Abstract
Isothermal and dynamic differential scanning calorimetry (DSC) was exploited to study the curing behavior of diglycidyl ether bisphenol-A epoxy resin with various combining ratios of dicyandiamide (DICY) and nadic methyl anhydride (NMA). Curves of prepared samples indicated that the enthalpy of the reaction decreased with increasing the molar ratios (NMA/DICY) up to 40% after which an exothermic peak peculiar to the effect of anhydride appeared at a higher temperature. The curing behavior examination of the samples containing the aforementioned molar ratio of NMA/DICY (= 40%) was carried out using isothermal condition at different temperatures (130–145 °C) and dynamic condition DSC at various heating rates (2.5–20 °C min−1). Under the isothermal condition, by constructing a master curve, the values of activation energy (Ea) and pre-exponential factor (A) were calculated 89.3 kJ mol−1 and 1.2 × 10+9 s−1, respectively. The activation energy of the curing reactions in a dynamic mode was obtained 85.32 kJ mol−1 and 88.02 kJ mol−1 using Kissinger and Ozawa methods, respectively. Likewise, pre-exponential factors were also calculated 3.35 × 10+8 and 7.4 × 10 +8 s−1, respectively. The overall order of reaction for both conditions was found to be a value around 3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For unique mechanical, chemical and heat-resistant properties, epoxy resins are widely used in prepregs composite applications. Epoxy resins belong to polyether families having more than one epoxy group capable of reacting with a curing agent to form three-dimensional cross-linked structures [1, 2]. The final properties of the product are strongly influenced by the degree of cure which, in turn, is largely determined by curing conditions (such as curing time and temperature) [3,4,5,6]. For this reason, determining the curing kinetic parameters and finally selecting an optimal curing cycle is a key factor for manufacturing high-performance epoxy composites. DSC analysis is a thermo-analytical technique that relates the heat flow changes with the reaction rates of the resin during the curing process [3, 4, 7]. Isothermal and dynamic DSC modes are two different approaches that could be applied to examine the cure kinetics of an epoxy resin. During isothermal scanning, the sample is maintained at a constant temperature for various time intervals, whereas dynamic measurements involve heating the sample at a constant rate over a desired temperature range [4, 5]. Dynamic DSC measuring the sample heat flow and total heat flow rates as a function of temperature provides more reliable information than the isothermal mode [1, 8]. In general, the degree of cure, α, is generally proportional to the ratio of the heat released by cross-linking at given time (\(\Delta H_{\text{t}}\)) to the total amount of heat generated during a complete reaction, \({\text{i}} . {\text{e}}\; \left( {\alpha = \frac{{\Delta H_{\text{t}} }}{{\Delta H_{\text{total}} }}} \right)\) [9, 10]. The heat of curing reaction is achieved by integrating the total area of the heat flow curve. Isothermal heat flow data are obtained either by the residual heat or by the reaction heat at a particular time [11, 12]. N-order and autocatalytic mechanisms are two kinetic models in DSC cure analysis which are able to anticipate the characteristic features of the curing system for a given cure cycle. Among several proposed models to characterize the curing conversion-dependence function, the simplest one is \(f\left( \alpha \right) = (1 - \alpha )^{n}\) for nth order and \(f\left( \alpha \right) = \alpha^{m} (1 - \alpha )^{n}\) for autocatalytic systems [8, 11, 12]. The values of m and n indicate the symmetry and shape of the observed reaction rate curve, respectively.
The maximum rate of an nth order reaction will take place at the beginning of the reaction, whereas the autocatalytic reaction will exhibit its maximum rate in the intermediate conversion stage of the reaction [2, 11]. Curing kinetic parameters of n-order could be obtained from both the isothermal and dynamic methods, in contrast, in the case of autocatalytic order reactions, they should be manipulated solely by isothermal method [13]. In an isothermal approach, the conversion-dependent part of the rate equation,\(f(\alpha )\), could be determined by transforming the experimental data obtained from different temperatures into a master curve according to the following equation [11, 13]:
where dimensionless time tr is the ratio of a particular time of the reaction (t) to the time (tm) in which the maximum reaction rate (\(\alpha_{m}\)) appears.
In non-isothermal DSC measurements carried out at different heating rates, it is common to express the reaction rate as a function of heating rate as the following equation [11, 13]:
where \(\beta = \frac{{{\text{d}}T}}{{{\text{d}}t}}\) and the subscript “p” stands for the maximum peak in DSC (\(T_{\text{p}}\)) for each heating rate. This equation relates the derivative of a degree of cure with respect to temperature (\(\frac{{{\text{d}}\alpha }}{{{\text{d}}T}}\)) to the heating rate and model parameters. From the dynamic DSC measurements, the Arrhenius parameters could be evaluated by the variable heating rate method of Ozawa and Kissinger in which the activation energy and pre-exponential factor are considered to be constant [2, 11]. In these methods, kinetic parameters are estimated with no knowledge of the degree of conversion and conversion-dependant function as ensuing [2]:
These methods, also, assume that more reliable data are obtained merely for the peak maxima in DSC (\(T_{\text{p}}\)) for each heating rate. By plotting the left side of each equation versus \(\frac{1}{{T_{\text{p}} }}\), activation energy could be calculated by the slope of the linear plot.
Several works have been reported to specify the cure reaction kinetic and monitor the curing process of either dicyandiamide (DICY)-cured [14,15,16,17,18,19] or Nadic methyl anhydride (NMA)-cured [20,21,22,23,24,25,26,27] epoxy resins.
Sacher [14] investigated the effect of DICY on the cure kinetics of epoxy resin based on bisphenol-A in a range of temperatures exploiting DSC technique. Schneider [16] studied the curing behavior of an epoxy resin containing commercial DICY as a curing agent by differential scanning calorimetry (DSC). The cure kinetics and synergistic impact of NMA along with some certain mercaptans upon the curing of epoxy resin were also studied [20]. The curing behavior of epoxy glass-reinforced prepreg/DICY system was examined by Hayati et al. [2, 11]. They reported the apparent activation energy and overall reaction order according to both isothermal and dynamic DSC methods. No report, however, upon investigation of the curing kinetic parameters of an epoxy resin based a mixture of DICY and NMA has yet been published in the literature. Therefore, the objective of this work is to study the effect of NMA on the cure kinetics of epoxy/DICY applied for prepreg composites. For this purpose, a series of epoxy samples based on diglycidyl ether of bisphenol-A (DGEBA), DICY and NMA were prepared. The curing behavior of the samples was characterized by the utilization of isothermally and dynamically obtained thermograms. Under the isothermal condition, the two techniques of isoconversion and a reduced plot was adopted to obtain the cure kinetic parameters. The kinetic parameters of epoxy resin in dynamic cure fashion are also derived by fitting the data upon the Kissinger and Ozawa approaches.
Experimental
Materials
Diglycidyl ether bisphenol-A (DGEBA, LY556, epoxy equivalent = 183–189 g eq−1) was purchased from Huntsman (Germany). The curing agents, dicyandiamide (DICY) and nadic methyl anhydride (NMA) are supplied by Sigma-Aldrich Co. and Korean Kukdo Co., respectively. 2-Methyl imidazole as an accelerator supplied by Sigma-Aldrich.
Sample preparation
All samples utilized for thermal analysis were prepared at room temperature as follows: The epoxy resin and the DICY were mixed together in a vial for 5 min. The NMA at different mole ratios of DICY/ NMA was added to the mixture and mixed for 10 min to prepare the samples. The mixing was slow enough to avoid air trapping. Table 1 represents the various mole ratios of DICY/ NMA per 100 g of epoxy resin in different samples (coded by ESx in which the index “x” represents the number of samples). The upper limits for the amount of hardener (7 g for DICY and 94 g for NMA) are selected based on several works from the literature [3, 5, 28].
Differential scanning calorimetric (DSC) analysis
Isothermal and non-isothermal curing kinetics were carried out using a DSC (Mettler toledo star, Swiss) instrument which was calibrated via indium. 10–20 mg sample was put into an aluminum pan, sealed and then placed in the DSC chamber. An empty pan was used as a reference. The experiments were performed under a constant flow of nitrogen of 20 mL min−1 used as purge gas. The sample was quickly heated up to the desired cure temperature (130, 135, 140, and 145 °C) and then isothermally kept at that temperature for various times. Following this scan, the DSC cell was rapidly cooled down to room temperature and then reheated to 275 °C at 10 °C min−1 to measure the residual heat of the reaction. The dynamic DSC runs were carried out at different heating rates ranging from 2.5 to 20 °C min−1. The curves were obtained and analyzed.
Results and discussion
Isothermal analysis
In order to study the curing behavior of the epoxy resins, the first step is to investigate the effect of the addition of NMA to DICY/epoxy by analyzing dynamic cure DSC curves (over the temperatures in the range from 20 to 400 °C using heating rate of 2.5 °C min−1). Figure 1 shows the changes of the enthalpy of reaction versus the moles of DICY/NMA. It could be seen that there is a decrease in the heat of reaction (\(\Delta H_{1}\)) once the mole ratio of DICY/NMA added in epoxy/DICY system is increased.
This could be explained by the endothermic nature of reaction between the epoxy and anhydride groups according to the following mechanism (Scheme 1).
As the mole ratio of DICY/NMA exceeds 40%, an exothermic reaction heat (\(\Delta H_{2}\)) starts at a higher temperature compared with that of (\(\Delta H_{1}\)). It reveals that the epoxy samples containing the higher content of NMA demand a post-curing operation. Consequently, an optimum mole of NMA should be chosen such that the heat reaction peak at the higher temperature (\(\Delta H_{2}\)) disappears. Hence, it is expected that the breakpoint of the \(\Delta H_{2}\) curve with 40% mole fraction of NMA should be selected as the optimal sample (i.e., EC5).
A DSC dynamic scan in a heating rate of 5°C min−1 (from 30 to 350 °C) was performed on this sample. It is essential to select appropriate temperatures for isothermal curing analysis. Table 2 presents the flow heat curve characteristics of the dynamic DSC curve of the sample with 40% mole fraction of NMA.
According to the table, the temperature, in which the isothermal DSC experiment fulfilled, could be selected in the first part of the exothermic peak, i.e., 130, 135, 140, and 145 °C. The cure integration of isothermal DSC experiments at higher temperatures may be inaccurate due to a significant fluctuation.
The evolution of conversion data calculated by Eq. (1) is shown in Fig. 2. It seems that the reactions at all four temperatures are incomplete. As expected, the conversion at any given time is increased with temperature. All curves exhibit a typical sigmoid shape indicating an autocatalytic curing kinetic mechanism in the epoxy samples.
The rates of cure reaction are achieved by virtue of differentiating the cure degree curve with respect to time. Figure 3 demonstrates the reaction rate versus conversion at various temperatures. As it is obvious, the reaction rates pass through a maximum at a conversion about 20% and then decreases with increasing curing degree. Such behavior is characteristic of an autocatalytic reaction mechanism in which initial reaction rate is zero.
The maximum value of the reaction rate is also found to increase with an increase in temperature.
However, the maximum rate conversion, \(\alpha_{\text{m}}\), where the maximum reaction rate is observed (~20%), remains virtually unaffected by the changes of temperature. The activation energy values of the curing could be acquired with the isoconversional method [29,30,31]. For this purpose, the logarithm of the times \((Ln(t))\) required for reaching the same conversion at various isothermal temperatures is plotted versus \(\frac{1}{T}\). Such plots are given in Fig. 4.
Iso- conversion times as a function of \(1/T\). The calculated lines slope corresponding to activation energies is summarized in Table 3
As shown, the time to reach the same conversion decreases in a linear fashion with increasing temperature. The slope of each line provides the reaction activation energy as given in Table 3.
It could be seen that the activation energies remain approximately constant and are close to their average value, i.e., 90.5 kJ mol−1. This activation energy is consistent with the experimental values found in the literature for different epoxy resin systems [8, 11, 13]. Extrapolating these lines back to the \(ln(t)\)-intercept could be utilized so as to calculate the pre-exponential factor A. Prior to that, the conversion-dependence function should be determined. In this regard, the times illustrating the maximum reaction rate \((t_{\text{m}} )\) at each isothermal temperature are obtained from Figs. 1 and 2.
The \({\text{d}}\alpha /{\text{d}}t\) values are multiplied by tm to acquire reduced rate and construct single master curve. Figure 5 demonstrates the experimental data obtained at different isothermal temperatures that are fitted onto a single curve (master curve) reflecting conversion-dependence contribution of the cure reaction \((f(\alpha ))\). In order to determine conversion-dependence function, the values of \(\alpha_{\text{m}} = 0.2\) and \((\frac{{{\text{d}}\alpha }}{{{\text{d}}t_{r} }})_{{\alpha = \alpha_{\text{m}} }} = 0.0092\) are read from Fig. 5 and used in Eq. 1.
Reaction rate reduced time plot (master curve) of epoxy composites with the reference time of maximum conversion (\(\alpha_{ \hbox{max} } = 0.2\)) compared with model Eq. (1)
Using a linear data regression based on Levenberg–Marquardt algorithm method, the reaction orders m=0.7, n = 2.3 are determined to fit the experimental master curve.
According to Eq. 2, the reaction rate (\({\text{d}}\alpha /{\text{d}}t\)) has a linear dependence on conversion-dependence function. Figure 6 indicates the linear plot of \({\text{d}}\alpha /{\text{d}}t\) vs \(f(\alpha )\) providing the reaction rate constants from the slopes for the four isothermal experiments.
Eventually, the kinetic parameters (E and A) are determined according to the Arrhenius equation by plotting the obtained reaction rate constants against 1/T as shown in Fig. 7. As a result, \(A = 1.2 \times 10^{9}\) and \(E = 89.3 \;{\text{kJ}}\; {\text{mol}}^{ - 1}\) are calculated from the intercept and the slope of the linear plot, respectively.
Non-isothermal analysis
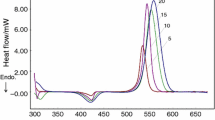
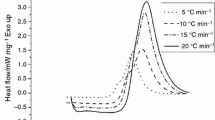
The heat flow diagrams obtained from DSC at different heating rates versus time and temperature are shown in Fig. 8.
By the integration of under-flow curves in Fig. 8a, the reaction heat at each heating rate is calculated. The values of total reaction heat and the major peak characteristics \(\left(\left( {\frac{{{\text{d}}\alpha }}{{{\text{d}}T}}} \right)_{\text{p}} , T_{\text{p}} , \alpha_{\text{p}} , t_{\text{p}} \right)\) contributing to model evaluation are presented in Table 4.
From Fig. 8b, it is conspicuous that the temperatures, where the beginning, the end and the maximum of exothermic peak occur, increase with the heating rate. The average cure reaction heat is calculated as 425.87 J g−1. The heat evolved up to a certain temperature, (\(\Delta H_{\text{t}}\)), divided by the total heat of reaction, \(({\text{total}}\; {\text{area}}\; {\text{under}}\; {\text{the}}\; {\text{heat}}\; {\text{flow}}\; {\text{curve}} =\Delta H_{\text{total}} )\) gives the extent of reaction, (\(\alpha\)) and its derivative \(\left(\frac{{{\text{d}}\alpha }}{{{\text{d}}t}} \right)\) for different times/temperatures. The flow curve characteristics are given in Table 4. While the estimated values for \((\frac{{{\text{d}}\alpha }}{{{\text{d}}T}})_{\text{p}}\) are insensitive to heating rate, the \(\alpha_{\text{p}}\) is decreased.
A logarithm plot of the heating rate against the reciprocal absolute temperature peak is represented in Fig. 9. From the slope of the well linear relationship between \(\beta\) and \(\frac{1}{{T_{\text{p}} }}\), activation energy is calculated regarding the Ozawa and Kissinger methods to be 88.015 and 85.32 kJ mol−1, respectively.
Figure 10 shows a number of isoconversional plots obtained from plotting \(\beta\) against \(\frac{1}{T}\). Apparently, a series of parallel lines is achieved up to the conversion of 70% reflecting almost the same activation energies. At higher conversions, however, the slope slightly deviates. From Fig. 10, it is quite clear that the temperature required to reach the same conversion increases with increasing heating rate. The apparent activation energies calculated from the slopes of the straight lines are evaluated to be 84.98 kJ mol−1.
The reason for the deviation in slopes observed at higher conversion may be account for the change in cure behavior due to the more activity of anhydride groups at higher temperatures.
The reduced pre-exponential factor (\(A_{\text{r}}\)), pre-exponential factor A and the orders of the cure reaction (m and n) were calculated for Ozawa and Kissinger methods based on the Levenberg–Marquardt algorithm multiple nonlinear least squares regression.
Data shown in Table 5 compare the average values of the cure kinetic parameters obtained from three different methods, i.e., isothermal, Kissinger and Ozawa. The E value obtained with the Kissinger approach is differently collated with the two other methods.
The lowest value for the overall reaction order was obtained with the isothermal method, whereas the highest value was given with the Kissinger method. The range of obtained overall reaction order with these three methods represents a third-order reaction.
Conclusions
The cure kinetics of an epoxy/ co-hardener system used in prepreg application was investigated. The kinetic parameters coupled with the conversion-dependent function were determined by constructing an isothermal cure master curve. An autocatalytic model (Kamal equation) was considered to describe the cure kinetics in the range of 130–145 °C. The activation energy and pre-exponential factor were determined as \(A = 1.2 \times 10^{9}\) and \(E = 89.3\) kJ mol−1 .The isoconversional method was also applied to calculate the activation energy, 90.5 kJ mol−1, for a comparison. It is found to be a desirable agreement between the obtained results. The kinetic parameters of the cure reaction were also investigated by a dynamic DSC technique using the Kissinger and Ozawa equations. Although the value of E obtained from Kissinger method indicates a deviation compared to other methods, it could be mentioned that there is a favorable consistency between all of the methods in predicting cure kinetic parameters of NMA/DICY/epoxy resin. The overall reaction order (m + n ≈ 3) with these three methods shows a third-order epoxy cure reaction for the discussed system.
References
Monteserín C, Blanco M, Laza JM, Aranzabe E, Vilas JL. Thickness effect on the generation of temperature and curing degree gradients in epoxy–amine thermoset systems. J Therm Anal Calorim. 2018;132(3):1867–81.
Morancho J, Fernández-Francos X, Acebo C, Ramis X, Salla J, Serra Àjjo TA, et al. Thermal curing of an epoxy-anhydride system modified with hyperbranched poly (ethylene imine) s with different terminal groups. J Therm Anal Calorim. 2017;127(1):645–54.
Flick EW. Epoxy resins, curing agents, compounds, and modifiers: an industrial guide. 2nd ed. William Andrew/Noyes: Park Ridge; 1993.
Hamed AA, Saad GR, Elsabee MZ. Synthesis and characterization of modified epoxy resins and novel E-glass-/spectra-reinforced composites. High Perform Polym. 2017;29(3):328–40.
Jaipuriar A, Bakis CE, Lopez MM, editors. Cure kinetics and physical aging of an ambient-curing epoxy resin. In: Proceedings of the 6th international conference on CICE; 2012; 6(13).
Pascault JP, Williams RJ. Epoxy polymers. Germany: Wiley; 2009.
Yousefi A, Lafleur P, Gauvin R. Kinetic studies of thermoset cure reactions: a review. Polym Compos. 1997;18(2):157–68.
Cai H, Li P, Sui G, Yu Y, Li G, Yang X, et al. Curing kinetics study of epoxy resin/flexible amine toughness systems by dynamic and isothermal DSC. Thermochim Acta. 2008;473(1):101–5.
Wan J, Li C, Bu Z-Y, Xu C-J, Li B-G, Fan H. A comparative study of epoxy resin cured with a linear diamine and a branched polyamine. Chem Eng J. 2012;188:160–72.
Chandran MS, Krishna M, Rai S, Krupashankara M, Salini K. Cure kinetics and activation energy studies of modified bismaleimide resins. ISRN Polym Sci. 2012;2012:1–8.
Hayaty M, Beheshty MH, Esfandeh M. Isothermal differential scanning calorimetry study of a glass/epoxy prepreg. Polym Adv Technol. 2011;22(6):1001–6.
Pagano RL, Calado VM, Tavares FW, Biscaia EC. Cure kinetic parameter estimation of thermosetting resins with isothermal data by using particle swarm optimization. Eur Polym J. 2008;44(8):2678–86.
Kim SW, Nam J-D. Study of cure kinetic modeling of RSL-1895 epoxy system using composite kinetic methodology. J Ind Eng Chem. 2002;8:212.
Sacher E. Kinetics of epoxy cure: 3. the systems bisphenol-A epoxides/dicy. Polymer. 1973;14(3):91–5.
LaLiberte BR, Bornstein J, Sacher RE. Cure behavior of an epoxy resin-dicyandiamide system accelerated by monuron. Ind Eng Chem Prod Res Dev. 1983;22(2):261–2.
Schneider N, Sprouse J, Hagnauer G, Gillham J. DSC and TBA studies of the curing behavior of two dicy-containing epoxy resins. Polym Eng Sci. 1979;19(4):304–12.
Ren R, Xiong X, Ma X, Liu S, Wang J, Chen P, et al. Isothermal curing kinetics and mechanism of DGEBA epoxy resin with phthalide-containing aromatic diamine. Thermochim Acta. 2016;623:15–21.
Hong S-G, Wu C-S. DSC and FTIR analysis of the curing behaviors of epoxy/DICY/solvent open systems. Thermochim Acta. 1998;316(2):167–75.
Güthner T, Hammer B. Curing of epoxy resins with dicyandiamide and urones. J Appl Polym Sci. 1993;50(8):1453–9.
Romão B, Diniz MF, Azevedo MF, Lourenço VL, Pardini LC, Dutra RC, et al. Characterization of the curing agents used in epoxy resins with TG/FT-IR technique. Polimeros. 2006;16(2):94–8.
Zee R, Huang Y, Chen J, Jang B. Properties and processing characteristics of dielectric-filled epoxy resins. Polym Compos. 1989;10(4):205–14.
Tadros R, Timm D. Fourier-transform infrared analysis of a linear, anhydride-cured epoxy. Macromolecules. 1995;28(22):7441–6.
Rocks J, George GA, Vohwinkel F. Curing kinetics and thermomechanical behaviour of co-anhydride cured aminoglycidyl epoxy resins. Polym Int. 2003;52(11):1758–66.
Hu J, Shan J, Zhao J, Tong ZJ. Water resistance and curing kinetics of epoxy resins with a novel curing agent of biphenyl-containing amine synthesized by one-pot method. Thermochim Acta. 2015;606:58–65.
Jain R, Choudhary V, Narula A. Studies on the curing kinetics of epoxy resins using mixture of nadic/or maleic anhydride and 4, 4′-diaminodiphenyl sulfone. J Therm Anal Calorim. 2007;90(2):495–501.
Jain R, Choudhary V, Narula A. Curing and thermal behavior of DGEBA in presence of dianhydrides and aromatic diamine. J Appl Polym Sci. 2007;105(6):3804–8.
Chian W, Timm DC. Chemical/mechanical analyses of anhydride-cured thermosetting epoxys: DGEBA/NMA/BDMA. Macromolecules. 2004;37(21):8098–109.
Stockel RF. Dicyandiamide (cyanoguanidine). J Chem Educ. 1969;46(6):391.
Vafayan M, Beheshty MH, Ghoreishy MHR, Abedini H. Advanced integral isoconversional analysis for evaluating and predicting the kinetic parameters of the curing reaction of epoxy prepreg. Thermochim Acta. 2013;557:37–43.
Fan M, Li X, Zhang J, Cheng J. Curing kinetics and shape-memory behavior of an intrinsically toughened epoxy resin system. J Therm Anal Calorim. 2015;119(1):537–46.
Jovičić M, Radičević R, Pavličević J, Bera O. Isoconversional kinetic analysis of the alkyd/melamine resins curing. Chem Ind Chem Eng Q. 2013;19(2):253–62.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerami, G., Bagheri, R. & Darvishi, R. Investigation of isothermal and dynamic cure kinetics of epoxy resin/nadic methyl anhydride/dicyandiamide by differential scanning calorimetry (DSC). J Therm Anal Calorim 137, 575–582 (2019). https://doi.org/10.1007/s10973-018-7961-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7961-9