Abstract
Purpose of review
The geriatric patient population represents an ever increasing proportion of a surgeon’s practice spectrum. With this comes the need to understand the factors largely impacting the geriatric surgical population. One of these factors is frailty. At first glance frailty appears as a simple concept. However, a review of the literature reveals a plethora of definitions, quantification endeavors, and theories attempting to delineate its principle attributes.
Recent findings
Despite the variety of theories, the literature supports a strong link between frailty and poor surgical outcomes. While this link is strong, the exact utility of frailty in preoperative assessments is still being appreciated both in the general surgery setting and in its subspecialties.
Summary
In this review we first discuss the background and defining attributes of frailty and their relation to concepts closely associated with frailty such as resilience, multi-morbidity, and accelerating factors of aging. Understanding the prominent defining features and close associations allows for a greater appreciation of frailty’s clinical impact on the geriatric perioperative surgical assessment. Ultimately, when including frailty in the geriatric surgical assessment its consideration allows for modification in the preoperative, intraoperative, and postoperative setting with the overall goal of improving surgical outcomes in the geriatric population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With an ever burgeoning older population, the elderly will be a large part of the surgeon’s practice. Interestingly, the attitude toward geriatric surgery has changed over the years. A threshold of 50 years was chosen to describe geriatric patients in a paper in 1907 [1], and 20 years later influential surgeons still wrote that elective herniorrhaphy in this age group was not warranted [2]. Today, many complex operations are successfully performed in octogenarians, nonagenarians, and even centenarians [3–5].
In 2010, over a third of inpatient procedures in the US were performed in patients 65 years and older [6]. This number is projected to rise as the population continues to live longer. With this increase in age also comes a range of comorbidities such as cataracts, recurrent cancers, and coronary artery disease—the frequency of which in the latter years of life exponentially increases. Beyond these comorbidities it has been shown that aging presents unique challenges that must be considered when contemplating surgical options.
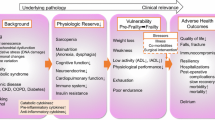
When viewing the challenges associated with aging, frailty emerges as one of the more exigent “phenotypic expressions” in the older population. Frailty is an age-related attenuation in multiple physiologic systems, culminating in greater susceptibility to minor stressors which can abruptly compromise an individual’s health. In other words, it is a vulnerable circumstance in which a “stressor event” causes a reduced likelihood of returning to homeostasis and an increased probability of encountering further “adverse outcomes.” The literature has repeatedly supported the association of frailty with poor health outcomes. Hence, frailty is becoming an indispensable part of a preoperative assessment in the older surgical patient.
Frailty Overview
Perioperative Frailty is Associated with Poor Surgical Outcomes
Although the concept of frailty has existed for a long time, its discussion in the medical literature has increased over the last three decades [7]. Since then three landmark studies have impacted our understanding of frailty and its association with suboptimal surgical outcomes. Dasgupta et al. found an association between high frailty scores (Edmonton Frail Scale) and increased postoperative complications [8]. Robinson et al. reported patients with increased frailty characteristics to have higher 6-month mortality [9]. Makary et al. described an association between frailty and increased postoperative complications [10]. Further examples of factors associated with frailty and subsequent poor surgical outcomes include prolonged length of stay, hospital readmission and discharge to institutional care facility (Table 1).
The advances in an ever-improving evidence-based framework with regard to frailty guide its use in perioperative management and risk stratification. In an effort to bridge the gap between the concept of frailty and its ramifications on surgical decisions and outcomes, the National Institute on Aging and the American Geriatrics Society sponsored a 2-day conference (March 2015) to discuss frailty and its implications in perioperative management [11]. Ultimately, comprehensive geriatric preoperative assessments help identify frail patients early and allow for a more comprehensive preoperative workup and preparation for surgery [12].
Frailty Defined
In 1908, Eli Metchnikoff posited, “How can we transform to a normal and physiological condition, old age, at present utterly pathological, unless we first understand the intimate details of its mechanism?” [13]. Currently, when viewing frailty in terms of “intimate” biologic underpinnings, examples of phenotypic expressions of frailty include telomere attrition, mitochondrial dysfunction, and epigenetic alterations [14].
Consensus opinion conceptualizes frailty as a multifaceted, age-related state of reduced multi-system, physiologic reserve causing increased susceptibility to stressors and decreased adaptive capacity [15–19]. The increased susceptibility to poor health outcomes include functional dependence, falls, mortality, and need for institutionalization [20]. Further, this “frail state” is associated with biologic rather than chronologic age.
While there is agreement on the concept of frailty no consensus has yet been established of how to best assess frailty [15]. A broad heterogeneous spectrum of tools has been developed in an attempt to quantify frailty’s operational definition. With differing measures of quantification, different operational definitions of frailty have emerged. The two most commonly cited are Fried’s phenotypic approach and the accumulation of deficit models by Mitnitski and Rockwood [17, 21, 22].
Fried’s phenotypic definition, also known as “physical frailty,” describes frailty as the consequence of phenotypic expression of the accumulated decline of multiple physiologic mechanisms. Frailty characteristics include unintentional weight loss, decreased muscle strength, self-reported exhaustion, low physical activity, and slow walking speed [17].
The accumulation of deficits model, also known as “frailty index,” defines frailty as a generalized, age-associated susceptibility exhibited by accumulated deficits entailing medical, social, and functional variables [23]. These deficits are quantified by medical diagnoses, symptoms, and lab abnormalities which are associated with increasing age and suboptimal health outcomes [24]. With this quantification Mitnitski showed a 3 % rate of deficit accumulation per year in community-dwelling individuals [25]. Kulminski et al. associated the frailty index with increasing age and mortality [26].
Considering these two theories of operational frailty the important difference is that phenotypic frailty is “driven” by biologic factors while the accumulation of deficit model is driven by an accretion of clinical deficits. Although there is general consensus on the conceptualization of frailty, no explicit consensus exists as to the most appropriate quantitative measure of operational frailty (that is clinically implementable and able to guide prevention and care) [15].
Frailty Quantified
A review of the literature reveals over 80 tools available to quantify frailty. Some “tools” examine over 60–70 items in an effort to quantify frailty. This reflects the multifaceted nature of variables illustrating the clinical needs for different specialties. Although this wide variety exists and despite differing opinions on frailty quantification tools, most experts agree that patients over age 70 should be screened for frailty. This is in agreement with the literature which supports interventions targeted towards the geriatric surgical population to ameliorate their frailty-associated adverse outcomes [27].
It is worthwhile to point out frailty screening and frailty assessments have differing purposes. Frailty screening is aimed at risk stratification. A frailty assessment provides a detailed formal evaluation to help define preoperative interventions to optimize surgical outcomes.
Frailty screening is often easily completed with single variable assessments such as Timed Get Up and Go Test and gait speed [28, 29]. Savva et al. showed that the Timed Get Up and Go Test could identify frail members of the older population [30]. In cardiac patients Afillalo et al. showed slower gait speed associated with increased postcardiac surgery morbidity and mortality [31].
Another screening tool often used is Frail Scale which takes less than 5 min to complete. It was developed by the Geriatric Advisory Panel of the International Academy of Nutrition and Aging in an effort to create a screening tool [29]. This tool reviews five simple questions quantifying frailty as robust, prefrail, and frail [32, 33].
Phenotypic frailty requires 10–15 min to complete, it is most commonly used in frailty research [17]. A diagnosis of frailty is made when three or more of the following five criteria are present: unintentional weight loss of 10 lb or more in the past year, self-reported exhaustion, weakness as measured by grip strength using a dynamometer, slow walking or gait speed, and low physical activity. The presence of two or three of these criteria would identify a person as “pre-frail.”
The deficit accumulation definition of frailty is quantified by criteria developed by Rockwood et al. in the Canadian Health and Aging Study [34]. Generally measuring between 21 and 70 deficits, the quantification is based on an index score which divides an individual’s number of deficits by the total number of measured characteristics. This test is more time consuming than aforementioned tests.
Perioperative Frailty and Closely Related Factors
Frailty and Resilience
A discussion of frailty would be incomplete without discussing resilience. Frailty and resilience are related, however not considered opposites. Resilience reflects an individual’s aptitude in dealing with stressors and challenges [35]. While resilience is not considered an opposite of frailty, it reflects an individual’s positive capacity in dealing with challenging events [14]. It is possible that the processes influencing frailty and resilience are closely entwined and reflected in genetic, environmental, and functional processes. More research is needed to assess whether quantifying “positive biologic reserve” will possibly improve surgical decisions and patient outcomes [35].
Frailty and Multi-Morbidity
It is important to discuss the difference between multi-morbidity and frailty. Multi-morbidity is considered the simultaneous presence of 2 or more chronic conditions in the same patient [36, 37]. Frailty and multi-morbidity are different syndromes although both entail elevated postsurgical risks [20]. One must understand that dealing with multi-morbidity in the frail geriatric surgical candidate requires medical optimization focusing on specific chronic medical conditions. The difficulty is that treating one disease may have ramifications ultimately not improving a patient’s overall health. An often cited example is improving the glucose control in a person with diabetes which may result in a fall secondary to hypoglycemia. Early postoperative feeding in a patient with undiagnosed gastroparesis can result in aspiration and its potential complications. When confronted with multiple clinical conditions, one should focus on the more clinically relevant conditions over less significant ones [38].
Frailty and Social Vulnerability
The deficit-driven definition of frailty also addresses social vulnerability. This definition looks beyond innate physiologic and biologic factors to include variables impacting from an “extrinsic social context.” The Social Vulnerability Index quantifies extrinsic factors such as socioeconomic status, living situation, and social engagement [39]. This index is related to a patient’s frailty. In other words it is part of how frail individuals are described through the accumulation of deficit model. Social vulnerability is higher in people who are frail. Social vulnerability is associated with higher mortality, independent of frailty. More work is needed to describe social vulnerability as it is now recognized that attention to social factors is essential to the care of the older patient [39].
Frailty and Reversible “Age Accelerating Causes”
While the biologic consequences of chronologic age can at times be influenced, it is often easier to target interventions on actual underlying diseases that are “age accelerating causes” of frailty. Malignancy, infections secondary to a chronic condition, and uncompensated single-end organ dysfunction are established causations leading to a frail state. An example is congestive heart failure secondary to aortic stenosis. Heart failure, a systemically impacting clinical condition, can lead to loss of skeletal muscle, immobility, and increased adipose tissue production to name a few. With continued heart failure exacerbations, frailty can easily develop or, if present, worsen [40]. Surgical intervention with an aortic valve replacement or left ventricular assist device, if successful, can reverse frailty developed secondary to the heart failure. This represents a disease-driven, end organ failure which differs from chronologic age-based frailty. Other examples of “accelerators” of clinical frailty that are reversible include HIV and end-stage renal disease.
Frailty Assessment in Surgical Practice
The utility of frailty in the spectrum of geriatric surgery is multifaceted. Overall the goals include maximizing quality of life while reducing comorbidity-associated disease burden, irreversible worsening of chronic disease, and “catastrophic” postsurgical events. Understanding frailty perioperatively allows evidence-supported risk assessments to assist in clinical decisions and allow for modification in the preoperative, intraoperative, and postoperative setting with the overall goal of improving surgical outcomes (Table 2).
Perioperative Interventions
Prehabilitation
Preoperative interventions aimed at improving a patient’s physiologic reserve to allow them to better tolerate surgical procedures has been shown to be successful. Carli et al. showed prehabilitation to enhance postoperative recovery of an octogenarian following robotic-assisted hysterectomy with endometrial cancer [41]. Prehabilitation has also been shown effective in colorectal, cardiac and abdominal surgery [42, 43].
Presurgical exercise programs in frail geriatric patients have shown shortened postoperative recovery, enhanced functional capacity, decreased mortality, and improved quality of life in cardiac and abdominal surgeries [43]. In elective cardiac surgery procedures, a Cochrane Review found that physical therapy reduced length of stay and decreased pulmonary complications [44]. In elective abdominal oncologic surgery, Dronkers et al. showed that preoperative therapeutic exercise was feasible and improved pulmonary function of patients when compared with home-based exercise instruction [45]. While the literature generally shows a very positive association with presurgical exercise interventions, more studies are needed to better understand the direct impact in the geriatric surgical population [46].
Preoperative multimodal approaches are also being trialed. Li et al. showed the positive impact tri-modal prehabilitation program (physical therapy, nutritional optimization, and anxiety reduction) has on functional recovery after colorectal cancer surgery [47].
More research is needed to better define what prehabilitation interventions are most appropriate. Further, practical questions that still remain include: 1. Who should be paying for it? 2. What setting is most appropriate (i.e., home versus care facility) and 3. Worth delaying something like an oncologic surgical resection for prehabilitation?
Delirium Prevention
Inouye et al. found that over a third of postoperative delirium is preventable [48]. Given the close link between postoperative delirium and frailty, it is crucial to address modifiable risk factors in the perioperative setting in order to minimize a patient’s risk of postoperative delirium. Evidence-based, postoperative delirium prevention protocols can be utilized in an anticipatory manner [49]. Also, when patients are flagged as having a higher chance of postoperative delirium, the anesthesiologist can consider alternatives such as regional block to possibly minimize postoperative narcotic analgesia or intraoperative EEG monitor to minimize depth of intraoperative sedation when possible [50, 51]. The Postoperative Delirium in Older Adults: Best Practice Statement from the American Geriatrics Society provides an excellent overview [52].
Palliative Care Discussion
While “palliative care” is often thought of as tantamount to withdrawal of care, its consideration is crucial when considering major surgical interventions in very frail patients. Incorporating principles of palliative care and having a palliative care discussion allows a patient to consider their personal goals, quality of life versus quantity of life in light of the procedure’s impact on quality of life, prognosis of recovery, and functional decline expectations [53, 54]. This conversation also allows a patient’s family to participate in discussing the pros and cons of undergoing a procedure and understanding realistic postoperative course expectations.
Intraoperative Setting Modifications
When a frailty assessment identifies a patient at increased surgical risk when undergoing certain procedures, less invasive procedures can be considered, different approaches can be discussed, or even medical management options can be considered.
Modification can include endoscopic versus open surgery or even pursuing medical management rather than surgical options. Endoscopic stenting of an obstructing pulmonary lesion may be considered over surgical resection. Biopsies could be performed under local rather than general anesthesia. A patient with acute appendicitis with high probability of poor operative outcomes could undergo medical management with antibiotics rather than surgical resection. While a diagnosis of frailty can prompt a surgeon to pursue less aggressive surgical options, research is still needed to evaluate the benefits of contouring surgical recommendations to frailty-based assessments. Further research is necessary to improve our understanding of outcome efficacy based on frailty assessments.
While best practice guidelines for an optimal anesthesia regimen have not been established, the literature supports minimizing general anesthesia sedation when possible. Further, utilization of regional and local anesthetics to allow for minimal sedation has shown great benefit particularly with regard to decrease in postoperative delirium. It has been most beneficial when an interdisciplinary approach between the surgeon and anesthesiologist discuss an operative plan and options [55].
Postoperative Expectations
Understanding frailty’s impact on a postsurgical course allows a surgeon to provide a more accurate depiction of expected surgical outcomes, possible complications, and recovery time. Knowing the reality of increased chance of certain complications or prolonged hospital admission can help a patient and their family have more realistic expectations of the postoperative recovery. It also enables them to plan expected stays at postacute care rehabilitation facilities or possibly arrange extra home care for a loved one. Also, acknowledging and addressing co-dependence and needs of a patient’s caregiver or family member before pursuing a major surgery with significant possible complications may relieve the patient and the caregiver/family’s apprehension and burden.
Conclusions
Discussions regarding surgical options, complications, and realistic postoperative outcomes can be taxing on both patient and their family. Shared decision-making models have helped patients understand their options and minimize decisional conflict [56]. Research is needed to understand the best use of shared decision making. Further, rather than focusing on procedural details, it is important for a surgeon to recognize and address what matters most to the patient and their family such as postoperative pain control, functional status, or being able to eat—quality versus quantity of life [57].
A multidisciplinary approach to the care of the geriatric surgical patient not only includes the longitudinal primary care provider, anesthesiologist, and surgeon, but also appropriate consultations from other health care professionals which may include oncology, cardiology, rehabilitation medicine, nutrition, and social work to help optimize the postsurgical outcomes of these challenging patients [55].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Smith OC. Advanced age as a contraindication to operation. Med Rec (NY). 1907;72:642–4.
Ochsner A. Is risk of operation too great in the elderly? Geriatrics. 1967;22:121–30.
Cohen JR, Johnson H, Eaton S, Sterman H, Wise L. Surgical procedures in patients during the tenth decade of life. Surgery. 1988;104(4):646–51.
Katlic MR. Surgery in centenarians. JAMA. 1985;253(21):3139–41.
Bridges CR, Edwards FH, Peterson ED, Coombs LP, Ferguson TB. Cardiac surgery in nonagenarians and centenarians. J Am Coll Surg. 2003;197(3):347–56.
United States Census Bureau—2014 National Population Projections. https://www.census.gov/population/projections/data/national/2014.html. Accessed Sep 22, 2015.
Hogan DB, MacKnight C, Bergman H. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15(Suppl 3):1–29.
Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009;48(1):78–83.
•• Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and comorbidity. Ann Surg. 2009;250:449–55.
Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–8.
•• Robinson TN, Walston JD, Brummel NE, Deiner S, Brown CH 4th, Kennedy M, Hurria A. Frailty for Surgeons: review of a National Institute on Aging Conference on Frailty for Specialists. J Am Coll Surg. 2015 Dec;221(6):1083–92.
Partridge JS, Collingridge G, Gordon AL, Martin FC, Harari D, Dhesi JK. Where are we in perioperative medicine for older surgical patients? A UK survey of geriatric medicine delivered services in surgery. Age Ageing. 2014;43(5):721–4.
Achenbaum WA. Crossing frontiers: gerontology emerges as a science. Cambridge University Press, Cambridge, 1995.
Lopez-Otin C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153:1194–217.
• Rodriguez-Manas L, Feart C, Mann G, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68:62–7.
Bortz WM 2nd. A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57:M283–8.
•• Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:146–56.
Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57:115–B125.
Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95.
Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63.
• Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World J. 2001;1:323–36.
•• Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–7.
Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26.
de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104–14.
Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–9.
Kulminski AM, Ukraintseva SV, Culminskaya IV, et al. Cumulative deficits and physiological indices as predictors of mortality and long life. J Gerontol A Biol Sci Med Sci. 2008;63:1053–9.
Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–7.
Robinson TN, Wu DS, Sauaia A, et al. Slower walking speed forecasts increased postoperative morbidity and 1-year mortality across surgical specialties. Ann Surg. 2013;258:582–588; discussion 588–590.
van Abellan Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9:71–2.
Savva GM, Donoghue OA, Horgan F, et al. Using timed up and-go to identify frail members of the older population. J Gerontol A Biol Sci Med Sci. 2013;68:441–6.
Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–76.
Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16(7):601–8.
Woo J, Yu R, Wong M, et al. Frailty screening in the community using the FRAIL scale. J Am Med Dir Assoc. 2015;16:412–9.
• Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–7.
De Alfieri W, Borgogni T. Through the looking glass and what frailty found there: looking for resilience in older adults. J Am Geriatr Soc. 2010;58:602–3.
Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for healthcare, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43.
Boeckxstaens P, Vaes B, Legrand D, Dalleur O, De Sutter A, Degryse JM. The relationship of multimorbidity with disability and frailty in the oldest patients: a cross-sectional analysis of three measures of multimorbidity in the BELFRAIL cohort. Eur J Gen Pract. 2015;21(1):39–44.
• Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351(27):2870–4.
Andrew MK, Mitnitski AB, Rockwood K. Social vulnerability, frailty and mortality in elderly people. PLoS ONE. 2008;3:22–32.
• McNallan SM, Singh M, Chamberlain AM, et al. Frailty and health care utilization among patients with heart failure in the community. JACC Heart Fail. 2013;(1)135–141.
Carli F, Brown R, Kennepohl S. Prehabilitation to enhance postoperative recovery for an octogenarian following robotic-assisted hysterectomy with endometrial cancer. Can J Anaesth. 2012;59(8):779–84.
Mayo NE, Feldman L, Scott S, Zavorsky G, Kim DJ, Charlebois P, Stein B, Carli F. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150(3):505–14.
Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care. 2005;8(1):23–32.
Hulzebos EH, Smit Y, Helders PP, van Meeteren NL. Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database Syst Rev 2012;11:CD010118.
Dronkers JJ, Lamberts H, Reutelingsperger IM, Naber RH, Dronkers-Landman CM, Veldman A, van Meeteren NL. Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: a randomized controlled pilot study. Clin Rehabil. 2010;24(7):614–22.
Halloway S, Buchholz SW, Wilbur J, Schoeny ME. Prehabilitation interventions for older adults: an integrative review. West J Nurs Res. Jan 2015;37(1).
Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS, Kaneva P, Augustin B, Wongyingsinn M, Gamsa A, do Kim J, Vassiliou MC, Feldman LS. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27(4):1072–82.
Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–76.
• American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. Jan 2015. 63(1):142–50.
Sieber FE, Zakriya KJ, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26.
Orena EF, King AB, Hughes CG. The role of anesthesia in the prevention of postoperative delirium: a systematic review. Minerva Anestesiol. 2016;82(6):669–83.
American Geriatric Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220(2):136–48.
•• Task Force on Surgical Palliative care; Committee on Ethics. Statement of principles of palliative care. Bull Am Coll Surg. 2005;90:34–5.
•• Dunn GP. Surgery, palliative care, and the American College of Surgeons. Ann Pallia Care. 2015;4(1):5–9.
Tan KY, Tan P, Tan L. A collaborative transdisciplinary ‘geriatric surgery service’ ensures consistent successful outcomes in elderly colorectal surgery patients. World J Surg. 2011;35:1608–14.
Stacey D, Légaré F, Col NF, Bennett CL, Barry MJ, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Thomson R, Trevena L, Wu JH. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014; (1) 28.
• Glance LG, Osler TM, Neuman MD. Redesigning surgical decision making for high-risk patients. N Engl J Med. 2014;370:1379–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Wozniak, Coleman, and Katlic declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Geriatric Surgery.
Rights and permissions
About this article
Cite this article
Wozniak, S.E., Coleman, J. & Katlic, M.R. The Utility of Preoperative Frailty Assessment. Curr Surg Rep 4, 36 (2016). https://doi.org/10.1007/s40137-016-0156-z
Published:
DOI: https://doi.org/10.1007/s40137-016-0156-z