Abstract
With the continued aging of the population, a rising proportion of surgical procedures will be performed in elderly patients. Current surgical decision-making tools rely on subjective judgments and were developed without special geriatric considerations. As a result, they have certain inadequacies that limit their utility in this population. Frailty, described for decades by the medical geriatric community, has just recently begun to be investigated as a surgical risk assessment tool. Identifying the frailty phenotype has been shown by multiple investigators to reliably predict which patients are at increased risk of adverse peri-operative outcomes. Perhaps, most importantly, it is a tool that can potentially discriminate among the elderly, identifying those that warrant heightened concern, "pre-habilitation", or special postoperative attention from those at no increased risk compared to the general population. Alternatively, some patients may be identified as “too frail” to undergo surgery, and alternative nonsurgical treatment options may be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the “baby boomer” population ages, there has been a concomitant rise in the share of surgical procedures performed in the elderly, with over half of surgeries in the United States performed on patients 65 years or older [1]. Accompanying the dramatic increase in life expectancy over the last century is an increase in the life expectancy of the very old, with an 85-year-old man and woman expected to live another 5.5 and 7 years, respectively [2•]. These facts introduce nuance to a practicing surgeon’s decision making: which elderly patients will benefit from lifesaving or life improving procedures, and which will be exposed to more harm than benefit? Exposing elderly frail patients to surgical procedures potentially puts them at risk for poor postoperative outcomes, while denying non-frail patients based on advanced age (“ageism”) or inaccurate assessment tools may deny them the benefits of surgery.
Physiology of Aging
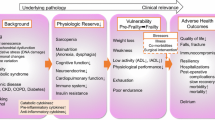
Although not the focus of this review, it is worthwhile to briefly discuss the physiologic derangements that accompany aging. As will be discussed in more detail later in the review, it is not the overt diagnosed comorbidities that present a challenge in preoperative risk assessment, but the subclinical deficits that are masked by compensatory mechanisms. Additionally, it is the inability to respond to the insults of surgery that may ultimately lead to adverse outcomes in the elderly following surgery.
Cardiac disease is most prevalent in the elderly. And, with 80 % of patients over 80 years of age showing evidence of cardiovascular disease, it also represents the most common cause of mortality in those 65 years and older [3]. In short, changes in cardiovascular physiology in the elderly are characterized by an increase in arterial intimal thickness, increase in left ventricle thickness with a concomitant decrease in contractility and compliance, and an increase in left atrial volume and pressure [4–6]. Taken together, although these physiologic derangements may not result in symptomatic heart disease, they represent a decrease in functional reserve and an impairment in a patient’s ability to respond to stressors, such as a surgical intervention.
Similarly, the elderly patient’s pulmonary function is characterized by decreased elastic recoil of the lung parenchyma, decreased compliance of the chest wall, and diminished strength in respiratory muscles [3, 7, 8]. In combination with impaired response to hypoxia and hypercapnia, elderly patients are susceptible to risk and complications of general anesthesia and mechanical ventilation [9]. As such, while age itself is not an independent risk factor for needing mechanical ventilation after intensive care unit admission, there is an increased risk of an elderly patient being unable to wean off a ventilator due to these aforementioned age-related changes.
Independent of nephrotoxic comorbidities such as hypertension and diabetes mellitus, advancing age is inevitably accompanied by a steady decrease in glomerular filtration rate (GFR) and creatinine clearance [10]. Additionally, aging kidneys demonstrate vascular dysautonomia, altered tubular handling of creatinine, and impaired sodium reabsorption and potassium secretion, reflected by the inclusion of age as a variable in the MDRD formula for calculation of GFR [11]. Although clinically silent until a critical threshold is passed, the gradually decreased reserve in renal function predisposes the elderly to electrolyte imbalances, volume disturbances of overload and dehydration, and drug toxicity [11]. While important in all elderly patients, these concerns hold special importance in those subjected to the acute stress of surgery.
Combined together, these decrements in physiologic reserve impair a patient’s ability to respond to stressors, whether unintended medical illness or the intended stress of surgery. Importantly, they not only predispose patients to postoperative complications, but also prolong an elderly's patient recovery from seemingly minor events that would be less concerning in healthy, younger patients.
Traditional Risk Assessments
Although large retrospective studies have shown that advanced age carries a modestly increased risk of peri-operative morbidity and mortality [1], others have demonstrated that mortality remains low and the elderly have outcomes similar to their younger counterparts for a variety of procedures [12–14]. The United States National Comprehensive Cancer Network (NCCN) and International Society of Geriatric Oncology (SIOG) both recommend that elderly cancer patients undergo some form of a geriatric assessment to help guide treatment decision making [15, 16]. Additionally, to avoid the pitfalls of ageism—offering or denying surgical procedures solely on the basis of age—many risk assessment tools and evaluations have been developed and currently exist in clinical practice. Unfortunately, the commonly employed risk assessment tools or comorbidity indices poorly capture the functional heterogeneity that characterizes the elderly. Aging is a very individualized phenomenon, and any comprehensive assessment must be multidimensional and multidisciplinary to fully characterize a patient’s functional, physical, cognitive, and socio-environmental factors [16, 17].
The American Society of Anesthesiology Physical Status Classification System (ASA) is a widely employed risk assessment scale of surgical patients, and was developed in the 1940s as a standardization tool to allow accurate data collection and analysis, not to quantify operative risk [18]. Designation of a patient to a particular ASA score relies only on the degree of systemic illness and symptomatology and not actual operative risk. Although large studies have demonstrated that an increasing ASA classification carries modestly increased risk for morbidity and mortality [1, 19], the ASA’s usefulness is limited by the subjective nature by which a patient's ASA score is assigned, as evidenced by its observed poor inter-observer consistency [20]. Similar to the ASA, the Eastern Cooperative Oncology Group Performance Status (ECOG) was developed as a tool not to estimate operative risk, but as a method to identify patients suitable for inclusion in clinical trials. It also relies on a subjective judgment of a patient's symptoms due to cancer and neglects subclinical deficits in physiologic reserve.
As cardiac disease is the most prevalent comorbidity in the elderly as well as the number one cause of mortality in this age group, the Eagle Criteria and subsequent Revised Cardiac Risk Index (RCRI) were developed to estimate the risk of cardiac complications in patients undergoing non-cardiac surgery. The obvious shortcomings of these risk indices are that they neglect non-cardiac organ systems and poorly capture the functional heterogeneity of the elderly surgical patient.
In an effort to remedy the deficiencies of the above mentioned risk assessment tools in the elderly, some investigators have advocated for the use of multidimensional assessments of the elderly, such as the Comprehensive Geriatric Assessment (CGA) [21]. The CGA encompasses physical, psychosocial, and environmental factors that impact outcomes, allowing a coordinated and optimized treatment plan [22]. Subsequently, the Preoperative Assessment of Cancer in the Elderly (PACE) was developed to predict postoperative outcomes, and incorporates the multifaceted CGA, Brief Fatigue Inventory (BFI), ECOG, and ASA [23]. In the original investigation, a multivariate analysis showed that Instrumental Activities of Daily Living (IADL), BFI, and ASA were significantly linked to comorbidities and useful for stratifying patients according to fitness for surgery. Although the PACE demonstrates reliable identification of those patients at risk for poor outcomes, its practicality is limited by the extensive time and expertise required for administration.
Frailty
Definitions
The medical geriatric community has for many years recognized frailty as an entity independent of diagnosed comorbidities and chronologic age in community dwelling adults that places these adults at increased risk for hospitalization, falls, institutionalization, and mortality [24••, 25••]. No single definition has been agreed upon, but two models have emerged: a frailty index of accumulated deficits [24••] and a phenotype characterized by the constellation of weight loss, weakness, slowed gait speed, exhaustion in daily activities, and low activity [25••].
The accumulated deficits model provides a method to estimate stepwise increasing odds of poor outcomes, most notably mortality, as deficits are accrued [26]. Conversely, the phenotypic definition offers a simple-to-measure index that gives a dichotomous result: at increased risk over the average population of a similar age or not. Using the phenotypic definition, a large prospective observational study with long-term follow-up demonstrated that baseline frailty was an independent predictor for death, hip fracture, dependence in activities of daily living, and hospitalization [27].
Investigations in Surgical Patients
Recently, a number of surgical series have employed the measurement of frailty in their respective patient populations and correlated these findings with patient peri-operative outcomes. The mounting evidence from retrospective and prospective studies have shown that measuring frailty as an accumulation of deficits, as well as the frailty phenotype, specifically described by Fried et al., has a robust ability to identify patients at risk for adverse outcomes: morbidity, mortality, institutionalization, and extended length of stay. Recently published surgical frailty series are summarized in Table 1.
Accumulated Deficits
Rockwood et al. have published extensively on their development of a frailty index based on itemized variables from the complete geriatric assessment (FI-CGA) encompassing frailty characteristics of symptoms, signs, disabilities, disease, and lab values [24••]. Their results demonstrate a reliable estimation of the likelihood of death, hospitalization, and the development of disability in community-dwelling older adults [28, 29]. Although calculation of a frailty index from an extensive itemized list of variables may have a high degree of validity and reliability, the applicability to a busy surgical setting is limited.
Correspondingly, investigators have adapted the deficit accumulation model to the surgical setting. Robinson et al. operationalized an eight-item frailty index to patients ≥ 65 years of age undergoing elective major surgery requiring intensive care unit (ICU) care postoperatively. Concordant with the geriatric community definition of frailty, the authors captured a multifactorial picture of their patients by measuring cognition, albumin, hematocrit, history of falls, dependence in activities of daily living, and comorbidities. Accumulation of these variables demonstrated stepwise increased risk of mortality and discharge to a nursing facility [30••].
In an effort to extend a currently practiced frailty assessment to a surgical population, Dasgupta et al. published their results utilizing the Edmonton Frail Scale (EFS) in a largely orthopedic surgical population. The EFS is a nine-item patient directed self-assessment that encompasses cognition, health status, functional independence, social support, medications, nutrition, mood, continence, and functional performance. Independent of age, the authors demonstrated increasing levels of frailty carried correspondingly increasing risk for postoperative complications, increased length-of-stay, and discharge to a nursing facility [31].
As further proof-of-concept of the accumulated deficit method of measuring frailty, Velanovich et al. undertook a large retrospective study matching 11 variables from the Canadian Study of Health and Aging Frailty Index (FI) to variables already captured in the National Surgical Quality Improvement Program (NSQIP) administrative database. A stepwise increase in the risk of morbidity and mortality was observed as patients accrued an increasing burden of frailty variables that was valid across a wide range of procedure and surgery types [32]. Despite the findings of these studies, the clinical utility of these results is limited by the lack of a single standard definition, and the complexity and extensiveness of measuring some of the frailty indices, limiting adoption into clinical practice.
Frailty Phenotype
Linda Fried first proposed the phenotypic definition of frailty, and it encapsulates the functional heterogeneity of the elderly population through an objective five-component scale that includes weight loss, poor grip strength, slowed gait speed, exhaustion, and low activity [25••]. In community dwelling adults, a designation of frailty using Fried's definition carries an increased risk for falls, hospitalizations, institutionalization, disability, and mortality [25••]. In 2010, Makary and colleagues published their experience measuring the Fried Frailty Criteria in a surgical population and correlating these measurements with peri-operative outcomes. Assessed during the preoperative anesthesia clinic, the study prospectively analyzed a large cohort of patients undergoing a wide variety of surgical procedures. After controlling for surgery type, demographic factors, and traditional risk assessments, frailty was shown to identify patients at increased risk for morbidity, mortality, extended length of stay, and discharge to a long-term care facility. Notably, although the frailty assessment independently outperformed the traditional risk assessments, the authors also demonstrated the additive value of combining frailty with the ASA, Lee score, and Eagle score [33••].
To further characterize the phenotypic definition of frailty and its use as a surgical risk assessment tool, our group undertook a large prospective trial evaluating patients undergoing major intra-abdominal surgery. Our data showed that when adjusted for age and traditional risk assessments, a designation of “intermediately frail” or “frail” places a patient at increased risk for any 30-day complication (OR = 2.07, 95 % CI 1.05 to 4.08, p = 0.036) [34]. Furthermore, a subset analysis also demonstrated that the risk conferred by frailty remained a statistically significant predictor of 30-day morbidity in patients undergoing minimally invasive surgery (OR = 5.914, 95 % CI = 1.25 – 27.96, p = 0.025) [35]. While our results reinforced others’ conclusions, we also evaluated a variety of other laboratory values and patient self-assessments not previously investigated in surgical patients. As a predictor for postoperative outcomes, we found no utility for the Katz Activities of Daily Living, Mini Nutritional Short Form, or the Center for Epidemiologic Studies Depression Scale [34].
Another unique aspect of our study was the inclusion of all patients aged 18 years or older, in contrast to others who have only investigated surgical frailty in patients 65 years or older. As illustrated in Fig. 1 the incidence of frailty increased with age; however, we also discovered a significant portion of patients in their 40s and 50s who were deemed intermediately frail or frail [34]. Our findings highlight the importance of considering patients of all ages with these types of risk assessments. Although numbers in our study were too small for subset analyses, other investigators have shown that even patients in their 40s who are frail have higher rates of mortality compared to their non-frail peers [36].
In contrast to the accumulated deficits model, the five-component Fried criteria may be more appropriate for the pre-operative setting. Although not thoroughly investigated in surgical patients at this point, each of the five components conceivably highlights an area amenable to optimization before surgery. Weight loss could be curbed with a nutrition program; poor grip strength, slow walking speed, and low activity may benefit from an exercise regimen; and exhaustion is likely co-linear with depression and may respond to appropriate psychiatric treatment. A summary of the literature on these considerations in non-surgical patients is below.
Interventions on Frailty
Nutrition
While frailty is certainly multifactorial with contributions across a wide range of organ systems, improving nutritional status is an attractive avenue to pursue improvement in patient outcomes. Anemia and hypoalbuminemia can be considered surrogates for poor nutrition and represent well-defined treatment targets in surgical patients [37, 38]. For elective orthopedic procedures, guideline concordant management includes treating anemic patients by replenishing iron, B12, and folate. Specifically, the Network for Advancement of Transfusion Alternatives (NATA) recommends that “anemia should be viewed as a serious and treatable medical condition, rather than simply an abnormal laboratory value” [39].
Similarly, sarcopenia is a well-recognized phenomenon that co-exists with hypoalbuminemia and often occurs in frail elderly patients. To counteract the muscle-wasting and disability that characterize sarcopenic patients, there are recommendations for increased protein intake in community dwelling adults [40]. These interventions could also be instituted pre-operatively. Also, although not a preoperative intervention, the colorectal and urology communities have recommended early enteral feeding with a carbohydrate and protein rich diet in colorectal resection and cystectomy patients, to promote healing, convalescence, and the prevention of disability [41–44].
Exercise
Several large, high-quality trials have demonstrated that exercise interventions in frail patients improve gait speed, balance, and functional abilities [45]. Furthermore, although yet to be investigated as a modifiable risk factor, cardiopulmonary exercise capacity has been shown to hold prognostic implications in patients undergoing major vascular surgery, even outperforming traditional risk indices [46]. Although no studies have evaluated the impact that preoperative exercise programs would have on postoperative adverse outcomes, it seems likely that "pre-habilitation" with exercise would improve the risk profile of surgical patients. Clearly, a "pre-habilitation" program that is low-cost and applicable across a wide range of socio-economic classes would be optimal.
Hypogonadism
Low serum testosterone levels have been observed in frail older men and may represent one of the few opportunities for pharmacologic intervention on the frailty phenotype [47, 48]. In a study of hypogonadal men who received 6 months of testosterone supplementation, these patients showed improvement in body mass index (BMI), waist circumference, fasting plasma glucose, and insulin resistance. Notably, these patients also exhibited an improvement their frailty scores, specifically grip strength, physical activity, and gait speed [49]. Further research is needed to assess the impact treatment of hypogonadism has on the outcomes of surgical patients.
Conclusions and Future Directions
Regardless of the approach, frailty is a dependable method to identify and distinguish between those patients at increased risk for adverse outcomes compared to the general population, regardless of age. Deficit accumulation and phenotypic frailty have proved useful for a variety of investigators, but perhaps there is a role for a combination of features of both, and even inclusion of biochemical variables. Ultimately, for a frailty assessment to prove valuable enough for wide dissemination into clinical practice it must fulfill two criteria. First, it must accurately estimate a patient’s risk for post-operative outcomes to facilitate patient counseling and shared decision-making. Secondly, a worthwhile frailty assessment will offer an opportunity for patient pre-habilitation to minimize risk and optimize outcomes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hamel MB, Henderson WG, Khuri SF, Daley J. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc. 2005;53(3):424–9.
Colloca G, Santoro M, Gambassi G. Age-related physiologic changes and perioperative management of elderly patients. Surg Oncol. 2010;19(3):124–30. This review article is a comprehensive summary of the age-related changes in physiology that place patients at increased peri-operative risk. It also includes recommendations for peri-operative management of patients’ with special physiologic and pharmacodynamic derangements.
Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68(6):1560–8.
Nagai Y, Metter EJ, Earley CJ, Kemper MK, Becker LC, Lakatta EG, et al. Increased carotid artery intimal-medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation. 1998;98(15):1504–9.
O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson Jr SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340(1):14–22.
Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung. Comparison with normal and emphysematous lungs. 2. Functional aspects. Chest. 1992;101(3):800–9.
Tolep K, Kelsen SG. Effect of aging on respiratory skeletal muscles. Clin Chest Med. 1993;14(3):363–78.
Krumpe PE, Knudson RJ, Parsons G, Reiser K. The aging respiratory system. Clin Geriatr Med. 1985;1(1):143–75.
Rowe JW, Andres R, Tobin JD, Norris AH, Shock NW. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976;31(2):155–63.
Musso CG, Oreopoulos DG. Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron Physiol. 2011;119 Suppl 1:1–5.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70.
Gardner GJ. Ovarian cancer cytoreductive surgery in the elderly. Curr Treat Options Oncol. 2009;10(3–4):171–9.
Berry MF, Hanna J, Tong BC, Burfeind Jr WR, Harpole DH, D'Amico TA, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg. 2009;88(4):1093–9.
Balducci L, Yates J. General guidelines for the management of older patients with cancer. Oncology (Williston Park). 2000;14(11A):221–7.
Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S, et al. Task Force on CGA of the International Society of Geriatric Oncology. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol. 2005;55(3):241–52.
Palmer RM. Geriatric assessment. Med Clin N Am. 1999;83(6):1503–23. vii-viii.
Leung JM, Dzankic S. Relative importance of preoperative health status versus intraoperative factors in predicting postoperative adverse outcomes in geriatric surgical patients. J Am Geriatr Soc. 2001;49(8):1080–5.
Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2:281–4.
Bowles TA, Sanders KM, Colson M, Watters DA. Simplified risk stratification in elective colorectal surgery. ANZ J Surg. 2008;78(1–2):24–7.
Aronson WL, McAuliffe MS, Miller K. Variability in the American Society of anesthesiologists physical status classification scale. AANA J. 2003;71(4):265–74.
Balducci L, Beghe C. The application of the principles of geriatrics to the management of the older person with cancer. Crit Rev Oncol Hematol. 2000;35(3):147–54.
Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342(8878):1032–6.
Pope D, Ramesh H, Gennari R, Corsini G, Maffezzini M, Hoekstra HJ, et al. Pre-operative assessment of cancer in the elderly (PACE): a comprehensive assessment of underlying characteristics of elderly cancer patients prior to elective surgery. Surg Oncol. 2006;15(4):189–97.
Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–7. A comprehensive review article on assessing frailty according to the deficit accumulation model. The authors summarize the literature, particularly the implications of considering signs, symptoms, diseases, and disabilities as markers of frailty placing patients at increased risk for poor outcomes.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. Large, prospective study that defined frailty as a distinct clinical syndrome characterized by weight loss, weakness, slowed gait speed, low activity, and exhaustion. Community dwelling frail patients were shown to be at increased risk for falls, hospitalizations, disability, institutionalization, and mortality. Particularly important as it led to the adaptation of frailty assessments to surgical cohorts.
Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev. 2002;123(11):1457–60.
Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, et al. Women's Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321–30.
Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54(6):975–9.
Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681–7.
Robinson TN, Eiseman B, Wallace JI, Church SD, McFann KK, Pfister SM, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250(3):449–55. Prospective study demonstrating that the accumulated deficit model of frailty, when measured in surgical patients requiring ICU care, is a reliable predictor of discharge to a nursing facility and mortality. Identifies important questions regarding the cost-effectiveness and appropriateness of pursuing surgical care in frail patients likely to need institutionalization or at high risk for mortality.
Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009;48(1):78–83.
Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res. 2013;183(1):104–10.
Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–8. This large, prospective study was the first to adapt Dr. Fried’s phenotypic frailty definition to a surgical cohort. Identification of the frailty syndrome was shown to be a reliable method for distinguishing patients at increased risk for post-operative complications, extended length of stay, discharge to a nursing facility, and mortality.
Revenig LM, Canter DJ, Taylor MD, Tai C, Sweeney JF, Sarmiento JM, et al. Too frail for surgery? Initial results of a large multidisciplinary prospective study examining preoperative variables predictive of poor surgical outcomes. J Am Coll Surg. 2013;217(4):665–70.
Revenig LM, Canter DJ, Master VA, Maithel SK, Kooby DA, Pattaras JG, et al. A prospective study examining the association between pre-operative frailty and post-operative complications in patients undergoing minimally invasive surgery. J Endourol. 2013. In press.
Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183(8):E487–94.
Balducci L. Anemia, fatigue and aging. Transfus Clin Biol. 2010;17(5–6):375–81.
Steensma DP, Tefferi A. Anemia in the elderly: how should we define it, when does it matter, and what can be done? Mayo Clin Proc. 2007;82(8):958–66.
Goodnough LT, Maniatis A, Earnshaw P, Benoni G, Beris P, Bisbe E, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106(1):13–22.
Waters DL, Baumgartner RN, Garry PJ, Vellas B. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: an update. Clin Interv Aging. 2010;5:259–70.
Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24 h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst Rev. 2006. doi:10.1002/14651858.CD004080.pub2.
Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J Gastrointest Surg. 2009;13(3):569–75.
Basse L, Raskov HH, Hjort Jakobsen D, Sonne E, Billesbølle P, Hendel HW, et al. Accelerated postoperative recovery programme after colonic resection improves physical performance, pulmonary function and body composition. Br J Surg. 2002;89(4):446–53.
Karl A, Buchner A, Becker A, Staehler M, Seitz M, Khoder K, et al. A new concept for Early Recovery After Surgery in patients undergoing radical cystectomy for bladder cancer—results of a prospective randomized study. J Urol. 2013. doi:10.1016/j.juro.2013.08.019.
Cadore EL, Rodríguez-Mañas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res. 2013;16(2):105–14.
Thompson AR, Peters N, Lovegrove RE, Ledwidge S, Kitching A, Magee TR, et al. Cardiopulmonary exercise testing provides a predictive tool for early and late outcomes in abdominal aortic aneurysm patients. Ann R Coll Surg Engl. 2011;93(6):474–81.
Yeap BB, Araujo AB, Wittert GA. Do low testosterone levels contribute to ill-health during male ageing? Crit Rev Clin Lab Sci. 2012;49(5–6):168–82.
Laosa O, Alonso C, Castro M, Rodríguez-Mañas L. Pharmaceutical interventions for frailty and sarcopenia. Curr Pharm Des. 2013;PMID:24079768.
Strollo F, Strollo G, Morè M, Magni P, Macchi C, Masini MA, et al. Low-intermediate dose testosterone replacement therapy by different pharmaceutical preparations improves frailty score in elderly hypogonadal hyperglycaemic patients. Aging Male. 2013;16(2):33–7.
Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121(8):973–8.
Robinson TN, Wallace JI, Wu DS, Wiktor A, Pointer LF, Pfister SM, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg. 2011;213(1):37–42.
Tan KY, Kawamura YJ, Tokomitsu A, Tang T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg. 2012;204(2):139–43.
Courtney-Brooks M, Tellawi AR, Scalici J, Duska LR, Jazaeri AA, Modesitt SC, et al. Frailty: an outcome predictor for elderly gynecologic oncology patients. Gynecol Oncol. 2012;126(1):20–4.
Compliance with Ethics Guidelines
Conflict of Interest
Louis M. Revenig, Kenneth Ogan, Thomas J. Guzzo, and Daniel J. Canter declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Revenig, L.M., Ogan, K., Guzzo, T.J. et al. The Use of Frailty as a Surgical Risk Assessment Tool in Elderly Patients. Curr Geri Rep 3, 1–7 (2014). https://doi.org/10.1007/s13670-013-0068-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13670-013-0068-z