Ann Biomed Eng 34:89–101
Ramalingam N, Natarajan TS, Rajiv S (2014) Preparation and characterization of electrospun curcumin loaded poly (2-hydroxyethyl methacrylate) nanofiber-A biomaterial for multidrug resistant organisms. J Biomed Mater Res A. doi:10.1002/jbm.a.35138
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY (2001) Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21:2895–2900
Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R (2008) Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res 14:4491–4499
Priyadarsini KI, Maity DK, Naik GH, Kumar MS, Unnikrishnan MK, Satav JG, Mohan H (2003) Role of phenolic O:H and methylene hydrogen on the free radical reaction and antioxidant activity of curcumin. Free Radic Biol Med 35:475–484
Thangaraju E, Srinivasan NT, Kumar R, Sehgal PK, Rajiv S (2012) Fabrication of electrospun poly (L-lactide) and curcumin loaded poly (L-lactide) nanofibers for drug delivery. Fibers Polym 13:823–830
Elakkiya T, Malarvizhi G, Rajiv S, Natarajan TS (2013) Curcumin loaded electrospun Bombyxmori silk nanofibers for drug delivery. Polym Int 63:100–105
Nagiah N, Sivagnanam UT, Mohan R, Srinivasan NT, Sehgal PK (2012) Development and characterization of electrospun poly (propylene carbonate) ultrathin fibers as tissue engineering scaffolds. Adv Eng Mater 14:B138–B148
Frey MW, Li L (2007) Electrospinning and porosity measurements of Nylon-6/poly(ethylene oxide) blended nonwovens. J Eng Fibers Fabr 2:31–37
Rockwood DN, Preda RC, Yucel T, Wang X, Lovett ML, Kaplan DL (2011) Materials fabrication from Bombyx mori silk fibroin. Nat Protoc 6:1612–1631
Donsi F, Wang Y, Li J, Huang Q (2010) Preparation of curcumin sub-micrometer dispersions by high-pressure homogenization. J Agric Food Chem 58:2848–2853
Kizhakkayil J, Thayyullathi F, Chathoth S, Hago A, Patel M, Galadari S (2010) Modulation of curcumin-induced Akt phosphorylation and apoptosis by PI3K inhibitor in MCF-7 cells. Biochem Biophys Res Commun 394:476–481
Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD (2008) Dual roles of Nrf2 in cancer. Pharmacol Res 58:262–270
Liu D, Chen Z (2013) The effect of curcumin on breast cancer cells. J Breast Cancer 16:133–137
Sa G, Das T (2008) Anticancer effects of curcumin: cycle of life and death. Cell Div 3:14
Prabaharan M, Jayakumar R, Nair SV (2012) Electrospun nanofibrous scaffolds-current status and prospectus in drug delivery. Adv Polym Sci 246:241–262
Acknowledgments
The authors acknowledge the Department of Science and Technology (DST) for financial assistance (Project No. SR/S1/PC-56/2009 (G) dated 12.07.2011). The instrumentation facility provided under FIST-DST and DRS-UGC to Department of Chemistry, Anna University, Chennai is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Thangaraju, E., Rajiv, S. & Natarajan, T.S. Comparison of preparation and characterization of water-bath collected porous poly L –lactide microfibers and cellulose/silk fibroin based poly L-lactide nanofibers for biomedical applications. J Polym Res 22, 24 (2015). https://doi.org/10.1007/s10965-015-0664-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-015-0664-z