Abstract
Purpose of Review
Obesity is related to several comorbidities such as type 2 diabetes mellitus, cardiovascular disease, heart failure, and various types of cancers. While the detrimental effect of obesity in both mortality and morbidity has been well established, the concept of the obesity paradox in specific chronic diseases remains a topic of continuous interest. In the present review, we examine the controversial issues around the obesity paradox in certain conditions such as cardiovascular disease, several types of cancer and chronic obstructive pulmonary disease, and the factors that may confound the relation between obesity and mortality.
Recent Findings
We refer to the obesity paradox when particular chronic diseases exhibit an interesting “paradoxical” protective association between the body mass index (BMI) and clinical outcomes. This association, however, may be driven by multiple factors among which the limitations of the BMI itself; the unintended weight loss precipitated by chronic illness; the various phenotypes of obesity, i.e., sarcopenic obesity or the athlete’s obesity phenotype; and the cardiorespiratory fitness levels of the included patients. Recent evidence highlighted that previous cardioprotective medications, obesity duration, and smoking status seem to play a role in the obesity paradox.
Summary
The obesity paradox has been described in a plethora of chronic diseases. It cannot be emphasized enough that the incomplete information received from a single BMI measurement may interfere with outcomes of studies arguing in favor of the obesity paradox. Thus, the development of carefully designed studies, unhampered by confounding factors, is of great importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of obesity is constantly rising, creating a major global health problem [1]. There are numerous studies and meta-analyses which demonstrate that obesity is related with several comorbidities such as type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD), heart failure (HF), hypertension, and various types of cancers [2]. We refer to the obesity paradox when particular chronic diseases, that will be reviewed hereunder, exhibit an interesting “paradoxical” protective association between body mass index (BMI) and clinical outcomes. Nevertheless, in the absence of coexisting conditions with may display these paradoxical associations, body weight has unequivocally been shown to display a J-shaped association with multiple causes of morbidity and mortality as well as with the leading causes of death globally, especially in the setting of obesity classes II and III [3, 4].
Overweight and obesity are generally defined using the body mass index, both in clinical practice and in epidemiological studies [5]. The need of an index describing the magnitude of adiposity was originally generated in 1832 by a Belgian mathematician, statistician, and astronomer named Quetelet who used the ratio of the weight in kilograms divided by the square of the height in meters. This index was validated by Ancel Keys in 1972 naming it body mass index (BMI) [6]. The World Health Organization defines overweight and obesity as abnormal or excess body adiposity that may impair health, underlining that BMI may not reflect the degree of fat percentage among different individuals [7]. Therefore, it is essential to include additional parameters in order to correctly define overweight and obesity, as a trait which reflects the sum of adiposity, such as waist and hip circumferences and, when feasible, assessments of fat/muscle percentage via body composition analysis [8]. Anthropometric and body composition analysis could showcase individuals with similar BMIs, but different body composition data in terms of lean and fat mass, thus differentiating health outcomes [9]. Furthermore, several medical conditions have been associated with low muscle mass and function or with the coexistence of reduced lean and increased fat mass, notably in the elderly population, a phenomenon known as sarcopenia or sarcopenic obesity, respectively [10, 11]. These observations could partly set the ground for understanding how the term “obesity paradox” was generated. Consequently, in order to clarify the controversial issues around obesity related outcomes, BMI determination does not suffice, and additional indices resulting from the quantification of different body compartments such as fat and lean mass are of utmost importance [12].
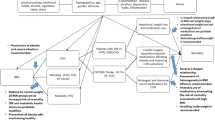
The obesity paradox has been described in a plethora of chronic diseases. That said, it is important to note that despite the literature pointing towards an obesity paradox, there is very strong evidence supporting obesity’s role in the pathogenesis of multiple diseases including CVD and is associated with reduced overall survival. The paradoxical “protective” effect of overweight or mild obesity has been reported mainly in CVD, including coronary heart disease (CHD), heart failure (HF), hypertension, and atrial fibrillation (AF) [13]. However, this paradoxical effect has appeared in several other conditions such as cancers, chronic kidney disease, chronic obstructive pulmonary disease (COPD), stroke, pulmonary hypertension, osteoporosis, critical illness, and sepsis (Fig. 1) [4, 14,15,16,17,18,19,20]. It cannot be emphasized enough that the incomplete information received from a single BMI measurement may interfere with outcomes of studies arguing in favor of the obesity paradox. A large meta-analysis of 10,625,411 participants that included 239 prospective studies from 4 continents showed that the relationship between BMI and all-cause mortality is generally J-shaped. This could point to the fact that if underweight were to be excluded from this analysis, the association of higher body weight with mortality would be even stronger. The nadir BMI for low mortality varied with age with participants aged 35–49 years old exhibiting a nadir mean BMI of 22 kg/m2. Participants aged 50–69 years old had a mean BMI equal to 23 kg/m2, and those aged 70–89 years old were found with a mean BMI of 24 kg/m2 [21]. Another meta-analysis of 230 cohort studies demonstrated that the BMI-associated mortality curve is U-shaped with an elevated mortality at the extremes of obesity and underweight among all participants. However, a J-shaped curve was observed among participants that were never smokers [22]. A large cohort study demonstrated that in middle aged-adults were smokers, obesity is associated with shorter longevity and increased cardiovascular mortality compared with normal weight patients. Of note, 48% of normal weight patients were smokers versus 32,7% in patients with obesity [23].
BMI might be a very easy, costless method to classify adiposity; however, there are various different obesity phenotypes, suggesting that people with similar BMIs may present with different body compositions. A first such example is the nonsarcopenic phenotype of obesity with both increased fat and lean mass. Then, there is the athlete’s phenotype with obesity, i.e., decreased fat and increased lean mass, and lastly, the sarcopenic phenotype of obesity with elevated fat and reduced lean mass [24, 25]. A substantial number of studies have shown that there were different health outcomes among individuals with the same BMI, but different body composition data, since BMI does not account for the degree or site of adiposity [12]. A prospective cohort study involving 10,265 men demonstrated that there is an inverse and independent association between muscular strength and all-cause mortality. After adjustment for age, the authors showed that the group with high muscular strength and cardiorespiratory fitness had significantly 60% lower mortality rates than the opposite group [26].
The concept of metabolically healthy obesity has also recently emerged, in which adults with obesity do not present with metabolic comorbidities and ultimately may have lower mortality than leaner individuals with cardiovascular risk factors [27] or may have a higher level of fitness [28]. One should consider the heterogeneous nature of obesity and thus should possibly regard metabolically healthy obesity as a transient state [27] which suggests that people living with obesity are at increased risk for adverse long-term outcomes even in the absence of metabolic abnormalities, i.e., no healthy pattern of obesity actually exists [29].
Methodological and study limitations may also interfere with the association of obesity with morbidity and mortality, originating from the lack of understanding that obesity is a chronic disease [30].
Obesity year exposure may play an important role. Relating obesity duration to cardiometabolic disease risk factors in mid-adulthood has shown that a larger obesity duration exposure is associated with worsening of cardiometabolic disease risk factors, although this positive association was attenuated when adjusted for obesity severity [31]. Another possible explanation includes the bias due to the so called reverse causation where antecedent weight loss due to chronic illness like cancer may elevate mortality risk [32]. It has been proposed that excess adipose tissue may act as a metabolic reservoir, in a way protecting exposure of additional tissues to lipotoxicity and ectopic fat deposition along with acting as a buffer for dietary fat influx [33]. Several of the aforementioned confounding factors, i.e., the subjects’ underlying health status, antecedent body weight loss due to chronic illness or ageing, and smoking, may on several occasions be challenging to surmount, and thus, caution should be exercised in interpreting study results [32]. The aim of the present review is to examine the controversial issues around the obesity paradox in certain conditions such as cardiovascular disease, several types of cancer and chronic obstructive pulmonary disease, and the factors that may confound the relation between obesity and mortality.
Obesity Paradox in Cardiovascular Disease
Coronary Heart Disease
The association of obesity with increased mortality risk has long been established [34], and multiple studies have pointed out that obesity has a detrimental effect on CVD. Pioneering research in the field was performed by Ellis et al., who tested the hypothesis that individuals with both BMI < 20 kg/m2 and BMI > 35 kg/m2 would possibly demonstrate increased mortality risk after percutaneous coronary intervention (PCI). They enrolled 3571 patients from a single center with a median follow-up of 1 year, and their initial hypothesis was found to be correct [35]. Diletti et al. enrolled 5127 patients with CAD and followed them up for 2 years. The analysis revealed that after drug-eluting stent implantation BMI had no association with mortality [36]. Another small cohort study observed the death rates after PCI and concluded that there were no significant differences after 30 days among all BMI groups [37]. Akin et al. also reported no obesity paradox after 1 year of follow-up in patients who underwent PCI with drug-eluting stents [38].
On the other hand, and in favor of the obesity paradox, there are multiple studies in the literature. In a cohort of 3634 patients, a subgroup of 1829 was randomized to either coronary artery bypass graft surgery (CABG) or percutaneous transluminal coronary angioplasty (PTCA) examining the short- and long-term outcomes after initial revascularization. Interestingly, with each unit increase of BMI, there was a 5.5% lower adjusted risk of a major in-hospital event after PTCA, whereas no significant differences were observed after CABG. In the long term, the mortality rates were lower among PTCA-treated patients with overweight or class I obesity compared with normal weight patients. In the CABG group, the 5-year mortality risk depicted a positive linear correlation with class II/III obesity [39]. The observation that in-hospital complications after PCI were higher in underweight and normal weight patients was also confirmed in a study of 9633 patients [40]. Additionally, a different cohort demonstrated that lean patients had the highest in-hospital mortality risk even though the multivariate analysis showed that BMI had no impact on it [41]. In agreement with the aforementioned came a large Swedish study in which the association between BMI and all-cause mortality was U-shaped in patients who underwent coronary angiography due to acute coronary syndromes, with the lowest mortality noted to be when BMI was between 30 and 40 kg/m2. Notably, the unadjusted survival in people with CAD was lower when BMI was under 18 kg/m2 as demonstrated by the Kaplan-Meir curve [42]. A Korean study including 3824 patients who underwent PCI after a ST-segment elevation myocardial infarction (STEMI) showed that serum N-terminal pro-brain natriuretic peptide (NT-pro-BNP) levels were significantly higher in underweight and normal weight patients than their counterparts with overweight and obesity. These laboratory findings suggested hemodynamic instability, and in combination with infrequent use of β-blockers and angiotensin-converting enzyme inhibitors, lean and underweight patients had significantly higher mortality risk [43].
However, there are many essential variables that need to be examined in order to determine whether obesity is actually responsible for better clinical outcomes in patients with CAD. Data from a multicenter, multinational population with CAD demonstrated that from 16 potential variables for cardiovascular mortality, the independent variables included age, BMI, current smoking habits, diabetes mellitus, total cholesterol levels, and history of myocardial infarction, congestive heart failure, revascularization, or stroke [44]. Numerous studies have shown that patients with obesity and CAD are more likely to be younger than lean patients with CAD [45]. Oreopoulos et al. examined 31,021 patients for a median follow-up of 46 months and showed that patients with obesity who had undergone PCI or CABG were younger and most likely never smokers. Patients with class I obesity in the CABG group and patients with class II obesity in the PCI group had the lowest mortality risk [45]. There is evidence suggesting that advanced age is related to worse health outcomes independent of course of treatment [46]. On the same note, substantial research in 409 US hospitals revealed that patients with obesity and CAD were younger and had more intensive guideline focused therapy amongst more comorbidities. Thereby, greater BMI was associated with increased use of aspirin, lipid lowering drugs, b-blockers, and antihypertensive medications both at hospitalization and in the long term [47]. Adiponectin might contribute to this paradoxical effect, thus proposing a different paradox [48]. Naturally, adiponectin is secreted by adipocytes, and its levels are decreased in people with obesity [49]. Whereas adiponectin is known, among others, for its cardioprotective effects, recent data have demonstrated the “adiponectin paradox,” wherein people with established CVD and elevated adiponectin had a greater mortality risk [50]. There is evidence indicating that elevated adiponectin levels in CAD are mediated by atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) [48]. Thus, this association may not in fact be causal.
Obesity is well known for its low-grade subclinical inflammatory state. Systemic inflammation is characterized by high-sensitivity C-reactive protein (hsCRP) levels, and a large number of studies have implicated its role in CVD. Anti-inflammatory therapy has been shown to reduce hsCRP levels and the risk for a major cardiovascular event or death [51]. On the other hand, there have been studies suggesting that mortality was significantly lower after cardiac rehabilitation in a study population that had high CRP and low body fat. The authors discussed the protective role that elevated CRP may have, or the increased lean mass, or the combination of both [52].
Heart Failure
By definition, the obesity paradox in heart failure (HF) indicates towards low mortality rates in patients with overweight or obesity. People with obesity are prone to be more often diagnosed with HF, but it appears that obesity exerts a protective effect after diagnosis [53]. Indeed, Curtis et al. studied 7767 patients with stable HF for 37 months and highlighted that increased BMI was associated with lower mortality risk [54]. Patients with a BMI over 45 kg/m2 presented with increased mortality rates, formulating a U-shaped relationship between mortality and BMI [55]. The association between BMI and mortality was also present in patients with chronic HF with both reduced ejection fraction (EF) and preserved EF (HfrEF, HfpEF) [56]. Additionally, a study investigating patients with both ischemic HF and non-ischemic HF showed that the paradox association between BMI and mortality was present only in patients with non-ischemic HF. The medical history of the non-ischemic patients included higher prevalence of AF, worse NYHA class, and a majority of women, whereas the ischemic patients had higher prevalence of diabetes, hypercholesterolemia, and peripheral vascular disease [57]. Interestingly, in order to further elucidate the obesity paradox phenomenon, the relationship of body surface area was investigated in patients with HF. The investigators highlighted that for each 0.1 m2 increase in body surface, there was an inverse association with mortality, but the aforementioned association did not apply to hospitalizations [58]. Specifically, a study enrolled 3,811 patients with HF and EF < 40% and found that the unadjusted analysis was in favor of the obesity paradox. On the contrary, after adjustments for several confounders, the results revealed that this paradox was present only in females, while the male population had a significantly increased mortality rate when overweight or obesity were present [59]. Notwithstanding, the obesity paradox was also confirmed by a Spanish study, and the authors stressed that the protective effect was lost when waist circumference was over 120 cm [60]. HF is characterized by a chronic catabolic state and in the long-term cardiac cachexia might occur, accompanied with high mortality risk [61]. To wit, people with obesity can cope better with the catabolic state due to higher metabolic reserves. In an attempt to assess the nutritional status in patients with HF, a couple of interesting studies found that undernourished patients had the highest mortality rates [62, 63]. Moreover, patients with HF and obesity seem to have increased prevalence of co-existing medical conditions like T2DM or hypertension. Those findings suggest that the more frequent use of β-blockers and antihypertensive drugs such as ACE inhibitors may play a favorable role in mortality rate [64]. ACE inhibitors reduce mortality in patients with HF, preventing deaths especially to those with high levels of catecholamines [65]. Another confounder in the obesity paradox is the greater prevalence of non-smokers or ex-smokers, as well as a lower incidence of previous STEMI or non STEMI in patients with obesity [64].
We mentioned previously that NT-proBNP and BNP levels were significantly higher in people with obesity and CAD. BNP is a neurohormone secreted by the cardiac ventricles, expressing pressure overload and volume expansion [66]. Mehra et al. investigated people with congestive heart failure, measuring BNP levels, and confirmed that people with obesity had significantly lower levels and thereby better prognosis. In order to exclude the age confounder, they divided the subjects to elderly and nonelderly, with no difference in the results [67].
Atrial Fibrillation
Several studies agree on the increased prevalence of atrial fibrillation among patients with obesity [68]. What is intriguing though is that in the last decade, an ample amount of research manifested a similar paradox among people with obesity and atrial fibrillation as described in CAD and HF. In a large study of 17,913 patients with AF, patients were randomized to receive warfarin or apixaban and were followed for 1.8 years. The multivariate analysis demonstrated a lower risk for all-cause mortality and significantly reduced adverse events like stroke, bleeding, MI, or systemic embolism in people with overweight and obesity compared with normal weight patients. Additionally, the patients that were randomized to the warfarin arm had increased incidence in all complications investigated compared to the apixaban arm. Patients in the apixaban subgroup with a normal BMI had lower prevalence of major bleeding and stroke compared with higher BMIs [69]. The paradox association between BMI and AF was also apparent in an elderly population of underweight patients, who exhibited two to three times higher risk for all-cause mortality [70].
Among others, one plausible explanation for the obesity paradox in patients with AF might be the dosage of anticoagulant drugs. Numerous studies have shown that underweight patients have increased incidence of major bleeding [71]. It is interesting that in patients with AF who were under 50 kg, there seemed to be a significant increase in plasma concentrations of rivaroxaban compared with normal weight patients, whereas there was no significant difference in concentrations between the normal weight group and the obesity group [72].
Obesity Paradox and Cancer
The role of obesity in cancer has been broadly researched, highlighting that overweight and obesity have contributed to increasing the prevalence of several types of cancers [73]. Almost 55% of cancers diagnosed in women and 24% diagnosed in men are considered overweight- and obesity-related cancers, i.e., breast and endometrial cancers, adenocarcinoma of the esophagus, and multiple other sites such as the gastric cardia, colon, rectum, liver, gallbladder, pancreas, kidney, thyroid gland, and multiple myeloma [74]. The insulin/IGF1 system, the effect of sex hormones, and adipocytokines are among the mechanisms that seem to be implicated [73]. On the other hand, there are studies proposing that obesity may play a protective role in some types of cancers regarding their incidence and mortality [75]. BMI has a different effect among premenopausal and postmenopausal women, and it seems that those with obesity have a lower risk of developing pre-menopausal breast cancer, especially the hormone sensitive type, compared to lean women, a finding attributed among others to the low levels of estrogen and progesterone [76]. There are, however, other meta-analyses that have pointed out a worse breast cancer survival in both premenopausal and post-menopausal women [77, 78]. A plethora of epidemiological studies have shown a paradoxical relationship between survival and BMI among people with certain cancers. Moreover, there have been conflicting results on the effect of BMI on the prognosis of several cancers such as colorectal cancer [79], renal cancer [80], gastrointestinal stromal tumors [81], leukemia [82], B cell lymphoma [83], lung cancer [84], and esophageal cancer [85]. There are other cancer types where a positive association with BMI has been found, like the aggressive form of prostate cancer [86], while an inverse relationship has been shown with the localized type [87, 88]. There are also types of cancer for which higher BMI has been associated with poor prognosis and lower survival like postmenopausal breast cancer [77], ovarian cancers [89], and type 1 endometrial cancers [90]. Data from the Framingham cohort study showed that patients with obesity had increased mortality risk by 6–7% for every 2 years lived with obesity and the mortality from cancer for patients with obesity increased by 3% for every 2 years lived with obesity [91].
In order to understand and explore the obesity paradox in cancer, several important factors need to be considered. Essential aspects are the time of the calculated BMI, the age of the patients, their smoking habits, and the type of treatment [92]. When patients with cancer are studied, it is of great importance to acknowledge the physical status before diagnosis. When weight loss is cancer-related, it could lead to cancer cachexia characterized by unintentional weight loss. In this case, skeletal muscle mass is lost through catabolism caused directly by tumor metabolism or indirectly by other tumor-mediated effects [93]. Sarcopenic obesity has been evaluated in patients with cancers of the respiratory or gastrointestinal tract. The reported BMI had been calculated in the first day in the clinic, and several patients had presented with a trend towards weight loss in the last 6 months. They underwent a CT scan in order to quantify and calculate the fat-free mass. The results showed that 15% had sarcopenic obesity with poor prognosis and moreover sarcopenic obesity was a significant independent predictor of survival [94]. An observational study has shown that having obesity before the diagnosis of colorectal cancer is associated with increased mortality [95], whereas increased post diagnosis BMI in patients with colorectal cancer is associated with decreased mortality risk [79].
The last 50 years have strongly highlighted the large negative effect of tobacco use on health and the increased risk of development of at least 12 types of cancers [96]. The influence of tobacco use on mortality is enormous; hence, the estimation of mortality risk on patients with cancer should preferably be made separately for smokers and non-smokers [97]. The use of tobacco stimulates the sympathetic nervous system resulting in an increase of resting metabolic rate and alters the central hypothalamic regulation of appetite, resulting in reduced energy intake [98]. Therefore, tobacco users in the majority are leaner. Interestingly, several cross-sectional studies demonstrated that waist to hip ratio (WHR) is higher in tobacco users than in non-tobacco users, thus pointing towards an alteration in fat distribution [99, 100].
Other factors to be considered include the age of participants and their therapeutic treatment. A meta-analysis of 22 clinical trials examined 14 different types of cancers and treatments. The results showed that living with obesity had no influence in survival compared with normal weight patients, except in those patients treated with certain chemotherapeutic drugs accompanied with elevated mortality risk potentially due to underdosing [101]. Moreover, the age of the participants may also be relevant when the obesity paradox is under investigation. The incidence of some types of cancer might have a wide age range, e.g., leukemia. A study that examined patients with acute myeloid leukemia demonstrated that increased mortality risk was significantly associated with advanced age and low BMI [82]. The American Cancer Society in 2012 published guidelines regarding nutritional and physical activity for cancer survivors. The general recommendation for patients that have active treatment is to maintain their baseline weight and in case of overweight or obesity to mildly reduce it. The guidelines encourage patients with cancer and survivors to engage in regular physical activity and avoid inactivity. The authors underline that obesity enhances several comorbidities and preventing them is of high importance [102].
Obesity Paradox and Chronic Obstructive Pulmonary Disease
A large cohort study in 1,213,829 Koreans demonstrated that mortality risk increased as BMI decreased in patients with respiratory diseases, including COPD [103]. Likewise, a meta-analysis of 22 studies in patients with diagnosed COPD demonstrated that lower BMI was markedly associated with increased mortality risk. The highest mortality was among underweight patients, and the lower overall mortality was found in patients with overweight and obesity, even after risk adjustment analysis [104]. A study by Lainscak et al. corroborated this finding. The authors followed 968 patients with COPD for a median of 3.26 years and showed that the lowest mortality was in patients living with overweight and more specifically between the BMI range of 25.09–26.56 kg/m2 [105]. Fat-free mass was found to be an independent predictor of mortality regardless of fat mass in a cohort study of 412 patients with moderate to severe COPD. Thus, fat-free mass could be an additional tool in the assessment of COPD severity, as increased fat-free mass was associated with lower mortality [106]. Furthermore, weight changes in patients with COPD might contribute to differential health outcomes. Patients with low BMI and COPD are frequently malnourished, and they might experience significant weight loss that could even lead to cachexia [107], underlining that significant weight loss is associated with increased mortality [108]. One should keep in mind that all these studies based their quantification of the degree of obesity on BMI measurement. Information about body composition and cardiorespiratory fitness (CRF) is lacking. Low CRF is associated with high all-cause mortality and may act as a limitation bias contributing to the obesity paradox [109].
Conclusion
While the detrimental effect of obesity in mortality and morbidity has been well established, the concept of the obesity paradox in particular chronic diseases is a topic of immense interest. In certain clinical settings, obesity exhibits an intriguing “paradoxical” protective effect. The association between BMI and clinical outcomes, however, may be driven by multiple factors among which the limitations of the BMI index itself, the unintended weight loss precipitated by chronic illness, the various phenotypes of obesity, i.e., sarcopenic obesity or the athlete’s obesity phenotype, and the cardiorespiratory fitness levels of the included patients. In order to clarify the controversial issues regarding the protective role of overweight and obesity revealed in some studies, determination of BMI is not adequate, and additional indicators such as quantitative determination of different body compartments such as fat and lean mass are of paramount importance. Also, previous cardioprotective medications, obesity year duration, and smoking status seem to play an important a role, thus increasing the need for carefully designed studies alleviated by such confounding factors.
Abbreviations
- BMI:
-
Body mass index
- T2DM:
-
Type 2 diabetes mellitus
- CVD:
-
Cardiovascular disease
- CHD:
-
Coronary heart disease
- HF:
-
Heart failure
- AF:
-
Atrial fibrillation
- COPD:
-
Cobstructive pulmonary disease
- PCI:
-
Percutaneous coronary intervention
- CABG:
-
Coronary artery bypass graft surgery
- PTCA:
-
Percutaneous transluminal coronary angioplasty
- STEMI:
-
ST-segment elevation myocardial infarction
- NT-pro-BNP:
-
N-terminal pro-brain natriuretic peptide
- hsCRP:
-
High-sensitivity C-reactive protein
- EF:
-
Ejection fraction
- HfrEF:
-
Heart failure with reduced ejection fraction EF
- HfpEF:
-
Heart failure with preserved ejection fraction
- CRF:
-
Cardiorespiratory fitness
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133:155217. https://doi.org/10.1016/j.metabol.2022.155217.
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. https://doi.org/10.1186/1471-2458-9-88.
Censin JC, Peters SAE, Bovijn J, Ferreira T, Pulit SL, Mägi R, et al. Causal relationships between obesity and the leading causes of death in women and men. PLoS Genet. 2019;15(10):e1008405. https://doi.org/10.1371/journal.pgen.1008405.
Karampela I, Chrysanthopoulou E, Christodoulatos GS, Dalamaga M. Is there an obesity paradox in critical illness? Epidemiologic and metabolic considerations. Curr Obes Rep. 2020;9(3):231–44. https://doi.org/10.1007/s13679-020-00394-x.
Weir CB, Jan A. BMI classification percentile and cut off points. 2022 Jun 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
Eknoyan G. Adolphe Quetelet (1796–1874)–The average man and indices of obesity. Nephrol Dial Transplant. 2008;23(1):47–51. https://doi.org/10.1093/ndt/gfm517.
WHO. Obesity Fact Sheet (No.311). 2014.
Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72(3):694–701. https://doi.org/10.1093/ajcn/72.3.694.
Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–82. https://doi.org/10.1093/ajcn/84.3.475.
Koliaki C, Liatis S, Dalamaga M, Kokkinos A. Sarcopenic obesity: epidemiologic evidence, pathophysiology, and therapeutic perspectives. Curr Obes Rep. 2019;8(4):458–71. https://doi.org/10.1007/s13679-019-00359-9.
Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts. 2022;15(3):321–35. https://doi.org/10.1159/000521241.
Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Sun Q, et al. Development and validation of anthropometric prediction equations for lean body mass, fat mass and percent fat in adults using the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Br J Nutr. 2017;118(10):858–66. https://doi.org/10.1017/s0007114517002665.
Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–32. https://doi.org/10.1016/j.jacc.2008.12.068.
Lee DH, Giovannucci EL. The obesity paradox in cancer: epidemiologic insights and perspectives. Curr Nutr Rep. 2019;8(3):175–81. https://doi.org/10.1007/s13668-019-00280-6.
Naderi N, Kleine CE, Park C, Hsiung JT, Soohoo M, Tantisattamo E, et al. Obesity paradox in advanced kidney disease: from bedside to the bench. Prog Cardiovasc Dis. 2018;61(2):168–81. https://doi.org/10.1016/j.pcad.2018.07.001.
Spelta F, Fratta Pasini AM, Cazzoletti L, Ferrari M. Body weight and mortality in COPD: focus on the obesity paradox. Eat Weight Disord. 2018;23(1):15–22. https://doi.org/10.1007/s40519-017-0456-z.
Forlivesi S, Cappellari M, Bonetti B. Obesity paradox and stroke: a narrative review. Eat Weight Disord. 2021;26(2):417–23. https://doi.org/10.1007/s40519-020-00876-w.
Zafrir B, Adir Y, Shehadeh W, Shteinberg M, Salman N, Amir O. The association between obesity, mortality and filling pressures in pulmonary hypertension patients; the “obesity paradox.” Respir Med. 2013;107(1):139–46. https://doi.org/10.1016/j.rmed.2012.10.019.
Fassio A, Idolazzi L, Rossini M, Gatti D, Adami G, Giollo A, et al. The obesity paradox and osteoporosis. Eat Weight Disord. 2018;23(3):293–302. https://doi.org/10.1007/s40519-018-0505-2.
Karampela I, Christodoulatos GS, Dalamaga M. The role of adipose tissue and adipokines in sepsis: inflammatory and metabolic considerations, and the obesity paradox. Curr Obes Rep. 2019;8(4):434–57. https://doi.org/10.1007/s13679-019-00360-2.
Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. https://doi.org/10.1016/s0140-6736(09)60318-4.
Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. https://doi.org/10.1136/bmj.i2156.
Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3(4):280–7. https://doi.org/10.1001/jamacardio.2018.0022.
Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ. Obesity paradox in cardiovascular disease: where do we stand? Vasc Health Risk Manag. 2019;15:89–100. https://doi.org/10.2147/vhrm.S168946.
•• Vecchié A, Dallegri F, Carbone F, Bonaventura A, Liberale L, Portincasa P, et al. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med. 2018;48:6–17. https://doi.org/10.1016/j.ejim.2017.10.020. Evaluates the association of BMI with mortality and examines obesity phenotypes with different CV risk, aiding to the understanding of the obesity paradox.
Ruiz JR, Sui X, Lobelo F, Morrow JR Jr, Jackson AW, Sjöström M, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337(7661):a439. https://doi.org/10.1136/bmj.a439.
Kim SA, Lim K, Lee JK, Kang D, Shin S. Metabolically healthy obesity and the risk of all-cause and cardiovascular disease mortality in a Korean population: a prospective cohort study. BMJ Open. 2021;11(9):e049063. https://doi.org/10.1136/bmjopen-2021-049063.
Ortega FB, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS, et al. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J. 2013;34(5):389–97. https://doi.org/10.1093/eurheartj/ehs174.
Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. Ann Intern Med. 2013;159(11):758–69. https://doi.org/10.7326/0003-4819-159-11-201312030-00008.
Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715–23. https://doi.org/10.1111/obr.12551.
Norris T, Cole TJ, Bann D, Hamer M, Hardy R, Li L, et al. Duration of obesity exposure between ages 10 and 40 years and its relationship with cardiometabolic disease risk factors: a cohort study. PLoS Med. 2020;17(12):e1003387. https://doi.org/10.1371/journal.pmed.1003387.
Tobias DK. Addressing reverse causation bias in the obesity paradox is not “one size fits all.” Diabetes Care. 2017;40(8):1000–1. https://doi.org/10.2337/dci17-0010.
Lee EY, Lee YH, Yi SW, Shin SA, Yi JJ. BMI and all-cause mortality in normoglycemia, impaired fasting glucose, newly diagnosed diabetes, and prevalent diabetes: a cohort study. Diabetes Care. 2017;40(8):1026–33. https://doi.org/10.2337/dc16-1458.
Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–78. https://doi.org/10.1056/NEJMoa055643.
Ellis SG, Elliott J, Horrigan M, Raymond RE, Howell G. Low-normal or excessive body mass index: newly identified and powerful risk factors for death and other complications with percutaneous coronary intervention. Am J Cardiol. 1996;78(6):642–6. https://doi.org/10.1016/s0002-9149(96)00386-4.
Diletti R, Garcia-Garcia HM, Bourantas C, Van Mieghem NM, van Geuns RJ, Muramatsu T, et al. Impact of body mass index on long-term clinical outcomes after second-generation drug eluting stent implantation: insights from the international global RESOLUTE program. Catheter Cardiovasc Interv. 2015;85(6):952–8. https://doi.org/10.1002/ccd.25828.
Iakobishvili Z, Danicek V, Porter A, Assali AR, Battler A, Hasdai D. Is increased body mass index associated with a cardioprotective effect after ST-segment-elevation myocardial infarction? Acute Card Care. 2006;8(2):95–8. https://doi.org/10.1080/17482940600768673.
Akin I, Tölg R, Hochadel M, Bergmann MW, Khattab AA, Schneider S, et al. No evidence of “obesity paradox” after treatment with drug-eluting stents in a routine clinical practice: results from the prospective multicenter German DES.DE (German Drug-Eluting Stent) Registry. JACC Cardiovasc Interv. 2012;5(2):162–9. https://doi.org/10.1016/j.jcin.2011.09.021.
Gurm HS, Whitlow PL, Kip KE. The impact of body mass index on short- and long-term outcomes inpatients undergoing coronary revascularization. Insights from the bypass angioplasty revascularization investigation (BARI). J Am Coll Cardiol. 2002;39(5):834–40. https://doi.org/10.1016/s0735-1097(02)01687-x.
Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39(4):578–84. https://doi.org/10.1016/s0735-1097(01)01802-2.
Kosuge M, Kimura K, Kojima S, Sakamoto T, Ishihara M, Asada Y, et al. Impact of body mass index on in-hospital outcomes after percutaneous coronary intervention for ST segment elevation acute myocardial infarction. Circ J. 2008;72(4):521–5. https://doi.org/10.1253/circj.72.521.
Angerås O, Albertsson P, Karason K, Råmunddal T, Matejka G, James S, et al. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J. 2013;34(5):345–53. https://doi.org/10.1093/eurheartj/ehs217.
Kang WY, Jeong MH, Ahn YK, Kim JH, Chae SC, Kim YJ, et al. Obesity paradox in Korean patients undergoing primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. J Cardiol. 2010;55(1):84–91. https://doi.org/10.1016/j.jjcc.2009.10.004.
Battes L, Barendse R, Steyerberg EW, Simoons ML, Deckers JW, Nieboer D, et al. Development and validation of a cardiovascular risk assessment model in patients with established coronary artery disease. Am J Cardiol. 2013;112(1):27–33. https://doi.org/10.1016/j.amjcard.2013.02.049.
Oreopoulos A, McAlister FA, Kalantar-Zadeh K, Padwal R, Ezekowitz JA, Sharma AM, et al. The relationship between body mass index, treatment, and mortality in patients with established coronary artery disease: a report from APPROACH. Eur Heart J. 2009;30(21):2584–92. https://doi.org/10.1093/eurheartj/ehp288.
Holmes DR Jr, White HD, Pieper KS, Ellis SG, Califf RM, Topol EJ. Effect of age on outcome with primary angioplasty versus thrombolysis. J Am Coll Cardiol. 1999;33(2):412–9. https://doi.org/10.1016/s0735-1097(98)00579-8.
Steinberg BA, Cannon CP, Hernandez AF, Pan W, Peterson ED, Fonarow GC. Medical therapies and invasive treatments for coronary artery disease by body mass: the “obesity paradox” in the Get With The Guidelines database. Am J Cardiol. 2007;100(9):1331–5. https://doi.org/10.1016/j.amjcard.2007.06.019.
Menzaghi C, Trischitta V. The adiponectin paradox for all-cause and cardiovascular mortality. Diabetes. 2018;67(1):12–22. https://doi.org/10.2337/dbi17-0016.
Zhao S, Kusminski CM, Scherer PE. Adiponectin, leptin and cardiovascular disorders. Circ Res. 2021;128(1):136–49. https://doi.org/10.1161/circresaha.120.314458.
Sook Lee E, Park SS, Kim E, Sook Yoon Y, Ahn HY, Park CY, et al. Association between adiponectin levels and coronary heart disease and mortality: a systematic review and meta-analysis. Int J Epidemiol. 2013;42(4):1029–39. https://doi.org/10.1093/ije/dyt087.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. https://doi.org/10.1056/NEJMoa1707914.
De Schutter A, Kachur S, Lavie CJ, Boddepalli RS, Patel DA, Milani RV. The impact of inflammation on the obesity paradox in coronary heart disease. Int J Obes (Lond). 2016;40(11):1730–5. https://doi.org/10.1038/ijo.2016.125.
Littnerova S, Parenica J, Spinar J, Vitovec J, Linhart A, Widimsky P, et al. Positive influence of being overweight/obese on long term survival in patients hospitalised due to acute heart failure. PLoS ONE. 2015;10(2):e0117142. https://doi.org/10.1371/journal.pone.0117142.
Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165(1):55–61. https://doi.org/10.1001/archinte.165.1.55.
Kapoor JR, Heidenreich PA. Obesity and survival in patients with heart failure and preserved systolic function: a U-shaped relationship. Am Heart J. 2010;159(1):75–80. https://doi.org/10.1016/j.ahj.2009.10.026.
Padwal R, McAlister FA, McMurray JJ, Cowie MR, Rich M, Pocock S, et al. The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta-analysis of individual patient data. Int J Obes (Lond). 2014;38(8):1110–4. https://doi.org/10.1038/ijo.2013.203.
Zamora E, Lupón J, de Antonio M, Urrutia A, Coll R, Díez C, et al. The obesity paradox in heart failure: is etiology a key factor? Int J Cardiol. 2013;166(3):601–5. https://doi.org/10.1016/j.ijcard.2011.11.022.
Zafrir B, Salman N, Crespo-Leiro MG, Anker SD, Coats AJ, Ferrari R, et al. Body surface area as a prognostic marker in chronic heart failure patients: results from the Heart Failure Registry of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18(7):859–68. https://doi.org/10.1002/ejhf.551.
Vest AR, Wu Y, Hachamovitch R, Young JB, Cho L. The heart failure overweight/obesity survival paradox: the missing sex link. JACC Heart Fail. 2015;3(11):917–26. https://doi.org/10.1016/j.jchf.2015.06.009.
Puig T, Ferrero-Gregori A, Roig E, Vazquez R, Gonzalez-Juanatey JR, Pascual-Figal D, et al. Prognostic value of body mass index and waist circumference in patients with chronic heart failure (Spanish REDINSCOR Registry). Rev Esp Cardiol (Engl Ed). 2014;67(2):101–6. https://doi.org/10.1016/j.rec.2013.06.022.
Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43(8):1439–44. https://doi.org/10.1016/j.jacc.2003.11.039.
Gastelurrutia P, Lupón J, de Antonio M, Zamora E, Domingo M, Urrutia A, et al. Body mass index, body fat, and nutritional status of patients with heart failure: the PLICA study. Clin Nutr. 2015;34(6):1233–8. https://doi.org/10.1016/j.clnu.2014.12.013.
Casas-Vara A, Santolaria F, Fernández-Bereciartúa A, González-Reimers E, García-Ochoa A, Martínez-Riera A. The obesity paradox in elderly patients with heart failure: analysis of nutritional status. Nutrition. 2012;28(6):616–22. https://doi.org/10.1016/j.nut.2011.10.006.
Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156(1):13–22. https://doi.org/10.1016/j.ahj.2008.02.014.
Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361(9363):1077–83. https://doi.org/10.1016/s0140-6736(03)12892-9.
Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–7. https://doi.org/10.1056/NEJMoa020233.
Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43(9):1590–5. https://doi.org/10.1016/j.jacc.2003.10.066.
Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity–results of a meta-analysis. Am Heart J. 2008;155(2):310–5. https://doi.org/10.1016/j.ahj.2007.10.004.
Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S, et al. The ‘obesity paradox’ in atrial fibrillation: observations from the ARISTOTLE (apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation) trial. Eur Heart J. 2016;37(38):2869–78. https://doi.org/10.1093/eurheartj/ehw124.
Yanagisawa S, Inden Y, Yoshida N, Kato H, Miyoshi-Fujii A, Mizutani Y, et al. Body mass index is associated with prognosis in Japanese elderly patients with atrial fibrillation: an observational study from the outpatient clinic. Heart Vessels. 2016;31(9):1553–61. https://doi.org/10.1007/s00380-015-0765-y.
Park CS, Choi EK, Kim HM, Lee SR, Cha MJ, Oh S. Increased risk of major bleeding in underweight patients with atrial fibrillation who were prescribed non-vitamin K antagonist oral anticoagulants. Heart Rhythm. 2017;14(4):501–7. https://doi.org/10.1016/j.hrthm.2016.12.036.
Uprichard J. Management of rivaroxaban in relation to bodyweight and body mass index. Ther Adv Cardiovasc Dis. 2016;10(5):294–303. https://doi.org/10.1177/1753944716643645.
• Argyrakopoulou G, Dalamaga M, Spyrou N, Kokkinos A. Gender differences in obesity-related cancers. Curr Obes Rep. 2021;10(2):100–15. https://doi.org/10.1007/s13679-021-00426-0. Examines the association of obesity with cancer according to gender.
Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T, et al. Vital signs: trends in incidence of cancers associated with overweight and obesity - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2017;66(39):1052–8. https://doi.org/10.15585/mmwr.mm6639e1.
Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–35. https://doi.org/10.1016/j.metabol.2018.11.001.
Key TJ, Pike MC. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol. 1988;24(1):29–43. https://doi.org/10.1016/0277-5379(88)90173-3.
Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–14. https://doi.org/10.1093/annonc/mdu042.
Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123(3):627–35. https://doi.org/10.1007/s10549-010-0990-0.
Schlesinger S, Siegert S, Koch M, Walter J, Heits N, Hinz S, et al. Postdiagnosis body mass index and risk of mortality in colorectal cancer survivors: a prospective study and meta-analysis. Cancer Causes Control. 2014;25(10):1407–18. https://doi.org/10.1007/s10552-014-0435-x.
Choi Y, Park B, Jeong BC, Seo SI, Jeon SS, Choi HY, et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer. 2013;132(3):625–34. https://doi.org/10.1002/ijc.27639.
Stiles ZE, Rist TM, Dickson PV, Glazer ES, Fleming MD, Shibata D, et al. Impact of body mass index on the short-term outcomes of resected gastrointestinal stromal tumors. J Surg Res. 2017;217:123–30. https://doi.org/10.1016/j.jss.2017.05.010.
Brunner AM, Sadrzadeh H, Feng Y, Drapkin BJ, Ballen KK, Attar EC, et al. Association between baseline body mass index and overall survival among patients over age 60 with acute myeloid leukemia. Am J Hematol. 2013;88(8):642–6. https://doi.org/10.1002/ajh.23462.
Carson KR, Bartlett NL, McDonald JR, Luo S, Zeringue A, Liu J, et al. Increased body mass index is associated with improved survival in United States veterans with diffuse large B-cell lymphoma. J Clin Oncol. 2012;30(26):3217–22. https://doi.org/10.1200/jco.2011.39.2100.
Zhang X, Liu Y, Shao H, Zheng X. Obesity paradox in lung cancer prognosis: evolving biological insights and clinical implications. J Thorac Oncol. 2017;12(10):1478–88. https://doi.org/10.1016/j.jtho.2017.07.022.
Kayani B, Okabayashi K, Ashrafian H, Harling L, Rao C, Darzi A, et al. Does obesity affect outcomes in patients undergoing esophagectomy for cancer? A meta-analysis. World J Surg. 2012;36(8):1785–95. https://doi.org/10.1007/s00268-012-1582-4.
Farris MS, Courneya KS, Kopciuk KA, McGregor SE, Friedenreich CM. Anthropometric measurements and survival after a prostate cancer diagnosis. Br J Cancer. 2018;118(4):607–10. https://doi.org/10.1038/bjc.2017.440.
Cao Y, Giovannucci E. Obesity and prostate cancer. Recent Results Cancer Res. 2016;208:137–53. https://doi.org/10.1007/978-3-319-42542-9_8.
Discacciati A, Orsini N, Andersson SO, Andrén O, Johansson JE, Wolk A. Body mass index in early and middle-late adulthood and risk of localised, advanced and fatal prostate cancer: a population-based prospective study. Br J Cancer. 2011;105(7):1061–8. https://doi.org/10.1038/bjc.2011.319.
Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2012;5(7):901–10. https://doi.org/10.1158/1940-6207.Capr-12-0048.
Secord AA, Hasselblad V, Von Gruenigen VE, Gehrig PA, Modesitt SC, Bae-Jump V, et al. Body mass index and mortality in endometrial cancer: a systematic review and meta-analysis. Gynecol Oncol. 2016;140(1):184–90. https://doi.org/10.1016/j.ygyno.2015.10.020.
Abdullah A, Wolfe R, Stoelwinder JU, de Courten M, Stevenson C, Walls HL, et al. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol. 2011;40(4):985–96. https://doi.org/10.1093/ije/dyr018.
Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18(9):56. https://doi.org/10.1007/s11912-016-0539-4.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95. https://doi.org/10.1016/s1470-2045(10)70218-7.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35. https://doi.org/10.1016/s1470-2045(08)70153-0.
Lee J, Meyerhardt JA, Giovannucci E, Jeon JY. Association between body mass index and prognosis of colorectal cancer: a meta-analysis of prospective cohort studies. PLoS ONE. 2015;10(3):e0120706. https://doi.org/10.1371/journal.pone.0120706.
National Center for Chronic Disease Promotion and Health Promotion, Office on Smoking and Health. Reports of the Surgeon General. The health consequences of smoking—50 years of progress: a report of the surgeon general. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014.
Song M, Giovannucci E. Estimating the influence of obesity on cancer risk: stratification by smoking is critical. J Clin Oncol. 2016;34(27):3237–9. https://doi.org/10.1200/jco.2016.67.6916.
Schwartz A, Bellissimo N. Nicotine and energy balance: a review examining the effect of nicotine on hormonal appetite regulation and energy expenditure. Appetite. 2021;164:105260. https://doi.org/10.1016/j.appet.2021.105260.
Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87(4):801–9. https://doi.org/10.1093/ajcn/87.4.801.
Canoy D, Wareham N, Luben R, Welch A, Bingham S, Day N, et al. Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obes Res. 2005;13(8):1466–75. https://doi.org/10.1038/oby.2005.177.
Greenlee H, Unger JM, LeBlanc M, Ramsey S, Hershman DL. Association between body mass index and cancer survival in a pooled analysis of 22 clinical trials. Cancer Epidemiol Biomarkers Prev. 2017;26(1):21–9. https://doi.org/10.1158/1055-9965.Epi-15-1336.
Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–74. https://doi.org/10.3322/caac.21142.
• Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355(8):779–87. https://doi.org/10.1056/NEJMoa054017. Examines the association of BMI with mortality.
Cao C, Wang R, Wang J, Bunjhoo H, Xu Y, Xiong W. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PLoS ONE. 2012;7(8):e43892. https://doi.org/10.1371/journal.pone.0043892.
Lainscak M, von Haehling S, Doehner W, Sarc I, Jeric T, Ziherl K, et al. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J Cachexia Sarcopenia Muscle. 2011;2(2):81–6. https://doi.org/10.1007/s13539-011-0023-9.
Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82(1):53–9. https://doi.org/10.1093/ajcn.82.1.53.
Koehler F, Doehner W, Hoernig S, Witt C, Anker SD, John M. Anorexia in chronic obstructive pulmonary disease–association to cachexia and hormonal derangement. Int J Cardiol. 2007;119(1):83–9. https://doi.org/10.1016/j.ijcard.2006.07.088.
Prescott E, Almdal T, Mikkelsen KL, Tofteng CL, Vestbo J, Lange P. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J. 2002;20(3):539–44. https://doi.org/10.1183/09031936.02.00532002.
McAuley PA, Beavers KM. Contribution of cardiorespiratory fitness to the obesity paradox. Prog Cardiovasc Dis. 2014;56(4):434–40. https://doi.org/10.1016/j.pcad.2013.09.006.
Acknowledgements
We wish to express our deepest thanks to Katerina Sotiropoulou for kindly designing Fig 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Simati, S., Kokkinos, A., Dalamaga, M. et al. Obesity Paradox: Fact or Fiction?. Curr Obes Rep 12, 75–85 (2023). https://doi.org/10.1007/s13679-023-00497-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-023-00497-1