Abstract
Purpose of Review
Although Glucagon-like peptide (GLP)-1 receptor agonists have been used for almost two decades in the treatment of diabetes mellitus type 2 and, lately, in obesity, recent years have seen an increasing interest in the pharmacological agonism of other proglucagon-derived peptides, including GLP-2. Herein, we aimed to review the available evidence on the effects of GLP-2 agonism from animal and clinical studies. Furthermore, we summarize the current clinical applications of GLP-2 agonists among patients with intestinal failure associated with short bowel syndrome (SBS-IF) as well as potential future expansion of their indications to other intestinal disorders.
Recent Findings
Evidence from preclinical studies has highlighted the cellular trophic and functional beneficial actions of GLP-2 on small intestinal and colonic mucosa. Subsequently, pharmacologic agonism of GLP-2 has gathered interest for the treatment of patients with conditions pertaining to the loss of intestinal anatomical and/or functional integrity to a degree requiring parenteral support, collectively referred to as intestinal failure. GLP-2 analogs positively influence nutrient absorption in animal models and humans, although continued therapy is likely needed for sustained effects. The degradation-resistant GLP-2-analog teduglutide has received approval for the treatment of SBS-IF, in which it may decisively reduce patient dependency on parenteral support and improve quality of life. Another two longer-acting analogs, glepaglutide and apraglutide, are currently undergoing phase III clinical trials.
Summary
The use of GLP-2 analogs is effective in the management of SBS-IF and may show promise in the treatment of other severe gastrointestinal disorders associated with loss of effective intestinal resorptive surface area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucagon and its hyperglycemic effects were originally discovered in 1922, while its precise amino acid sequence was determined as early as 1957 [1, 2]. Since then, our knowledge regarding its origin and properties has substantially expanded. The most important milestone in this course was likely the identification of its precursor protein proglucagon in the early 1980s, which paved the way for the characterization of the family of proglucagon-derived peptides (PGDP).
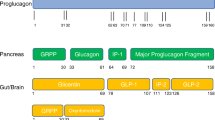
The proglucagon gene (GCG) is located on chromosome 2 and its expression leads to synthesis of pro-proglucagon, which is subsequently cleaved to proglucagon [3]. Depending on the tissue of expression, different sets of PGDPs are obtained through further enzymatic processing by the pro-hormone convertases (PCs); cleavage by PC2 (most abundant in pancreatic alpha-cells) results in production of glucagon and major proglucagon fragment (MPFG), while the action of PC1/3 in L enteroendocrine cells leads to the formation of Glucagon-like peptide-1 and Glucagon-like peptide-2 (GLP-1 and GLP-2, respectively), oxyntomodulin and glicentin [3]. PGDPs exert unique physiological actions in terms of metabolism and energy regulation via their binding to special G-protein-coupled membrane receptors, which renders them promising candidates for the treatment of several clinical entities. In particular, GLP-1 receptor agonists have been approved for the treatment of diabetes mellitus type 2 (T2DM) since 2005 [4], while GLP-2 analogs are being investigated for their potential use in various gastrointestinal disorders. Figure 1 depicts the different roles of GLP-1 and GLP-2 analogs in human physiology [3] (Fig. 1).
Main biological actions of GLP-1 and GLP-2. Abbreviations: GLP, glucagon-like peptide. (All images are originated from the free medical site http://smart.servier.com/ (accessed on June 15, 2022) by Servier licensed under a Creative Commons Attribution 3.0 Unported License)
GLP-2 receptors, just like GLP-1 receptors, are G-protein-coupled transmembrane receptors. The binding of GLP-2 to its receptor in the gastrointestinal (GI) tract leads to increased intracellular cyclic adenosine monophosphate (c-AMP) production, which, in turn, stimulates intestinal cell proliferation, while inhibiting apoptosis. Thus, GLP-2 exhibits intestinotrophic effects [5]. On the contrary, GLP-1 receptors, which are mainly located in the pancreatic beta cells, possess insulin-releasing properties, which are mediated by the accumulation of cytosolic Ca2 + and the c-AMP-related stimulation of protein kinase A (PKA). In this way, exocytosis of insulin granules is achieved leading to an incretin effect [4, 5].
GLP-1 together with gastric inhibitory peptide or glucose-dependent insulinotropic polypeptide (GIP), which is secreted by the enteroendocrine K-cells, are the main mediators of the incretin effect, namely, the induction of a glucose-dependent insulin secretion from pancreatic beta cells, while GLP-1 exhibits strong satiety-promoting properties, which are exploited for the medical treatment of obesity [6, 7]. GLP-2 is released from the enteroendocrine L-cells together with the rest of PC1/3-cleaved PGDPs and Peptide YY after ingestion of nutrients [5]. Although there is also ongoing interest regarding the potential application of GLP-2 in the treatment of diabetes mellitus, mounting evidence has advocated its main action as a regulator of growth and proliferation of cells lining the gastrointestinal (GI) tract as well as its key properties in increasing intestinal and portal blood flow and decreasing GI motility. These GI attributes of GLP-2 render it a useful agent for the therapy of several debilitating GI disorders, especially short bowel syndrome-intestinal failure (SBS-IF) [7, 8].
In this narrative review, we aim to (i) present the mechanisms of action of GLP-2 analogs, with a special focus on their intestinotrophic properties and effects on nutrient absorption; (ii) appraise their current therapeutic applications in patients with SBS-IF; (iii) discuss their therapeutic potential for other GI disorders; and (iv) review potential future perspectives of this promising category of agents.
Methodology
In July 2022, a literature search of two bibliographical databases (MEDLINE and Scopus) was conducted to assess the characteristics and the therapeutic potential of GLP-2 analogs. This search used the following terms: “Glucagon-like peptide-2 AND (treatment OR therapy OR drug OR diet OR nutrition).” A search of the abovementioned terms yielded a total of 903 results, most of which were published between 2012 and 2022 (during the past 10 years). Of these, 298 studies were excluded, as they dealt with the metabolic syndrome, obesity, diabetes, hypertension, bone disorders, and neurohormonal aspects.
GLP-2 and Their Mechanisms of Action
GLP-2 is secreted postprandially by enteroendocrine L-cells, which are located in the distal small intestine, the colon, and to a much lesser extend in the duodenum. Expression of the GLP-2 Receptor (GLP-2R), a G-protein-coupled receptor, is restricted to the intestine with higher levels in the jejunum, lower levels in the distal gut, and even lower expression in the duodenum. Notably, even within the gut, considerable GLP-2R mRNA transcript levels are rarely found [9,10,11,12,13]. Moreover, it has been demonstrated that the GLP-2R is localized to only a few enteroendocrine cells as well as subepithelial myofibroblasts and enteric neurons, whereas it has not been found in either the proliferative crypt cells or any other enterocyte surface. This finding is most suggestive of an indirect, instead of a direct, role regarding the growth and functional effects of GLP-2 on the gut, mediated by its actions on neuroendocrine cells and probably by means of other intestinal growth factors, such as Insulin-like Growth Factor-1 (IGF-1), Epidermal Growth Factor (EGF), and Keratinocyte Growth Factor (KGF). Figure 2 depicts the effects of endogenous GLP-2 on the gut under normal circumstances as well as after exogenous GLP-2 administration [10,11,12,13,14] (Fig. 2). As the low levels of the GLP-2R cannot exemplify the prominent effects of GLP-2R activation, it seems likely that these intestinal growth factors interact with each other to magnify the intestinotrophic actions of GLP-2 [9].
A–B GLP-2 is a key enteric hormone released from enteroendocrine L-cells that activate mucosal enteric neuron to release nNOS as well as subepithelial fibroblasts to release EGF and IGF-1. GLP-2 analogs have known therapeutic applications in patients with SBS-IF and a potential therapeutic use for other moderate to severe gastrointestinal disorders. Abbreviations: EGF, epidermal growth factor; GLP, glucagon-like peptide; GLP-2R, glucagon-like peptide 2 receptor; nNOS, neuron nitric oxide synthetase; IGF-1, insulin-like growth factor-1; SBS-IF, short bowel syndrome-intestinal failure. (All images are originated from the free medical site http://smart.servier.com/ (accessed on June 15, 2022) by Servier licensed under a Creative Commons Attribution 3.0 Unported License)
The intestinotrophic actions of GLP-2 are mainly mirrored as growth effects, measured as an increase in villus height and crypts depth as well as prevention of enterocyte apoptosis. The abovementioned growth effects of GLP-2 result in a proliferation of the enterocytes and expansion of the epithelial surface area [9, 15]. Apart from its intestinotrophic actions, GLP-2 also affects functional properties, as evidenced by the inhibition of gastric acid secretion and gastric emptying, stimulation of the intestinal blood flow, enhancement in intestinal barrier functions, anti-inflammatory potential, and increases in nutrient and fluid absorption. In particular, the delay in gastric emptying together with the increase in intestinal and portal blood flow and the amelioration of the intestinal barrier function may lead to the enhancement in nutrient absorption [9, 16, 17]. It should nonetheless be noted that a prominent GLP2 effect on gastric emptying has not been universally reported from all available studies [18]. Regarding the improvement in intestinal barrier function, it has been documented that it may be mediated by the increased expression of the tight-junction proteins, claudin 3 and 7 [19, 20]. More specifically, tight junctions are composed of at least 40 different transmembrane and cytoplasmic proteins. The three main transmembrane proteins are occludin, claudins, and junction adhesion molecule (JAM) proteins. Tight junctions prevent leakage of water and solutes between the epithelial cells and their function has been demonstrated to improve by enhancement in the expression of several claudins [21].
Despite its valuable properties, the clinical applications of the native GLP-2 peptide are limited by its degradation by dipeptidyl-peptidase 4 (DPP-4), which inactivates both GLP-2 and GLP-1 very rapidly. Thus, exogenous administration of GLP-2 in healthy volunteers results in an elimination half-life of 7 min, approximately [22, 23]. Nevertheless, this major drawback of GLP-2 has been overcome by the advent of GLP-2 analogs, which are resistant to the degradation by DPP-4. Three GLP-2 analogs have been developed and are currently being investigated for their intestinotrophic and adaptive mechanisms, in terms of intestinal growth and functions: teduglutide, glepaglutide, and apraglutide [24,25,26].
Teduglutide is also a 33 amino acid peptide, which differs from the native GLP-2 only in a N-terminus substitution of glycine for alanine at position 2 ([Gly2]GLP-2). This glycine substitution renders teduglutide resistant to enzymatic degradation by DPP-4 in vivo and prolongs its elimination half-life to 3 h approximately. Teduglutide is synthesized by genetically modified Escherichia coli strains by recombinant DNA technology. It is administered subcutaneously (sc) at doses of 0.05 to 0.10 mg/kg once daily, and has approximately 87% bioavailability after sc administration and is eliminated via the kidneys. Therefore, a 50% dose reduction is recommended in patients with moderate to severe renal impairment (creatinine clearance < 50 mL/min) [24, 27].
Glepaglutide, a novel long-acting GLP-2 analog, differs from endogenous GLP-2 by having 9 amino acid substitutions and a C-terminal tail consisting of 6 lysine residues. After sc administration of 1 mg or 10 mg glepaglutide, a sc depot is formed at the site of the injection, from which glepaglutide is released into the bloodstream [25, 28].
Apraglutide is the third and most recently developed long-acting GLP-2 analog, which is administered at a dose of 5 mg or 10 mg sc every week. It differs from endogenous GLP-2 by four amino acid substitutions, and has a longer elimination half-life (72 h), on account of its slower clearance due to the resistance to DPP-4 degradation and higher plasma protein binding ability. Therefore, apraglutide has the advantage of less frequent injections than teduglutide which renders it a candidate for once weekly dosing regimens [26, 29].
Absorption of Various Nutrients, Parenteral Nutrition Needs, and GLP-2 Analogs
There is a paucity of literature regarding the role of GLP-2 analogs and their association with nutrition. Accumulating evidence has supported the utility of GLP-2 analogs in increasing glucose absorption, both in an acute and in a chronic manner. In particular, an enhancement of glucose transport has been documented through the jejunal lateral side membrane of the intestinal epithelial cell, while increased glucose uptake has also been documented in animal models [30, 31].
Regarding lipids, the administration of GLP-2 analogs has been associated with increases in serum triglyceride levels as well as in free fatty acid release post-prandially in healthy humans [32]. However, there are inconsistent results with regard to serum triglycerides levels, as experiments in neonatal pigs have not shown any enhancement in lipids absorption with the chronic usage of GLP-2 analogs [33]. It is plausible that there are species-specific differences regarding the chronic use of GLP-2 analogs and their effects on lipid absorption.
In terms of amino acid absorption, it has been documented that there is an increase in the absorption of glycine and leucine in rodents [34]. Moreover, Lee et al. have also demonstrated an enhancement in the absorption of even more essential amino acids in mice [35].
Apart from the enhancement of absorption of glucose, lipids, and amino acids with the use of GLP-2 analogs, the administration of these agents has also been related to an increased digestion of macronutrients in animal models [36]. Therefore, the chronic use of GLP-2 analogs may lead to improvements in digestion as well as absorption of various nutrients, mainly by means of increasing the length of the microvillus by twofold, approximately [37]. In addition, GLP-2 analogs have been shown to strengthen the intestinal epithelial barrier, thereby mitigating local inflammation and ameliorating intestinal permeability. This feature contributes to the beneficial potential of GLP-2 analogs to improve intestinal permeability among patients on parenteral nutrition [31]. More specifically, the administration of GLP-2 analogs may result in diminishing the needs for parenteral nutrition. Despite the fact that the administration of GLP-2 analogs results in mitigation of the needs for parenteral nutrition, the clinical course after discontinuation of GLP-2 analogs requires further investigation. Only recently, it has been suggested that the discontinuation of GLP-2 analogs may lead again to increased needs for parenteral nutrition, especially 9 years after treatment cessation [38]. Therefore, chronic administration of GLP-2 analogs may be necessary in order to avoid re-institution of parenteral nutrition after the discontinuation of treatment with GLP-2 analogs.
GLP-2 Analogs and Their Therapeutic Effects
Based on their pharmacological properties, GLP-2 analogs are useful in the management of disorders pertaining to a reduction of effective intestinal resorptive surface area, most prominently among patients with SBS-IF. In 2015, the European Society for Clinical Nutrition and Metabolism (ESPEN) has published recommendations on the “definition and classification of IF in adults” [39]. According to these recommendations, IF has been defined as “the reduction of gut function below the minimum necessary for the absorption of macronutrients and/or water and electrolytes, such that intravenous supplementation (IVS) is required to maintain health and/or growth.” In this definition, two parameters are a prerequisite for diagnosing IF: first, decreased absorption of macronutrients and second, necessity for IVS [39].
A “functional classification” of IF has also been proposed based upon onset of appearance and expected outcomes. (1) Type I: an acute, short-term, and usually self-limited condition. This is relatively common, occurring peri-operatively after abdominal surgery and in association with critical illness. Patients usually require IVS for a few days or weeks. (2) Type II: an acute condition with a prolonged course, often found in metabolically unstable patients, which requires multidisciplinary care and IVS for weeks or months. (3) Type III: a chronic status in metabolically stable patients, who require IVS for months or years. This could be reversible or irreversible [39].
Short Bowel Syndrome
SBS is defined as a remaining small bowel with a length in continuity of less than 200 cm in adults and less than 25% of the remaining small bowel that is expected according to age among pediatric patients [8]. Many pathological processes can lead to SBS, such as mesenteric ischemia, Crohn’s disease, radiation enteritis, other surgical complications, and familial polyposis in adults, while another spectrum of congenital or acquired causes, such as gastroschisis, intestinal atresia, midgut volvulus, extensive aganglionosis, or necrotizing enterocolitis, may present soon after birth in the pediatric population [40, 41].
Animal Studies
Since it was the first GLP-2 analog to be developed, data from animal studies on the effects of teduglutide treatment are considerably more abundant compared to glepaglutide and apraglutide. Table 1 depicts a detailed description of studies regarding GLP-2 analogs in animal models, mainly piglets and, to a lesser extent, mice. Evidently, GLP-2 analogs exhibit positive effects on intestinal growth and nutrient digestion and absorption, while some reports have documented the synergistic effects of EGF, KGF, and IGF-1 on mediating GLP-2 intestinotrophic properties and enhanced absorptive and adaptive mechanisms [42,43,44].
Of note, a shift of the intestinal epithelial cell morphology is noted after chronic GLP-2 analog treatment, resulting in thinner and more elongated epithelial cells [36, 45]. The microvilli on the apex of the epithelial cells constitute the main functional unit of the small intestine, harboring more than 22 digestive enzymes and 53 ion channels and nutrient transporters. In mice, teduglutide treatment has been related to increased epithelial paracellular pore function as well as augmented claudin-10 expression in tight junctions in the villus tips, where it is localized together with sodium-glucose co-transporter 1 (SGLT-1) [46]. It could be therefore speculated that the effects of GLP-2 may also facilitate sodium, glucose, and water absorption. These findings in animal models suggest the promotion of both intestinal growth and function with chronic GLP-2 analog treatment [47,48,49].
Human Studies
Table 2 presents a plethora of studies in humans regarding the use of GLP-2 analogs in pediatric as well as in adult populations with SBS. It is noteworthy that to date only teduglutide has FDA and EMA approval, which was granted for the treatment of SBS in adults in 2012 by both agencies [50]. FDA has approved teduglutide for pediatric patients aged > 1 year old in 2019 [51••].
Teduglutide has been demonstrated to increase microvilli length as well as crypt depth by approximately 50% among adult patients with SBS-IF, while its administration resulted in enhanced macronutrient and fluid absorption in the gut. In addition, it decreased the requirements for parenteral support (PS) by 1–2 days weekly, but most notably it led to complete weaning off PS in 20.5% of patients [50, 52].
Moreover, teduglutide therapy has been associated with an improved quality of life (QoL) among patients with SBS-IF [53].
The effect of teduglutide on PS needs among patients with SBS-IF with ≥ 3 days PS requirements for at least 12 month was examined in the randomized, placebo (PBO)-controlled STEPS study series. These included the original STEPS study, its 2-year, open-label extension, STEPS-2 and the STEPS-3, a 1-year, open-label extension study in patients who completed STEPS-2. Among patients who completed STEPS-2, 14 were enrolled in STEPS-3 and 13 completed STEPS-3 after having received teduglutide for a total of 42 months. Regarding the results of STEP-3, 8 of 14 patients had a ≥ 1 day while 6 of 14 patients had a ≥ 3-day reduction in weekly PS requirements. At the completion of the STEP-3 study, 4 patients were independent from PS [52]. Long-term teduglutide treatment exhibited a safety profile consistent with previous shorter-term studies, while there was sustained efficacy, and a further decline in PS needs over time [54].
Inflammatory Bowel Disorder Without Short Bowel Syndrome-Intestinal Failure
Crohn’s disease (CD) is a chronic inflammatory, immune-mediated disorder, which is characterized by focal, asymmetric, transmural inflammation at any part of the luminal GI tract. Its causes remain elusive, while it typically exhibits a variable clinical course. Genetic and environmental factors in concert with increased intestinal permeability, activation of the immune system and an enhanced inflammatory response are suggested to contribute to the development of CD [55,56,57]. The currently available medications for the treatment of patients with CD include aminosalicylates, corticosteroids, antibiotics, immunomodulators (thiopurines, methotrexate), anti-TNFa agents (infliximab, certolizumab pegol, adalimumab), anti-integrin therapy with vedolizumab or natalizumab, and lately anti-IL-12 and IL-23 therapy with ustekinumab. Treatment with biologic agents (anti-TNF-a, anti-integrins, anti-IL-12, and IL-23) is indicated for patients with moderate to severe CD, who do not respond to prior conventional therapy [58,59,60].
On account of their intestinotrophic properties as well as their positive effects on the intestinal barrier function, there has been growing interest regarding the therapeutic potential of GLP-2 analogs among patients with CD. Indeed, in a multicenter study, Buchman et al. examined the effects of teduglutide treatment among patients with moderate to severe CD, defined as a CD Activity Index (CDAI) of 220–450. Among the initially recruited 100 participants, 71 completed the study. Higher response and remission rates were reported in all teduglutide-treated groups (0.05 mg/kg/day, 0.10 mg/kg/day, and 0.20 mg/kg/day) as compared with placebo, while these positive effects were evident as early as from the second week of treatment in the highest dose (0.20 mg/kg/day) group (44% response and 32% remission vs. 32% response and 20% remission in the placebo group). Among subjects who did not achieve remission during the 8-week placebo-controlled phase in the higher-dose group, 50% achieved remission during the more prolonged, open-label treatment phase. Plasma citrulline levels, a biomarker of small bowel enterocyte mass and small bowel absorptive capacity [61], were steady across all groups at baseline, but increased substantially over time in all teduglutide-treated groups when compared with placebo at week 8. The authors concluded that teduglutide could be effective in inducing remission as well as mucosal healing among patients with moderate to severe CD. However, it should be noted that further studies are lacking, in particular with the combined use of GLP-2 analogs and agents already approved for this indication, which act by means of their anti-inflammatory and immune-modulatory properties [62•].
Chemotherapy or Radiation-Induced Enteritis Without SBS-IF
Available data on the potential utility of GLP-2 analogs in chemotherapy- and radiation-induced enteritis stem exclusively from preclinical studies. Tavakkolizadeh et al. have demonstrated that treatment with a GLP-2 analog for 3 consecutive days after administration of 5-FU in a mouse model results in increased body weight, villus length, and crypt depth, findings that are not observed in control mice not having received a GLP-2 analog [63]. Dong et al. have shown that irinotecan-induced enteritis in mice provoked intestinal epithelial barrier damage, which was reversible with the use of GLP-2 analog [20]. Pini et al. have reported that among mice receiving long-term cisplatin treatment, administration of a GLP-2 analog led to the amelioration of both the gastric fundus mucosal damage, by preventing the epithelium thickness decrease, and of cisplatin-induced neuropathy, by salvaging Nitric Oxide Synthetase (nNOS)-producing neurons [64]. Only recently, in 2020, Nardini et al. have documented that cisplatin-treated mice show alterations in their intestinal morphology, which are reversible by the administration of a GLP-2 analog [65••].
To date, there are only two published studies to address the efficacy of GLP-2 administration in animal models of radiation-induced enteritis. In these studies by Zhang et al. and Torres et al., it was demonstrated that administration of longer half-life GLP-2 analogs reduced the histological severity of both acute and chronic radiation-induced enteritis [66, 67].
Overall, although studies in humans are lacking, chemotherapy- as well as radiation-induced enteritis could be a new focus for GLP-2 analogs based on their promising results in animal models.
Dumping Syndrome
Dumping syndrome is a frequent debilitating complication of esophageal and gastric surgery, which is attributable to an accelerated gastric emptying (GE) following meal ingestion [68]. The fast delivery of undigested nutrients in the small bowel causes a fluid shift from the intravascular to the intestinal luminal compartment and induces a robust increased release of GI peptide hormones, resulting in GI and vasomotor symptoms (early dumping) and/or reactive hypoglycemia (late dumping) [69]. Despite the fact that the majority of patients with mild symptoms respond to dietary measures and nutritional counseling, a significant subgroup will still require medical treatment, particularly somatostatin analogs. Moreover, as the number of patients undergoing bariatric/metabolic surgery continues to rise owing to the increasing prevalence of obesity and related comorbidities, the occurrence of dumping syndrome is likewise expected to increase worldwide. Therefore, apart from somatostatin analogs, which act by delaying GE and have proven benefits regarding QoL in patients with dumping syndrome, novel therapeutic alternatives are mandatory to further enrich our armamentarium against dumping syndrome [70]. In this context, the combination of somatostatin analogs with GLP-2 or GLP-1 analogs would be very interesting.
Although GLP-2 and GLP-1 analogs act via different receptors, which account for their subsequent biological actions, they likely share similar properties regarding their diminishing effects on GI motility [70, 71]. This feature that GLP-2 and GLP-1 analogs share might imply a synergistic potential of these analogs which together with a somatostatin analog may be promising in the management of dumping syndrome. Nevertheless, it should be noted that although there are available data from isolated case reports and case series on the efficacy of GLP-1 receptor agonists for the management of dumping syndrome under certain clinical circumstances [72, 73], evidence for the potential utility of GLP-2 analogs for this indication are to date lacking.
Safety Concerns of the GLP-2 Analogs
Adverse side effects are not uncommon with GLP-2 analogs, but are usually mild, self-limited, and of gastrointestinal origin, such as abdominal pain, nausea, vomiting, GI stoma complications, and abdominal distension [54, 74]. The abovementioned abdominal adverse effects are similar to those typically seen in patients with SBS-IF treated with anti-diarrheal agents [75]. GI stoma complications are expected to occur with GLP-2 analogs, especially stoma nipple enlargement. Patients should be aware of this complication and instructed accordingly, in order to enlarge and properly adjust the hole in the stoma pad, thus, mitigating any discomfort by the protruding stoma nipple. In addition, injection site mild adverse effects, such as pain, pruritus, erythema, edema, and hematoma, have been reported. Leg edema or rarely generalized edema has also been observed, likely as a consequence of increased intestinal fluid absorption capacity, especially among patients with preexisting heart failure and in particular if PS is not discontinued timely. This complication is usually seen during the first 4 weeks of treatment with a GLP-2 analog, while its incidence recedes thereafter [76]. Serious adverse effects have been reported very rarely: there are reports of 3 cases of acute cholecystitis necessitating cholecystectomy, 2 cases of self-limited intestinal pseudo-obstruction among adults, and 1 case of ileus and 1 case of intestinal obstruction due to fecal impaction in pediatric populations [75, 77].
Nevertheless, the intestinotrophic properties of GLP-2 agonism may harbor the presumed risk of progression of pre-existing tumors in patients under long-term treatment with a GLP-2 analog. Therefore, according to teduglutide’s summary of product characteristics (spc), a colonoscopy with removal of polyps should be performed at the time of teduglutide treatment initiation, and yearly colonoscopic surveillance is recommended during the first 2 years of therapy. Subsequent colonoscopies are recommended at a minimum of 5 year’s interval. In case of occurrence of malignancy under treatment, teduglutide therapy should be discontinued. However, there have been no reports of colon tumorigenesis in human studies, whereas in rat carcinogenicity models, benign tumors in the small bowel and the extra-hepatic bile ducts have been documented.
Limitations of Studies
Studies regarding GLP-2 analogs are challenging to perform, as until today, they are mostly restricted to patients with SBS-IF, who may have limited access to special health care providers with expertise in SBS-IF. Therefore, patients with SBS-IF are rather scarce to find and enroll. This problem accounts for the majority of the human studies being multi-centered, which are often difficult to organize and conduct. In addition, even though the high cost of chronic GLP-2 analog treatment may seem to pose an obstacle against their use, the overall health care burden of SBS and its complications is likely considerably greater. In particular, the estimated cost of teduglutide is reported to be approximately $300,000/year/patient. Although this over-weighs the annual costs of SBS annual conservative treatment expenses (estimated to be as high as US $150,000 per patient, mainly on account of PS requirements), teduglutide is expected to offset some of the economic burden of SBS-IF patients, which has an overall estimated health care expenditure of up to $500,000/year/patient [78]. It is noteworthy that no relevant pharmaco-economic studies are yet available, while the burden of the patients with SBS-IF is likely much higher than currently estimated, as assessments are chiefly based upon reports from specialized centers to which only a minority of patients have access to, mainly due to their scarcity and limited patient information.
Conclusion
SBS-IF confers a considerable burden for the prognosis and QoL of affected patients, largely due to the continuous need for chronic PS. Additional supportive measures are of proven value, such as the promotion of adequate protein (especially as a source of glutamine) and caloric intake, bile acid and carbohydrate supplementation (starch and fiber), and anti-diarrheal agents and probably somatostatin analogs [79, 80]. Nevertheless, there is an imperative need for the development and availability of agents which may aid to the reduction, or even elimination of the need for PS. GLP-2 analogs seem to possess promising properties towards that end and may be currently the best candidates for the medicinal management of SBS-IF patients. GLP-2 agonism exerts a positive effect on intestinal nutrient absorption, although continued therapy is likely necessary for a sustained benefit. Therapy with GLP-2 analogs with subsequent reduction in PS needs has demonstrated benefits regarding the QoL of this laden patient population [81]. Based upon their GI tract-restricted actions and their overall good tolerability, these agents could be also effective as adjunct therapies in other GI disorders, such as moderate to severe IBD or chemotherapy/radiation-induced enteritis, especially in combination with other intestinal growth factors, such as IGF-1, EGF, and KGF [42, 75, 82]. Moreover, the need for daily sc administration which could avert a subset of patients may be overcome by the advent of novel, long-acting GLP-2 analogs which are already in the pipeline and may be suitable for once-weekly administration [26, 29]. Further pharmacological research on GLP-2 analogs will aim to improve their pharmacokinetic/pharmacodynamic properties, while more studies are mandatory regarding their usage alone or in combination with other agents for patients with severe GI disorders.
Abbreviations
- Anti-TNFα:
-
Anti-tumor necrosis factor-α
- Anti-IL12 and anti-IL23:
-
Anti-interleukin-12 and anti-interleukin-23
- c-AMP:
-
Cyclic adenosine monophosphate
- CD:
-
Crohn’s disease
- CDAI:
-
Crohn’s Disease Activity Index
- DNA:
-
Deoxy-ribonucleic acid
- DPP-4:
-
Dipeptidyl peptidase-4
- EGF:
-
Epidermal growth factor
- ESPEN:
-
European Society for Clinical Nutrition and Metabolism
- GE:
-
Gastric emptying
- GI:
-
Gastrointestinal
- GLP-2:
-
Glucagon-like peptide-2
- GLP-2R:
-
Glucagon-like peptide 2 receptor
- GIP:
-
Glucose-dependent insulinotropic polypeptide
- GRPP:
-
Glicentin-related pancreatic peptide
- IBD:
-
Inflammatory bowel disease
- IGF-1:
-
Insulin-like growth factor-1
- IVS:
-
Intravenous supplementation
- KGF:
-
Keratinocyte growth factor
- nNOS:
-
Neuronal nitric oxide synthase
- PC:
-
Pro-hormone convertase
- PGDP:
-
Pro-glucagon-derived peptide
- PKA:
-
Protein kinase A
- PS:
-
Parenteral support
- QoL:
-
Quality of life
- SBS-IF:
-
Short bowel syndrome-intestinal failure
- SC:
-
Subcutaneous
- SGLT-1:
-
Sodium-glucose transporter-1
- T2DM:
-
Diabetes mellitus type 2
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Seige K. Glucagon, its discovery and description and the work of Max Burger. Z Gesamte Inn Med. 1986;41:568–71.
Bromer WW, Sinn LG, Staub A, Behrens OK. The amino acid sequence of glucagon. Diabetes. 1957;6:234–8. https://doi.org/10.2337/diab.6.3.234.
Lafferty RA, O’Harte FPM, Irwin N, Gault VA, Flatt PR. Proglucagon-derived peptides as therapeutics. Front Endocrinol (Lausanne). 2021;12: 689678. https://doi.org/10.3389/fendo.2021.689678.
Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143:559–69. https://doi.org/10.7326/0003-4819-143-8-200510180-00006.
Sjolund K, Sanden G, Hakanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–30.
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. https://doi.org/10.1053/j.gastro.2007.03.054.
Kuhre RE, Deacon CF, Holst JJ, Petersen N. What is an L-cell and how do we study the secretory mechanisms of the L-cell? Front Endocrinol (Lausanne). 2021;12: 694284. https://doi.org/10.3389/fendo.2021.694284.
Billiauws L, Joly F. Emerging treatments for short bowel syndrome in adult patients. Expert Rev Gastroenterol Hepatol. 2019;13:241–6. https://doi.org/10.1080/17474124.2019.1569514.
Austin K, Markovic MA, Brubaker PL. Current and potential therapeutic targets of glucagon-like peptide-2. Curr Opin Pharmacol. 2016;31:13–8. https://doi.org/10.1016/j.coph.2016.08.008.
Berkowitz DE, Steenbergen C, O’Rourke B. Hibernating squirrels: SIRTin clues for organ protection after ischemia-reperfusion. Anesthesiology. 2016;124:1215–7. https://doi.org/10.1097/ALN.0000000000001114.
Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol. 2014;76:561–83. https://doi.org/10.1146/annurev-physiol-021113-170317.
Rowland KJ, Brubaker PL. The “cryptic” mechanism of action of glucagon-like peptide-2. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1-8. https://doi.org/10.1152/ajpgi.00039.2011.
Yusta B, Holland D, Koehler JA, Maziarz M, Estall JL, Higgins R, et al. ErbB signaling is required for the proliferative actions of GLP-2 in the murine gut. Gastroenterology. 2009;137:986–96. https://doi.org/10.1053/j.gastro.2009.05.057.
Austin K, Imam NA, Pintar JE, Brubaker PL. IGF binding protein-4 is required for the growth effects of glucagon-like peptide-2 in murine intestine. Endocrinology. 2015;156:429–36. https://doi.org/10.1210/en.2014-1829.
Van Landeghem L, Santoro MA, Mah AT, Krebs AE, Dehmer JJ, McNaughton KK, et al. IGF1 stimulates crypt expansion via differential activation of 2 intestinal stem cell populations. FASEB J. 2015;29:2828–42. https://doi.org/10.1096/fj.14-264010.
Iturrino J, Camilleri M, Acosta A, O’Neill J, Burton D, Edakkanambeth Varayil J, et al. Acute effects of a glucagon-like peptide 2 analogue, teduglutide, on gastrointestinal motor function and permeability in adult patients with short bowel syndrome on home parenteral nutrition. JPEN J Parenter Enteral Nutr. 2016;40:1089–95. https://doi.org/10.1177/0148607115597644.
Bremholm L, Hornum M, Andersen UB, Hartmann B, Holst JJ, Jeppesen PB. The effect of glucagon-like peptide-2 on mesenteric blood flow and cardiac parameters in end-jejunostomy short bowel patients. Regul Pept. 2011;168:32–8. https://doi.org/10.1016/j.regpep.2011.03.003.
Berg JK, Kim EH, Li B, Joelsson B, Youssef NN. A randomized, double-blind, placebo-controlled, multiple-dose, parallel-group clinical trial to assess the effects of teduglutide on gastric emptying of liquids in healthy subjects. BMC Gastroenterol. 2014;14:25. https://doi.org/10.1186/1471-230X-14-25.
Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–103. https://doi.org/10.1136/gut.2008.165886.
Dong CX, Zhao W, Solomon C, Rowland KJ, Ackerley C, Robine S, et al. The intestinal epithelial insulin-like growth factor-1 receptor links glucagon-like peptide-2 action to gut barrier function. Endocrinology. 2014;155:370–9. https://doi.org/10.1210/en.2013-1871.
Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1–44. https://doi.org/10.1016/s0079-6107(02)00037-8.
Hartmann B, Harr MB, Jeppesen PB, Wojdemann M, Deacon CF, Mortensen PB, et al. In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J Clin Endocrinol Metab. 2000;85:2884–8. https://doi.org/10.1210/jcem.85.8.6717.
Drucker DJ, Shi Q, Crivici A, Sumner-Smith M, Tavares W, Hill M, et al. Regulation of the biological activity of glucagon-like peptide 2 in vivo by dipeptidyl peptidase IV. Nat Biotechnol. 1997;15:673–7. https://doi.org/10.1038/nbt0797-673.
Ferrone M, Scolapio JS. Teduglutide for the treatment of short bowel syndrome. Ann Pharmacother. 2006;40:1105–9. https://doi.org/10.1345/aph.1G419.
Naimi RM, Hvistendahl M, Enevoldsen LH, Madsen JL, Fuglsang S, Poulsen SS, et al. Glepaglutide, a novel long-acting glucagon-like peptide-2 analogue, for patients with short bowel syndrome: a randomised phase 2 trial. Lancet Gastroenterol Hepatol. 2019;4:354–63. https://doi.org/10.1016/S2468-1253(19)30077-9.
Eliasson J, Hvistendahl MK, Freund N, Bolognani F, Meyer C, Jeppesen PB. Apraglutide, a novel glucagon-like peptide-2 analog, improves fluid absorption in patients with short bowel syndrome intestinal failure: findings from a placebo-controlled, randomized phase 2 trial. JPEN J Parenter Enteral Nutr. 2021. https://doi.org/10.1002/jpen.2223.
Kocoshis SA, Merritt RJ, Hill S, Protheroe S, Carter BA, Horslen S, et al. Safety and efficacy of teduglutide in pediatric patients with intestinal failure due to short bowel syndrome: a 24-week, phase III study. JPEN J Parenter Enteral Nutr. 2020;44:621–31. https://doi.org/10.1002/jpen.1690.
Hvistendahl MK, Naimi RM, Enevoldsen LH, Madsen JL, Fuglsang S, Jeppesen PB. Effect of glepaglutide, a long-acting glucagon-like peptide-2 analog, on gastrointestinal transit time and motility in patients with short bowel syndrome: findings from a randomized trial. JPEN J Parenter Enteral Nutr. 2020;44:1535–44. https://doi.org/10.1002/jpen.1767.
Hargrove DM, Alagarsamy S, Croston G, Laporte R, Qi S, Srinivasan K, et al. Pharmacological characterization of apraglutide, a novel long-acting peptidic glucagon-like peptide-2 agonist, for the treatment of short bowel syndrome. J Pharmacol Exp Ther. 2020;373:193–203. https://doi.org/10.1124/jpet.119.262238.
Cheeseman CI, Tsang R. The effect of GIP and glucagon-like peptides on intestinal basolateral membrane hexose transport. Am J Physiol. 1996;271:G477–82. https://doi.org/10.1152/ajpgi.1996.271.3.G477.
Brubaker PL. Glucagon-like peptide-2 and the regulation of intestinal growth and function. Compr Physiol. 2018;8:1185–210. https://doi.org/10.1002/cphy.c170055.
Dahly EM, Gillingham MB, Guo Z, Murali SG, Nelson DW, Holst JJ, et al. Role of luminal nutrients and endogenous GLP-2 in intestinal adaptation to mid-small bowel resection. Am J Physiol Gastrointest Liver Physiol. 2003;284:G670–82. https://doi.org/10.1152/ajpgi.00293.2002.
Dash S, Xiao C, Morgantini C, Connelly PW, Patterson BW, Lewis GF. Glucagon-like peptide-2 regulates release of chylomicrons from the intestine. Gastroenterology. 2014;147:1275–84 e4. https://doi.org/10.1053/j.gastro.2014.08.037.
Schwartz LK, O’Keefe SJ, Fujioka K, Gabe SM, Lamprecht G, Pape UF, et al. Long-term teduglutide for the treatment of patients with intestinal failure associated with short bowel syndrome. Clin Transl Gastroenterol. 2016;7: e142. https://doi.org/10.1038/ctg.2015.69.
Lee SJ, Lee J, Li KK, Holland D, Maughan H, Guttman DS, et al. Disruption of the murine Glp2r impairs Paneth cell function and increases susceptibility to small bowel enteritis. Endocrinology. 2012;153:1141–51. https://doi.org/10.1210/en.2011-1954.
Benjamin MA, McKay DM, Yang PC, Cameron H, Perdue MH. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut. 2000;47:112–9. https://doi.org/10.1136/gut.47.1.112.
Sigalet DL, de Heuvel E, Wallace L, Bulloch E, Turner J, Wales PW, et al. Effects of chronic glucagon-like peptide-2 therapy during weaning in neonatal pigs. Regul Pept. 2014;188:70–80. https://doi.org/10.1016/j.regpep.2013.12.006.
Zaczek Z, Jurczak-Kobus P, Panczyk M, Braszczynska-Sochacka J, Majewska K, Kunecki M, et al. Changes in parenteral nutrition requirements and BMI in patients with parenteral nutrition-dependent short bowel syndrome after stopping teduglutide-9 years of follow-up. Nutrients. 2022;14. https://doi.org/10.3390/nu14081634.
Pironi L, Arends J, Baxter J, Bozzetti F, Pelaez RB, Cuerda C, et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015;34:171–80. https://doi.org/10.1016/j.clnu.2014.08.017.
Billiauws L, Corcos O, Joly F. What’s new in short bowel syndrome? Curr Opin Clin Nutr Metab Care. 2018;21:313–8. https://doi.org/10.1097/MCO.0000000000000473.
Goulet O, Abi Nader E, Pigneur B, Lambe C. Short bowel syndrome as the leading cause of intestinal failure in early life: some insights into the management. Pediatr Gastroenterol Hepatol Nutr. 2019;22:303–29. https://doi.org/10.5223/pghn.2019.22.4.303.
Lim DW, Levesque CL, Vine DF, Muto M, Koepke JR, Nation PN, et al. Synergy of glucagon-like peptide-2 and epidermal growth factor coadministration on intestinal adaptation in neonatal piglets with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2017;312:G390–404. https://doi.org/10.1152/ajpgi.00281.2016.
Rowland KJ, Trivedi S, Lee D, Wan K, Kulkarni RN, Holzenberger M, et al. Loss of glucagon-like peptide-2-induced proliferation following intestinal epithelial insulin-like growth factor-1-receptor deletion. Gastroenterology. 2011;141:2166–75 e7. https://doi.org/10.1053/j.gastro.2011.09.014.
Burrin DG, Stoll B, Guan X, Cui L, Chang X, Hadsell D. GLP-2 rapidly activates divergent intracellular signaling pathways involved in intestinal cell survival and proliferation in neonatal piglets. Am J Physiol Endocrinol Metab. 2007;292:E281–91. https://doi.org/10.1152/ajpendo.00129.2006.
Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O’Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902–14. https://doi.org/10.1136/gut.2010.218271.
Reiner J, Berlin P, Wobar J, Schaffler H, Bannert K, Bastian M, et al. Teduglutide promotes epithelial tight junction pore function in murine short bowel syndrome to alleviate intestinal insufficiency. Dig Dis Sci. 2020;65:3521–37. https://doi.org/10.1007/s10620-020-06140-6.
Pauline ML, Nation PN, Wizzard PR, Hinchliffe T, Wu T, Dimitriadou V, et al. Comparing the intestinotrophic effects of 2 glucagon-like peptide-2 analogues in the treatment of short-bowel syndrome in neonatal piglets. JPEN J Parenter Enteral Nutr. 2021;45:538–45. https://doi.org/10.1002/jpen.1853.
Slim GM, Lansing M, Wizzard P, Nation PN, Wheeler SE, Brubaker PL, et al. Novel long-acting GLP-2 analogue, FE 203799 (apraglutide), enhances adaptation and linear intestinal growth in a neonatal piglet model of short bowel syndrome with total resection of the ileum. JPEN J Parenter Enteral Nutr. 2019;43:891–8. https://doi.org/10.1002/jpen.1500.
Suri M, Turner JM, Sigalet DL, Wizzard PR, Nation PN, Ball RO, et al. Exogenous glucagon-like peptide-2 improves outcomes of intestinal adaptation in a distal-intestinal resection neonatal piglet model of short bowel syndrome. Pediatr Res. 2014;76:370–7. https://doi.org/10.1038/pr.2014.97.
Burness CB, McCormack PL. Teduglutide: a review of its use in the treatment of patients with short bowel syndrome. Drugs. 2013;73:935–47. https://doi.org/10.1007/s40265-013-0070-y.
•• Rosete BE, Wendel D, Horslen SP. Teduglutide for pediatric short bowel syndrome patients. Expert Rev Gastroenterol Hepatol. 2021;15:727–33. https://doi.org/10.1080/17474124.2021.1913052. Expert review regarding the usefulness of teduglytide for short bowel syndrome in pediatric patients.
Seidner DL, Gabe SM, Lee HM, Olivier C, Jeppesen PB. Enteral autonomy and days off parenteral support with teduglutide treatment for short bowel syndrome in the STEPS trials. JPEN J Parenter Enteral Nutr. 2020;44:697–702. https://doi.org/10.1002/jpen.1687.
Chen K, Mu F, Xie J, Kelkar SS, Olivier C, Signorovitch J, et al. Impact of teduglutide on quality of life among patients with short bowel syndrome and intestinal failure. JPEN J Parenter Enteral Nutr. 2020;44:119–28. https://doi.org/10.1002/jpen.1588.
Pape UF, Iyer KR, Jeppesen PB, Kunecki M, Pironi L, Schneider SM, et al. Teduglutide for the treatment of adults with intestinal failure associated with short bowel syndrome: pooled safety data from four clinical trials. Therap Adv Gastroenterol. 2020;13:1756284820905766. https://doi.org/10.1177/1756284820905766.
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. https://doi.org/10.1056/NEJMra020831.
Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–7. https://doi.org/10.1136/gut.2005.065557.
D’Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–7. https://doi.org/10.1016/S0140-6736(08)60304-9.
Blonski W, Buchner AM, Aberra F, Lichtenstein G. Teduglutide in Crohn’s disease. Expert Opin Biol Ther. 2013;13:1207–14. https://doi.org/10.1517/14712598.2013.815721.
Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–60. https://doi.org/10.1056/NEJMoa1602773.
Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–21. https://doi.org/10.1056/NEJMoa1215739.
Fragkos KC, Forbes A. Citrulline as a marker of intestinal function and absorption in clinical settings: a systematic review and meta-analysis. United European Gastroenterol J. 2018;6:181–91. https://doi.org/10.1177/2050640617737632.
• Buchman AL, Katz S, Fang JC, Bernstein CN, Abou-Assi SG, Teduglutide Study G. Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn’s disease. Inflamm Bowel Dis. 2010;16:962–73. https://doi.org/10.1002/ibd.21117. This is the first manuscript regarding the therapeutic potential of GLP-2 analogs among patients with severe Crohn’s disease.
Tavakkolizadeh A, Shen R, Abraham P, Kormi N, Seifert P, Edelman ER, et al. Glucagon-like peptide 2: a new treatment for chemotherapy-induced enteritis. J Surg Res. 2000;91:77–82. https://doi.org/10.1006/jsre.2000.5917.
Pini A, Garella R, Idrizaj E, Calosi L, Baccari MC, Vannucchi MG. Glucagon-like peptide 2 counteracts the mucosal damage and the neuropathy induced by chronic treatment with cisplatin in the mouse gastric fundus. Neurogastroenterol Motil. 2016;28:206–16. https://doi.org/10.1111/nmo.12712.
•• Nardini P, Pini A, Bessard A, Duchalais E, Niccolai E, Neunlist M, et al. GLP-2 prevents neuronal and glial changes in the distal colon of mice chronically treated with cisplatin. Int J Mol Sci. 2020;21. https://doi.org/10.3390/ijms21228875. This report highlights the therapeutic potential of GLP-2 analogs in a rodent model of chemotherapy-induced gastrointestinal damage.
Zhang T, Shi L, Xu Y, Li Y, Li S, Guan B, et al. Purified PEGylated human glucagon-like peptide-2 reduces the severity of irradiation-induced acute radiation enteritis in rats. J Radiat Res. 2019;60:7–16. https://doi.org/10.1093/jrr/rry076.
Torres S, Thim L, Milliat F, Vozenin-Brotons MC, Olsen UB, Ahnfelt-Ronne I, et al. Glucagon-like peptide-2 improves both acute and late experimental radiation enteritis in the rat. Int J Radiat Oncol Biol Phys. 2007;69:1563–71. https://doi.org/10.1016/j.ijrobp.2007.08.051.
Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol. 2009;6:583–90. https://doi.org/10.1038/nrgastro.2009.148.
Wauters L, Vanuytsel T. Applications of peptide hormone ligands for the treatment of dumping and short bowel syndrome. Curr Opin Pharmacol. 2018;43:118–23. https://doi.org/10.1016/j.coph.2018.09.005.
Scarpellini E, Arts J, Karamanolis G, Laurenius A, Siquini W, Suzuki H, et al. International consensus on the diagnosis and management of dumping syndrome. Nat Rev Endocrinol. 2020;16:448–66. https://doi.org/10.1038/s41574-020-0357-5.
van Furth AM, de Heide LJM, Emous M, Veeger N, van Beek AP. Dumping syndrome and postbariatric hypoglycemia: supporting evidence for a common etiology. Surg Obes Relat Dis. 2021;17:1912–8. https://doi.org/10.1016/j.soard.2021.05.020.
Abrahamsson N, Engstrom BE, Sundbom M, Karlsson FA. GLP1 analogs as treatment of postprandial hypoglycemia following gastric bypass surgery: a potential new indication? Eur J Endocrinol. 2013;169:885–9. https://doi.org/10.1530/EJE-13-0504.
Ding B, Hu Y, Yuan L, Yan RN, Ma JH. Effectiveness of beinaglutide in a patient with late dumping syndrome after gastrectomy: a case report. Medicine (Baltimore). 2021;100: e26086. https://doi.org/10.1097/MD.0000000000026086.
Marier JF, Beliveau M, Mouksassi MS, Shaw P, Cyran J, Kesavan J, et al. Pharmacokinetics, safety, and tolerability of teduglutide, a glucagon-like peptide-2 (GLP-2) analog, following multiple ascending subcutaneous administrations in healthy subjects. J Clin Pharmacol. 2008;48:1289–99. https://doi.org/10.1177/0091270008320605.
Jeppesen PB, Gabe SM, Seidner DL, Lee HM, Olivier C. Factors associated with response to teduglutide in patients with short-bowel syndrome and intestinal failure. Gastroenterology. 2018;154:874–85. https://doi.org/10.1053/j.gastro.2017.11.023.
Jeppesen PB, Pertkiewicz M, Messing B, Iyer K, Seidner DL, O'Keefe S J, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143:1473–81 e3. https://doi.org/10.1053/j.gastro.2012.09.007.
Hill S, Carter BA, Cohran V, Horslen S, Kaufman SS, Kocoshis SA, et al. Safety findings in pediatric patients during long-term treatment with teduglutide for short-bowel syndrome-associated intestinal failure: pooled analysis of 4 clinical studies. JPEN J Parenter Enteral Nutr. 2021;45:1456–65. https://doi.org/10.1002/jpen.2061.
Billiauws L, Bataille J, Boehm V, Corcos O, Joly F. Teduglutide for treatment of adult patients with short bowel syndrome. Expert Opin Biol Ther. 2017;17:623–32. https://doi.org/10.1080/14712598.2017.1304912.
Carroll RE, Benedetti E, Schowalter JP, Buchman AL. Management and complications of short bowel syndrome: an updated review. Curr Gastroenterol Rep. 2016;18:40. https://doi.org/10.1007/s11894-016-0511-3.
Jeppesen PB. The novel use of peptide analogs in short bowel syndrome. Expert Rev Gastroenterol Hepatol. 2013;7:197–9. https://doi.org/10.1586/egh.13.2.
Jeppesen PB, Pertkiewicz M, Forbes A, Pironi L, Gabe SM, Joly F, et al. Quality of life in patients with short bowel syndrome treated with the new glucagon-like peptide-2 analogue teduglutide–analyses from a randomised, placebo-controlled study. Clin Nutr. 2013;32:713–21. https://doi.org/10.1016/j.clnu.2013.03.016.
Bortvedt SF, Lund PK. Insulin-like growth factor 1: common mediator of multiple enterotrophic hormones and growth factors. Curr Opin Gastroenterol. 2012;28:89–98. https://doi.org/10.1097/MOG.0b013e32835004c6.
Tsai CH, Hill M, Drucker DJ. Biological determinants of intestinotrophic properties of GLP-2 in vivo. Am J Physiol. 1997;272:G662–8. https://doi.org/10.1152/ajpgi.1997.272.3.G662.
Brubaker PL, Izzo A, Hill M, Drucker DJ. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol. 1997;272:E1050–8. https://doi.org/10.1152/ajpendo.1997.272.6.E1050.
Litvak DA, Hellmich MR, Evers BM, Banker NA, Townsend CM Jr. Glucagon-like peptide 2 is a potent growth factor for small intestine and colon. J Gastrointest Surg. 1998;2:146–50. https://doi.org/10.1016/s1091-255x(98)80005-x.
Scott RB, Kirk D, MacNaughton WK, Meddings JB. GLP-2 augments the adaptive response to massive intestinal resection in rat. Am J Physiol. 1998;275:G911–21. https://doi.org/10.1152/ajpgi.1998.275.5.G911.
Burrin DG, Stoll B, Jiang R, Petersen Y, Elnif J, Buddington RK, et al. GLP-2 stimulates intestinal growth in premature TPN-fed pigs by suppressing proteolysis and apoptosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1249–56. https://doi.org/10.1152/ajpgi.2000.279.6.G1249.
Guan X, Stoll B, Lu X, Tappenden KA, Holst JJ, Hartmann B, et al. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology. 2003;125:136–47. https://doi.org/10.1016/s0016-5085(03)00667-x.
Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2004;286:G964–72. https://doi.org/10.1152/ajpgi.00509.2003.
Washizawa N, Gu LH, Gu L, Openo KP, Jones DP, Ziegler TR. Comparative effects of glucagon-like peptide-2 (GLP-2), growth hormone (GH), and keratinocyte growth factor (KGF) on markers of gut adaptation after massive small bowel resection in rats. JPEN J Parenter Enteral Nutr. 2004;28:399–409. https://doi.org/10.1177/0148607104028006399.
Burrin DG, Stoll B, Guan X, Cui L, Chang X, Holst JJ. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology. 2005;146:22–32. https://doi.org/10.1210/en.2004-1119.
Cottrell JJ, Stoll B, Buddington RK, Stephens JE, Cui L, Chang X, et al. Glucagon-like peptide-2 protects against TPN-induced intestinal hexose malabsorption in enterally refed piglets. Am J Physiol Gastrointest Liver Physiol. 2006;290:G293-300. https://doi.org/10.1152/ajpgi.00275.2005.
Sigalet DL, Bawazir O, Martin GR, Wallace LE, Zaharko G, Miller A, et al. Glucagon-like peptide-2 induces a specific pattern of adaptation in remnant jejunum. Dig Dis Sci. 2006;51:1557–66. https://doi.org/10.1007/s10620-006-9077-5.
Vegge A, Thymann T, Lund P, Stoll B, Bering SB, Hartmann B, et al. Glucagon-like peptide-2 induces rapid digestive adaptation following intestinal resection in preterm neonates. Am J Physiol Gastrointest Liver Physiol. 2013;305:G277–85. https://doi.org/10.1152/ajpgi.00064.2013.
Jeppesen PB, Sanguinetti EL, Buchman A, Howard L, Scolapio JS, Ziegler TR, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54:1224–31. https://doi.org/10.1136/gut.2004.061440.
O’Keefe SJ, Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B. Safety and efficacy of teduglutide after 52 weeks of treatment in patients with short bowel intestinal failure. Clin Gastroenterol Hepatol. 2013;11(815–23):e1-3. https://doi.org/10.1016/j.cgh.2012.12.029.
Tappenden KA, Edelman J, Joelsson B. Teduglutide enhances structural adaptation of the small intestinal mucosa in patients with short bowel syndrome. J Clin Gastroenterol. 2013;47:602–7. https://doi.org/10.1097/MCG.0b013e3182828f57.
Carter BA, Cohran VC, Cole CR, Corkins MR, Dimmitt RA, Duggan C, et al. Outcomes from a 12-week, open-label, multicenter clinical trial of teduglutide in pediatric short bowel syndrome. J Pediatr. 2017;181:102–11 e5. https://doi.org/10.1016/j.jpeds.2016.10.027.
Naimi RM, Hvistendahl M, Nerup N, Ambrus R, Achiam MP, Svendsen LB, et al. Effects of glepaglutide, a novel long-acting glucagon-like peptide-2 analogue, on markers of liver status in patients with short bowel syndrome: findings from a randomised phase 2 trial. EBioMedicine. 2019;46:444–51. https://doi.org/10.1016/j.ebiom.2019.07.016.
Joly F, Seguy D, Nuzzo A, Chambrier C, Beau P, Poullenot F, et al. Six-month outcomes of teduglutide treatment in adult patients with short bowel syndrome with chronic intestinal failure: a real-world French observational cohort study. Clin Nutr. 2020;39:2856–62. https://doi.org/10.1016/j.clnu.2019.12.019.
Jeppesen PB, Gabe SM, Seidner DL, Lee HM, Olivier C. Citrulline correlations in short bowel syndrome-intestinal failure by patient stratification: analysis of 24 weeks of teduglutide treatment from a randomized controlled study. Clin Nutr. 2020;39:2479–86. https://doi.org/10.1016/j.clnu.2019.11.001.
Ramos Boluda E, Redecillas Ferreiro S, Manrique Moral O, Garcia Romero R, Irastorza Terradillos I, Nunez Ramos R, et al. Experience with teduglutide in pediatric short bowel syndrome: first real-life data. J Pediatr Gastroenterol Nutr. 2020;71:734–9. https://doi.org/10.1097/MPG.0000000000002899.
Hvistendahl MK, Naimi RM, Hansen SH, Rehfeld JF, Kissow H, Pedersen J, et al. Bile acid-farnesoid X receptor-fibroblast growth factor 19 axis in patients with short bowel syndrome: the randomized, glepaglutide phase 2 trial. JPEN J Parenter Enteral Nutr. 2021. https://doi.org/10.1002/jpen.2224.
Solar H, Doeyo M, Ortega M, De Barrio S, Olano E, Moreira E, et al. Postsurgical intestinal rehabilitation using semisynthetic glucagon-like peptide-2 analogue (sGLP-2) at a referral center: can patients achieve parenteral nutrition and sGLP-2 independency? JPEN J Parenter Enteral Nutr. 2021;45:1072–82. https://doi.org/10.1002/jpen.1983.
Author information
Authors and Affiliations
Contributions
All authors meet authorship criteria. N.G. Vallianou and M. Dalamaga developed the concept of the manuscript. Literature research was carried out by D. Kounatidis, N.G. Vallianou, and D. Tsilingiris. D. Kounatidis, N.G. Vallianou, D. Tsilingiris, G.S. Christodoulatos, and T. Stratigou wrote the draft. Artwork was prepared by G.S. Christodoulatos. E. Geladari, I. Karampela, and M. Dalamaga edited and reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Diabetes and Obesity
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kounatidis, D., Vallianou, N.G., Tsilingiris, D. et al. Therapeutic Potential of GLP-2 Analogs in Gastrointestinal Disorders: Current Knowledge, Nutritional Aspects, and Future Perspectives. Curr Nutr Rep 11, 618–642 (2022). https://doi.org/10.1007/s13668-022-00433-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-022-00433-0