Abstract
This article presents archaeometallurgical research of three types of metal objects excavated underwater from two shipwrecks in Israel: Tantura F (mid-seventh-end of eighth centuries ad) and Akko 1 (first third of nineteenth century). Both non-destructive and destructive methods were employed. The finds were manufactured by joining processes; therefore, the studies concentrated on metallurgical processes. However, these researches were multidisciplinary, combining typological analyses of the archeological objects, as well as the historical perspective. The first case study is of an iron anchor from the Tantura F shipwreck. This anchor has a typical heterogeneous wrought iron microstructure of ferrite–pearlite–cementite and Widmanstätten plates, manufactured from several blooms made by the direct process. The blooms were joined using forge-welding by an expert blacksmith, resulting in a high-quality iron product. The blooms used in the anchor’s circular cross-section shank were forge-welded from iron and steel pieces, producing a composite material with superior mechanical properties. The second case study presents a 12-pdr cannonball from the Akko 1 shipwreck. The cannonball was manufactured from high-quality wrought iron, with a homogenous microstructure of iron matrix and rather large equiaxed α-ferrite grains, produced by an indirect technique, using the hot-forge-welding process. As its production technique pre-dates that of the ship, it is suggested that this cannonball was manufactured in a different place and by a different technology from the other cannonballs found in the shipwreck. It is also possible that the 12-pdr cannonball might have been used as ballast. The third case study deals with brass cases from the Akko 1 shipwreck. The cases were made of brass containing equiaxed α-brass grains with twins, manufactured from rolled sheets that may have originated in Great Britain. The parts were joined by soldering with tin–lead alloy, and it is suggested that the cases were made in an Egyptian workshop.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Archaeometallurgical studies of ancient metal objects manufactured by joining techniques have been carried out [1–4]; however, it is rare to find a broad review on the subject. It is even more challenging to review joining techniques of metal objects retrieved from shipwrecks which have lain for centuries on the seabed. In the current study, modern techniques have been used to provide information regarding the technologies of joining of metal artifacts retrieved from shipwrecks along the Mediterranean coast of Israel. The information gathered includes microstructure and composition of the various metals, and provides essential data concerning the manufacturing processes. The metallurgical research combines typological studies of the archeological finds and their historical contexts, illuminating the technological abilities of the societies of Late Antiquity and the Industrial Revolution. This multidisciplinary approach assists in dating shipwrecks, providing clues to their origin, and contributes to unraveling the mysteries surrounding the wrecking events.
Ancient Wrought Iron and Forge-Welding Techniques
Iron is one of the most common elements in Earth, and the second metallic element in the Earth’s crust; yet it appeared later in the history of mankind than less abundant non-ferrous metals (such as gold, silver, copper, tin, and lead), due to its complicated smelting techniques [5]. Production of ancient iron was made by smelting the ore in a reducing atmosphere at temperatures below the melting point of pure iron (1538 °C). This direct process is known as ‘bloomery’ [6]. In comparison to the more meltable and castable non-ferrous metals, it was unavoidable that a solid-state technology was developed for iron and its alloys, due to the limited performance of early furnaces (with the maximum temperature obtainable of 1200 °C) [5, 7].
When a chemical reaction occurs, the evaluation of the spontaneity of the reaction is estimated from the change of the overall free energy. The illustration of the standard Gibbs free energy change needed for a metal oxide formation as a function of the temperature is known as an Ellingham diagram; and the thermochemical conditions for the reduction of iron oxides are presented by the well-known Chaudron diagram and the Boudouard Fe–C–O equilibrium curve [5, 6, 8]. Based on the Ellingham diagram, it was concluded that haematite (Fe2O3) is reduced to magnetite (Fe3O4), wüstite (FeO), and metallic iron. The reduction process is driven by carbon, but since a solid–solid reaction has extremely low kinetics, carbon monoxide (CO) is the actual reducing agent, and the chemical reactions are 3Fe2O3 + CO ⇔ 2Fe3O4 + CO2, Fe3O4 + CO ⇔ 3FeO + CO2, FeO + CO ⇔ Fe + CO2 [5].
When high-quality haematite ores were used, superior iron metal was achieved, containing only minor quantities of unwanted slag and impurities. The use of poor quality ores resulted in the production of iron with relatively large amounts of slag inclusions [5]. The iron sponge (‘bloom’) was then hot worked to force the slag remains out of the bulk iron, and to reduce the amount of slag inclusions in the iron matrix, consolidating the metal into a denser and more manageable material. In order to obtain solid iron ingots, innovative technologies of hot working and forge-welding had to be developed. Solid-state joining of two different ferrous alloys has been used since the beginning of the 1st millennium bc [9]. A forge-welded cutting tool, found at Al-Mina, a Greek trading colony located on the south-eastern coast of Turkey, was made about 400 bc [9, 10]. Merovingian blades were manufactured in Europe from the end of the second century ad by forge-welding strips of unalloyed iron and carbon steel alloy. The strips were forged (hammered) together in a technique involving folding (or twisting). In the period around 500 ad, iron to steel pattern-welded daggers and swords were produced, including Viking blades in about 600 ad [9].
The product was wrought iron with an average carbon content of 0.1 wt% [11, 12]. In some direct reduction processes, high carbon wrought iron containing 0.4–1.5 wt% carbon was obtained (depending on temperature and time). However, these products were not defined as ‘steels’, because they were made of heterogeneous iron produced by direct reduction and not by cementation [6]. The metal was quite pure, containing minor metallic impurities, since oxides of phosphorus, silicon, and manganese were not reduced at the temperatures reached in the direct reduction process, but it did contain some preferentially oriented slag inclusion remains, as shown in Fig. 1(a) [6, 12]. The typical ancient bloom was small, due to furnace limitations, and in order to shape large objects, several blooms of a few kilograms each were joined together by forge-welding [13, 14].
Schematic illustration of: (a) equiaxed α-ferritic grains and few preferred oriented non-metallic slag inclusions; and (b) two equiaxed α-ferritic layers and the welding zone between them, characterized by a finer grain size and higher volume fraction of preferred oriented inclusions, parallel to the joint line
Secondary smithing was the process in which the metallic bar or billet produced during the primary smithing was shaped into a finished product by repeatedly heating the iron in a brick or stone hearth and hammering it on an anvil. In order to weld two or more pieces of iron together, it was essential that the metal was first heated to a temperature at which it was soft but not molten (in the austenitic phase). It was also essential to have a clean metal surface to allow diffusion bonding [15]. The welding zone between two different iron layers of dissimilar compositions is characterized by a higher volume fraction of the non-metallic inclusions and a finer grain size than in the main body of the metal. This is not the case when the compositions of the layers are similar [2, 3, 16]. When two or more strips are forge-welded together, the oxides are trapped between them, forming a relatively continuous layer [17].
When alignments of elongated precipitates and/or round inclusions are observed in the microstructure, they may reveal the track of the ancient weld [15]. Chemical analysis, combined with identification of wüstite and fayalite inclusion particles, would be the preferred way to determine whether an object was made from a single bloom or several different blooms [13, 14]. The forge-welded zones are quite often distinguished by the presence of yellowish ghost lines 10–20 μm wide between joined parts [14]. Apparently the ghost lines, which appear after etching, are caused by selective oxidation of the iron due to different welding temperatures, whereby dissolved elements, mostly phosphorus and arsenic, typically concentrate in the exposed surface [14]. Iron silicate slag inclusions could have resulted from the smelting process, or perhaps were added during sand fluxing to facilitate the welding by inhibiting accelerated corrosion at high temperatures [13, 18, 19]. Analysis of wrought iron objects (Fig. 1) may indicate welding zones between two or more different α-ferritic layers due to the high volume fraction of slag inclusions, and the finer α-ferritic grains at the welding zone, as shown in Fig. 1(b) [2, 3].
In order to manufacture steels with carbon contents up to 1.5 wt% carbon, several methods were employed: (1) the cementation process, before 1,000 bc; (2) puddling: the combination of wrought and cast iron in a semi-solid state, before the sixteenth century ad; (3) melting in a crucible, about 1,740; and (4) conversion of cast iron to steel by the Bessemer process, about 1,855 [7].
In the indirect technique, a two-stage process was employed. Firstly, the cast iron was produced, and then the carbon content was reduced. The molten cast iron was converted into iron or steel by being decarburized in a finery hearth by subjecting the molten metal to an oxidizing environment, or by allowing it to drip through an air blast onto a charcoal hearth to create a bloom [20]. Some iron oxides were formed when the carbon content of the iron was reduced, and might have reacted with the lining of the hearth. Subsequently, the wrought iron produced would contain some finery slags, which might have been a little different in composition from the extraction slag [20, 21].
The bloom was next transferred to the ‘chafery’ open furnace, where it was reheated and forged into a wrought iron bar. During the eighteenth century, wrought iron was often used in the production of small parts and plates, whereas cast iron was used for the production of large structural material parts. However, there were some large objects (such as anchors), for which wrought iron was preferably suited, due to its better corrosion resistance. As a result, anchor smiths had to deal with increased ship and anchor dimensions [22]. Therefore, advanced techniques were developed, such as manufacturing large pieces of wrought iron by forge-welding wrought iron strips together in order to obtain massive pieces with an additional uniform finish [7, 23]. By the middle of the nineteenth century, large forged-welded wrought iron objects, weighing as much as 30 tons, could be manufactured by joining 100 kg wrought iron parts, using mechanical handling facilities and steam hammers [7, 23].
Tin–Lead Soldering of Ancient Objects
During the 1st Dynasty of Ur (around 5,000 years ago), the smiths of Mesopotamia, skilled in soldering, were experienced in transforming gold leaf into delicate jewelry. They prepared gold and silver filigree patterns made of tiny beads arranged in artistic motifs and soldered together [24].
A solder is defined as metal or alloy, whose melting point is lower than that of the metal or alloy to be soldered, which runs between the parts to be joined together. In high-quality soldering, ancient as well as modern, two crucial factors are as follows: (1) that the amount of solder used to join the metal parts is minor relative to the total weight of the object and (2) no trace of the soldering flux (when flux is used) should remain on the surface of the object once the soldering process is completed [25].
Lead–tin solder (or soft solder) was commonly used by metal smiths during Antiquity to join pieces of metal together at low temperatures, and produce joints in copper, bronze, brass, and silver objects [24, 26]. In Classical Antiquity, the solder was usually an alloy of tin and lead, commonly two parts of tin and one part of lead (tertiarium); and the flux was tree resin, tallow, or other oily substance [1]. The Romans used tin and its alloys as a soldering material for joining lead products such as pipes, canisters, braziers, and coffins [27]. Due to the relatively low strength of the tin–lead solder alloy compared with the joined metals, lap joints were usually used, as shown in Fig. 2. This ensured an area of solder alloy sufficient to carry joint loads. Such joints required three conditions for successful soldering: (a) the proper heating cycle to allow the molten solder to flow and completely fill the joint; (b) the provision of an accurate joint gap, clearances of 0.1 mm being optimum for good capillarity and joint strength. During soldering and solidification of the molten solder, there had to be no relative movement between joint faces. This was obtained by the use of jigs and self-locating designs; (c) clean metal surfaces obtained by mechanical degreasing, abrading, and using an appropriate flux [28].
The solder alloys used in production of brass objects required the following main characteristics: suitable melting point and flow range, good metal-wetting properties, adequate strength, and reasonable ductility [29, 30]. The tin–lead soft solder was a suitable choice for this kind of application, while tree resin or tallow usually served as the soldering fluxes [1]. Soldering of the components was most probably performed ‘in the fire’—pieces of copper alloy were coated with solder-flux mixture, held together with copper alloy wires, and heated in the coals [25]. Repair work, however, was done on a soldering bench, holding a hot coal with tongs over the defective joint to be reworked. The flux material was usually removed when the soldering work was completed.
Test Cases of Joining Processes of Metal Objects from Shipwrecks
This paper describes three multidisciplinary archaeometallurgical test cases of metal artifacts, which were retrieved from two shipwrecks excavated under water in Israel, and were manufactured by joining techniques. The artifacts are (1) a forge-welded iron anchor from the Tantura F shipwreck, dated to between the mid-seventh and the end of the eighth centuries ad [13]; (2) a 12-pdr wrought iron cannonball from the nineteenth century Akko 1 shipwreck [33, 34]; and (3) 158 soldered brass cases from the Akko 1 shipwreck [29, 33, 35].
In all three case studies, modern archaeometallurgical methods were used, and a methodology was developed to provide information regarding the manufacturing techniques of the objects. In the first stage, non-destructive testing (NDT) techniques were used, including methods such as visual testing to detect any visible macroscopic level discontinuities, x-ray fluorescence (XRF) measurements to provide macroscopic scale elemental and chemical analyses of the objects, and gamma-ray or x-ray radiography in order to reveal forge-welding joints, locate forge-welding lines, and determine welding and soldering qualities. In the second stage, destructive testing techniques were employed, including density measurement, in order to examine the quality of the objects. Metallographic samples were taken from different parts of the objects in all three planes—P (planar), T (transverse), and L (longitudinal), as recommended by the ASTM E3-01 standard. The sample surfaces were ground with silicon carbide 240–600 grit papers, then polished with alumina paste from 5 to 0.05 μm, and then polished with 0.05 μm colloidal silica suspension pastes. The samples were cleaned with ethanol and dried in order to remove contaminants. Metallographic samples were examined using a LM and a scanning electron microscope (SEM) to quantify and identify slag inclusions. Vickers microindentation hardness measurements were made in order to examine homogeneity and mechanical properties. The metallographic samples were analyzed by energy dispersive spectroscopy (EDS) in order to examine the local composition of different phases and the type of slag inclusions (glass inclusion, wüstite–glass inclusion and fayalite–wüstite–glass inclusion). Chemical analysis was performed by optical emission spectroscopy (OES) to detect the presence of light elements, such as carbon.
Although the metallurgical study was the focus of the research, the various items were also studied by archeological-typological methods. In addition to measuring and detailed recording, they were compared with similar contemporary objects (where available). All the above studies were carried out in the proper historical contexts, where historical sources were investigated, which in a few instances answered technological questions [32, 36].
Test Case I: The T-shaped Iron Anchor from the Tantura F Shipwreck
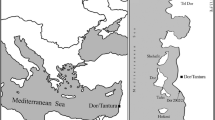
The Tantura F shipwreck was discovered in 1995 in Dor lagoon, Israel, and was excavated over five seasons in 2004–2008. Based on 14C dating, combined with typological study of the pottery, the shipwreck was dated to be between the mid-seventh and the end of the eighth centuries ad [31, 37]. Two T-shaped iron anchors were discovered. Both anchors (A and B) were found broken, with parts of the shank and the anchor cable ring missing. One of these anchors, anchor A (Fig. 3), was found under the wooden hull, and studied thoroughly in order to determine its manufacturing procedure, focusing on the forge-welding process, and identifying the locations of the forge-welding joints and their quality. This study was more comprehensive than previous similar studies of anchors [4, 38].
The anchor was retrieved from the seabed covered by a 4 cm-thick layer of encrustation coating and concretion composed of sea sand, shells, and small stones (Fig. 3a, b). The core of the iron shank and its arms was of solid, shiny, and relatively hard metal (Fig. 3b, c). The anchor shank had a circular cross-section (Fig. 4a), whereas its arms had rectangular cross-sections, typical of T-shaped anchors [13, 32, 38–40].
Anchor A cross-sections: (a) sectional plan (the hole at the center of the anchor was made by Eliyahu et al. [32] during the preliminary study of the anchor); (b) parts of anchor A after cutting; and (c) the radiograph image of section A2–A2, showing the forge-weld line
Visual inspection of the anchor’s surface showed forge-welding joints [32]. Ten radiography tests were carried out on the anchor, revealing one suspected forge-welding area (Fig. 4b), characterized in the radiographs by a tilted inner surface [13]. Eight metallographic cross-sections, A1-A1 to A8–A8, were taken from different zones of the anchor according to ASTM E3-01 (Fig. 4a). Macroscopic inspection of the external surface of A2–A2 (Figs. 4b, 5a) revealed a fine line, possibly corresponding to the forge-welded oblique inner surface previously observed by the radiographic examination. Examination of section A2–A2 after it was cut (Fig. 5b, c), revealed an inner cavity located as shown by the radiographs, indicating a forge-welding interlayer [13]. The metallographic samples were etched with Nital acid. LM and SEM observations of the metallographic sample prepared from the A2–A2 cross-section after polishing and etching showed a heterogeneous microstructure of an all allotriomorphic ferrite–pearlite–cementite network and Widmanstätten plates, as well as slag inclusions. SEM–EDS analysis of the A2–A2 cross-section revealed that the anchor was mostly made of iron, with the presence of some carbon, aluminum, silica, phosphorus, sulfur, and manganese. A high volume fraction of inclusions was observed near the forge-weld line of cross-section A2–A2 (Fig. 5e), indicating a forged-welding zone [2, 3]. Higher magnification of the slag inclusion demonstrated two-phase wüstite–glass slag inclusions (Fig. 5f), as expected for iron produced by the direct process [2, 3, 13].
Metallographic sections of anchor A: (a) a crack in the external part of the forge-weld line (section A2–A2, white arrows); (b) a defect in the internal part of the L cross-section before etching (section A2–A2); (c) the defect shown in (b) after polishing and etching; (d) section A1–A1 (T cross-section); (e) LM photograph showing high volume fraction of inclusions near the forge-weld line (section A2–A2); and (f) at a higher magnification (×500) two-phase wüstite–glass slag inclusions can be seen
The anchor shank had a core of about 5 cm diameter iron [13], indicating that the shank was prepared from forged cylindrical blooms about 60 mm in diameter [13]. Each bloom was produced by forge-welding of iron and steel pieces, resulting in a composite material macrostructure (Fig. 6). The hardness of the α-ferritic phase was 89 ± 11 HV (Fig. 6a, point 1) and the hardness of the pearlite was 219 ± 13 HV (Fig. 6a, point 2). Such a macrostructure provides higher strength and toughness than a homogeneous material macrostructure. The bloom was manufactured from various types of iron of different compositions. Carbon enrichment was observed around the weld joints, creating a transition interlayer between the iron and steel pieces. The presence of pearlite and ferrite with a Widmanstätten morphology in the transition zones exhibited local carbon enrichment of the iron pieces, with a corresponding decrease in carbon in the adjacent areas in the steel. The result of local carbon diffusion across the iron and steel austenite phase interfaces created the improved mechanical properties of the bloom macrostructure. Macroscopic inspection of the external surface of the anchor around the throat (the junction between the shank and the arms) revealed additional indications of forge-welded joints. Metallographic examination of section A8–A8 after etching presented a sharp border between the two different microstructures (Fig. 7). The transition interlayer suggested that two pieces with different compositions and microstructures, iron and steel, were forge-welded together.
Metallographic A2–A2 section (L cross-section) of anchor A: (a) sample of the L cross-section showing the interface of forge-weld joint; (b) sample of the T cross-section showing the interface of forge-weld joint; (c) α-ferritic grains; (d) pearlite Widmanstätten microstructure; and (e) pearlite microstructure
(a) Forge-weld joint (white dotted line) between the shank and the right arm of anchor A in section A8–A8, with high concentration of inclusions parallel to the joint line; (b) SEM image showing border between two different microstructures (white dotted line)—equiaxed ferrite grains at the left side of the photo, and ferrite with pearlite Widmanstätten microstructure zone that changes to pearlite at the right of the photo, with inclusions parallel to the joint line (white arrows)
From a metallurgical viewpoint, the blooms are of interest because their manufacturing process involved the solid-state bonding of iron to itself and to steels of radically different carbon contents by forge-welding. This was an early example of the simplest form of the manufacture of a composite material. Based on the analyses results, it seems that a ‘straight scarf weld’ technique was used to join the anchor blooms. Each arm of the anchor was made from a single piece weighing 2–3 kg, while the shank was made from at least five blooms (Fig. 8) weighing 1.5–2 kg each [13]. During the primary manufacturing stage, the circular cross-sections of the shank and the crown (Figs. 3c, 4a) were manufactured and combined. The arms were manufactured separately, and shaped to their rectangular cross-section with the flukes at their ends. At a subsequent stage, each arm was connected to the shank to form the complete anchor. Combining the radiographic and metallographic examinations, it was concluded that anchor A was of high quality, and was apparently manufactured by skilled blacksmiths.
Test Case II: A 12-pdr Cannonball from the Akko 1 Shipwreck
The Akko 1 shipwreck is the remains of an Egyptian armed vessel or a naval auxiliary brig, which sailed the Mediterranean during the first decades of the nineteenth century. It was discovered in Akko harbor and excavated over three seasons (2006–2008). Small arms and ammunition found in the shipwreck hinted at the ship’s involvement in a naval battle [33, 36]. Three cannonballs, covered with a thick layer of encrustation and concretion, were retrieved from the shipwreck, and were studied using archaeometallurgical methods. The cannonballs were identified as 9-, 12-, and 24-pdr, and theoretically, any or all of them could have hit the ship [33, 41].
The 12-pdr cannonball was discovered in the bow section of the shipwreck, trapped between framing timbers, with a ceramic sherd attached to the encrustation coating. It was identified as a 12-pdr, based on its dimensions following removal of the coating layer (Fig. 9): 345 mm in circumference and about 4.4 kg in weight ([42], Roth, pers. comm. 2007). This cannonball was studied in order to determine its manufacturing processes, and to locate forge-welding lines [36]. Ten metallographic cross-sections, B1–B6 (Fig. 10a, b) and C1–C4 (Fig. 10c, d), were cut from zones B and C of the cannonball. The cross-sections were made in the P, T, and L planes according to ASTM E3-01 (Fig. 10c, d). No manufacturing defects and/or forge-welding remains were detected by the gamma radiographic (RT) examination, indicating that the cannonball was apparently manufactured by a highly skilled blacksmith. The XRF measurements revealed that the cannonball was manufactured of iron containing low concentrations of molybdenum, nickel, copper, and silicon. The OES examination confirmed that it was forged from wrought iron containing minor amounts of molybdenum, nickel, copper, and cobalt. These metallic elements (molybdenum, nickel, copper, and cobalt) are invariably reduced into the iron during the metal extraction, while silicon is removed rapidly during the early stages of fining [6]. The low concentration of impurities indicates that the wrought iron was extracted by the indirect process—casting and fining.
The metallographic samples were etched with Nital acid. The LM inspection of the 12-pdr cannonball samples after etching demonstrated a homogenous microstructure of iron matrix with rather large (50–250 µm) equiaxed α-ferrite grains, which contained dark slag inclusions (elongated plate-like inclusions and spherical inclusions), as shown in Fig. 11(a) of sample B1 (T cross-section). No signs of pearlite and/or ferrite Widmanstätten plates were found. Higher LM magnifications of Fig. 11(a) revealed two-phase slag inclusions made of wüstite phase surrounded by a glassy matrix (Fig. 11b), as observed by Buchwald and Wivel [14]. Three-phase slag inclusions made of fayalite–wüstite phase surrounded by a glassy matrix were also observed (sample B5, Fig. 11c). Since the welding zone between two ferrite layers is characterized by a higher volume fraction of slag inclusions [2, 16], the alignment of elongated inclusions observed in Fig. 11(a) may identify the location of a forge-welding line.
LM metallographic observation (T cross-section, after etching) showing: (a) high concentration of preferred oriented inclusions near the external surface of the cannonball, surrounded by an equiaxed α-ferrite phase matrix (sample B1); (b) higher magnification of one of the inclusions revealed a two-phase wüstite–glass slag inclusion; and (c) three-phase slag inclusion of fayalite–wüstite–glass slag inclusion (sample B5)
The two other cannonballs which were retrieved from the shipwreck, 9- and 24-pdr, were made of cast iron. The 9-pdr cannonball was homogeneous, and included only white cast iron, while the cast iron in the 24-pdr cannonball was heterogeneous, and included white cast iron in the inner part of the cannonball, and gray cast iron in the external shell. A manganese concentration higher than 0.5 wt%, which must have been an addition, was found in both cannonballs, and was interpreted as indicating a post-1839 manufacturing date [41]. The 12-pdr cannonball was made of relatively pure iron containing slag inclusions, typical of forged wrought iron. Therefore, the use of wrought iron, unlike the cast iron 9- and 24-pdr cannonballs, suggests that the 12-pdr cannonball was manufactured in a different place and by a different technology. As its production technique pre-dates that of the ship (first third of the nineteenth century), it is possible that the 12-pdr cannonball might have been used as ballast [36].
Test Case III: The Brass Cases from the Akko 1 Shipwreck
During the underwater excavations of the Akko 1 shipwreck, 158 brass cases were found, mainly between midships and the aft extremity. The cases were semi-circular in cross-section; all of them had lids and two straps with loops on their body. All items were studied typologically, recorded and measured in detail. No similar parallel has yet been found. The 158 cases were 6.4–7.9-cm long, 4.8–5.8 cm wide, and 3.0–4.2 cm thick. The surviving lids were 4.2–6.0 cm wide and 1.1–1.9 cm high. The strap widths varied from 0.6 to 1.8 cm; the lower strap averaged 1.0 cm, and the upper strap averaged 1.0 cm wide. The distance between them varied from 1.0 to 2.3 cm (Fig. 12). Two identical crescent-shaped stamped marks were found in the center of the upper section of the backs of two of the cases. Small pieces of leather were discovered in some of the loops, suggesting that the brass cases were carried on a leather strap [29, 33, 35]. The brass cases were studied in order to determine their manufacturing processes, including examination of the quality of the soldering joint.
Visual observation of one of the brass cases revealed the soldering areas (Fig. 13a, white arrows). Another case was found covered with marine encrustation and connected to two iron nails. X-ray radiographic (RT) inspection of this case revealed that the joining zone in the left side of the case had begun to open. This may indicate poor quality soldering, probably the work of unskilled workers (Fig. 13b, white arrows). The radiographic examination of another brass case presented the soldering line between two joined parts of the case (Fig. 13c).
Observation of a typical brass case: (a) the soldering areas at the front of the case; (b) radiographic image (front view) of the brass case which was found covered with marine encrustation and connected to two iron nails, showing the joining zone soldered at the left side of the case (white arrows); and (c) radiographic image of the soldering zone between the two joined sides of another brass case
The XRF and SEM–EDS chemical analyses demonstrated that the brass cases were made of a brass alloy containing around 30 wt% Zn. The SEM–EDS analysis of the soldering material between two brass sheets revealed that it was made mostly of tin–lead. According to LM and SEM observations of the soldering material, its thickness varied between 140 and 250 µm (Fig. 14a, L cross-section). The metallographic samples were etched with ferric chloride acid. Higher LM magnification of the brass sheet (after etching) revealed that it was made of equiaxed α-brass grains with twins (Fig. 14b). Higher LM magnification of the soldering material showed bright and dark phases. The bright regions based on the LM notation were composed of tin, whereas the dark regions were composed of lead (Fig. 14c).
Light microscope images of the metallographic samples of a brass cases: (a) a layer of soldering metal filler between two brass sheets (after polishing and before etching); (b) α-brass phase with twins in the brass sheet (after etching); and (c) LM magnification indicating that the filler was made of eutectic tin–lead solder, where the bright phase is Sn and the dark phase is Pb
The relatively uniform thickness (0.50–0.55 mm) and microstructure of α-brass phase, observed after etching, with the presence of twins, in addition to the existence of preferentially oriented inclusions, indicates that the cases were made of brass sheets, manufactured by a rolling process, a technique that requires appropriate equipment [29, 35]. The presence of preferentially oriented slag inclusions combined with equiaxed grains (without the presence of preferentially oriented grains), indicated that all brass sheets were annealed after the rolling process [29, 35]. The rolled sheets used for manufacturing the cases were produced according to British standards, and could have been imported. This is also supported by the lead isotope analysis which suggests that the origin of the ore used for the body of the case was northern England—the Pennines or Cumbria [35]. The cases themselves were crudely hand-made with simple tools, and were most probably manufactured in Egypt.
Discussion
Three test cases of metal objects retrieved from two shipwrecks, Tantura F and Akko 1, are presented here. The recovered objects were examined in terms of their joining processes, by a multidisciplinary analysis, both non-destructive and destructive methods, focusing on the objects’ microstructure.
In the first test case, of the T-shaped iron anchor A from the Tantura F shipwreck, dozens of metallographic cross-sections in different locations and orientations were studied in order to reveal microstructure, composition, slag inclusions, and forge-welding junctions. The archaeometallurgical analysis demonstrated that anchor A was made of direct process wrought iron, with a heterogeneous multi-phase microstructure of ferrite, Widmanstätten plates, pearlite, and cementite rich in slag inclusions (glassy, fayalite, wüstite). The equiaxed ferrite grains and pearlite grains, and the parallel elongated slag inclusions, indicated that the anchor was manufactured by hot working (Fig. 7b). The silica slag particles which were observed suggest the use of silica as a flux material in the forge-welding areas (Fig. 5e, f).
From both radiographic and metallographic inspections, it was observed that the anchor was of high quality, and consequently was manufactured by skilled blacksmiths. Only the combination of visual observation, radiographic inspection, and analysis of the metallographic cross-sections by LM and SEM/EDS examination made it possible to locate the forge-welds of this anchor. It was determined that the ‘straight scarf weld’ technique was used to join the anchor blooms [13]. The typological study facilitated a viable reconstruction of the anchor, its weight, and size. The various tests on the anchor enabled us to reach a better understanding, and enriched our knowledge concerning the manufacturing processes of the late Byzantine and local early Islamic period anchors.
The second study, of the 12-pdr cannonball from the Akko 1 shipwreck, presents a cannonball made of relatively pure iron, with a homogenous microstructure of iron matrix and rather large equiaxed α-ferrite grains, which included three kinds of slag inclusions of different sizes up to 1 mm: glassy, glass–wüstite, and glass–wüstite–fayalite. However, a majority of the inclusions were less than 50 µm. The rather homogeneous microstructure of the cannonball indicated that it was made of indirect process wrought iron that was most probably produced by a two-step indirect technique [7, 14, 18]. The preferentially oriented slag inclusions embedded in the randomly oriented grains of α-ferrite matrix are typical of wrought iron manufactured by the hot-forging process [43]. The XRF and OES chemical analyses revealed that the cannonball was made of iron with the presence of other elements such as silicon molybdenum, nickel, copper, and cobalt. The local high concentration of preferentially oriented non-metallic inclusions, which was observed (LM) on sample B1 near the external surface of the cannonball (Fig. 10a), indicates the presence of forge-welded joints between two ferritic layers (Fig. 11) [2, 3, 16]. Furthermore, the wüstite–glass slag inclusions may be an indication of the use of silica as the flux material in the forge-welding process.
The combination of the radiographic and metallographic examinations indicated that the 12-pdr cannonball was manufactured by professional blacksmiths using hot forging [36]. The microstructure and microindentation hardness values of the ferrite phase suggest that this cannonball was produced by a thermo-mechanical process of hammering and annealing sequences to improve its hardness and toughness. Bearing in mind that it was much easier to cast a spherical cannonball than it was to make one by hammering, this cannonball can be considered as a unique object, especially for the nineteenth century. Moreover, ferrite is softer than cast iron, and thus it was probably necessary to apply surface cementation to the wrought iron cannonball to increase its hardness, a step which increased the complexity and cost of production. The technological process used to manufacture the 12-pdr cannonball suggests that it was made not later than the middle of the nineteenth [6, 36].
In the third study, it was found that the brass cases were manufactured from a binary copper-zinc rolled alloy [29, 35]. The brass sheets were fabricated from flat cast blanks by rolling [44]. The α-brass phase with twins indicated that the metal was annealed several times before the sheet reached the required thickness. The nominal thickness of the brass sheets prior to the fabrication of the cases (0.50–0.55 mm) corresponded to British 24 BWG (0.56 mm). A rather small kit of hand tools was required for manufacturing a brass case: first the various parts were cut and shaped, and then they were joined by a common tin–lead alloy [29, 35]. In order to clean the metal and remove the oxide film, a soldering flux was used, acting as a protective film until the solder wetted the metal alloy surface. The flux had to have sufficient affinity to the metal surface in order to remain in place during heating, but had to be weak enough to be displaced by the molten solder. Frequently, the metal parts were pre-heated, and only then was the solder material applied and melted directly during its contact with the hot metal [45]. The tin–lead soft solder used in the production of the brass cases from in the Akko 1 shipwreck had a suitable melting point and good wetting properties, and was a good selection for this kind of application.
The relationship between microstructure and mechanical properties can assist in understanding the manufacturing processes of metal objects. Information regarding ancient finds, including their microstructure and composition, provides important data regarding their production, as well as clues for their dating, thus contributing to the analysis of the wrecking event. These three studies illuminate the joining techniques of the metal objects, and enhance our knowledge regarding the technological abilities of the societies that produced them.
Conclusions
Three archaeometallurgical test cases of metal objects retrieved from shipwrecks were presented, focusing on their manufacturing processes according to their microstructure, and specifically concentrating on their joining processes. The research was a multidisciplinary project, involving archeological-typological methods, as well as the historical context.
Anchor A retrieved from the Tantura F shipwreck was made of forge-welded wrought iron produced by the direct technique, while the 12-pdr cannonball retrieved from the Akko 1 shipwreck was manufactured of wrought iron made by the indirect technique. In both cases, the objects themselves were manufactured by highly skilled blacksmiths. The metallurgical technology used for the 12-pdr cannonball suggests a relatively early manufacturing date—apparently before the date of the ship, which was built during the first third of the nineteenth century. The use of wrought iron, unlike the cast iron 9- and 24-pdr cannonballs, suggests that the 12-pdr cannonball was manufactured in a different place and by a different technology. This may support the possibility that not all the cannonballs belonged to the same ship, or that the three cannonballs were manufactured in different locations. However, the 12-pdr cannonball may have been used as ballast.
The brass cases from the Akko 1 shipwreck were manufactured from rolled sheets of brass (about 30 wt% Zn). The parts were first cut and shaped, then they were joined by a common soldering of tin–lead alloy. The brass sheets used for manufacturing of the cases may have originated from Great Britain or were produced according to British standards. The cases could have been manufactured in an Egyptian workshop near a shipyard, which adopted European working methods.
The study provides information regarding the joining techniques of metal objects retrieved from shipwrecks and dated to Late Antiquity and the eighteenth–nineteenth centuries. This information can assist in learning more about the technological abilities of these civilizations, as well as providing additional clues in deciphering the puzzle of these shipwrecks.
References
D. Ashkenazi, M. Fischer, A. Stern, O. Tal, Manufacturing technology of an ancient ship brazier: a unique example from the southern Levant. Skyllis 12(1), 85–93 (2013)
C. Mapelli, W. Nicodemi, R.F. Riva, Microstructural investigation of a medieval sword produced in 12th century ad. ISIJ Int. 47(7), 1050–1057 (2007)
W. Nicodemi, C. Mapelli, R. Venturini, R. Riva, Metallurgical investigations on two sword blades of 7th and 3rd century bc found in Central Italy. ISIJ Int. 45(9), 1358–1367 (2005)
F.H. van Doorninck Jr., in: Serçe Limani: an eleventh-century shipwreck. The ship and its anchorage, crew, and passengers, vol. I., ed. by G.F. Bass, S.D. Matthews, J.R. Steffy, F.H. van Doorninck Jr. (Texas A&M University Press, College Station, 2004), pp. 189–233
M. Cavallini, Thermodynamics applied to iron smelting techniques. Appl. Phys. A 113(4), 1049–1053 (2013)
J.L. Coze, Purification of iron and steels a continuous effort from 2000 bc to ad 2000. Mater. Trans. 41(1), 219–232 (2000)
R.F. Tylecote, A History of Metallurgy, 2nd edn. (The Metals Society, London, 1992)
J. Bénard, A. Michel, J. Philibert, J. Talbot, Métallurgie Générale (Masson, Paris, 1984)
J. Wadsworth, D.R. Lesuer, Ancient and modern laminated composites: from the Great Pyramid of Gizeh to Y2K*. Mater. Charact. 45, 289–313 (2000)
R. Maddin, J.D. Muhly, T.S. Wheeler, How the iron age began. Sci. Am. 237, 122–131 (1977)
R. Pleiner, Iron in Archaeology: The European Bloomery Smelters (Archaeologicky Uśtav Avěr, Praha, 2000)
R.F. Tylecote, J.W.B. Black, The effect of hydrogen reduction on the properties of ferrous materials. Stud. Conserv. 25, 87–96 (1980)
A. Aronson, D. Ashkenazi, O. Barkai, Y. Kahanov, Archaeometallurgical investigation of the iron anchor from the Tantura F shipwreck. Mater. Charact. 78, 108–120 (2013)
V.F. Buchwald, H. Wivel, Slag analysis as a method for the characterization and provenancing of ancient iron objects. Mater. Charact. 40, 73–96 (1998)
E. Blakelock, M. Martinón-Torres, H.A. Veldhuijzen, T. Young, Slag inclusions in iron objects and the quest for provenance: an experiment and a case study. J. Archaeol. Sci. 36(1), 1745–1757 (2009)
S. Barella, C. Mapelli, W. Nicodemi, A leap into the beginning of the metal age: recrystallization and carburizing. La Metallurgia Italiana 4, 9–16 (2008)
J. Perttula, Wootz Damascus steel of ancient orient. Scand. J. Metall. 33, 92–97 (2004)
J.V. Gimeno Adelantado, M.A. Ferrer Eres, F.M. Valle Algarra, J. Peris Vicente, F. Bosch Reig, Analytical study by SEM/EDX and metallographic techniques of materials used in the iron production process during the Iberian period. Talanta 60, 895–910 (2003)
H.J.W. Jobling, The History and Development of English Anchors C.A. 1550 to 1850. MA Thesis, Texas A&M University, College Station (1993)
A.R. Williams, The steel of the Negroli. Metrop. Mus. J. 34, 101–124 (1999)
L.F. Stenvik, Iron production in Scandinavian archaeology. Nor. Archaeol. Rev. 36(2), 119–134 (2003)
L.E. Samuels, The metallography of a wrought iron anchor from the Bark Endeavor. Metallography 13, 357–368 (1980)
J. Aston, E.B. Story, Wrought Iron. Its Manufacture, Characteristics and Applications (A. M. Byers, Pittsburgh, 1936)
H. Maryon, Archaeology and metallurgy. I welding and soldering. Man 41, 118–124 (1941)
D.L. Carroll, Antique metal-joining formulas in the “Mappae Clavicula”. Proc. Am. Philos. Soc. 125(2), 91–103 (1981)
H. Galli, R. Knopf, R. Gordon, The aging of solder joints over 1,600 years: evidence from Nubian bronze artifacts. JOM 59, 35–40 (2007)
E. Paparazzo, Surface and interface analysis of a Roman lead pipe “fistula”: microchemistry of the soldering at the join, as seen by scanning Auger microscopy and X-ray photoelectron spectroscopy. Appl. Surf. Sci. 74, 61–72 (1994)
A. Dahlgren, Study of international published experiences in joining copper and copper-alloys. SKI Rep. 97(12), 1–78 (1997)
D. Ashkenazi, D. Cvikel, N. Iddan, E.D. Mentovich, Y. Kahanov, M. Levinstain, Archaeometallurgical study of the brass cases from the Akko 1 shipwreck. J. Archaeol. Sci. 38(9), 2410–2419 (2011)
W.J. Tomlinson, N.J. Bryan, The strength of brass/Sn–Pb–Sb solder points containing 0–10% Sb. J. Mater. Sci. 21(1), 103–109 (1986)
O. Barkai, Y. Kahanov, The Tantura F shipwreck, Israel. Int. J. Naut. Arch. 36(1), 21–31 (2007)
E. Eliyahu, O. Barkai, Y. Goren, N. Eliaz, Y. Kahanov, D. Ashkenazi, Metallurgy analyzes of ancient iron anchor from Tantura F shipwreck. J. Archaeol. Sci. 38(2), 233–245 (2011)
D. Cvikel, Y. Kahanov, The Akko 1 shipwreck, Israel: the first two seasons. Int. J. Naut. Arch. 38(1), 38–57 (2009)
D. Cvikel, D. Ashkenazi, A. Stern, Y. Kahanov, Characterization of a 12-pdr wrought-iron cannonball from the Akko 1 shipwreck. Mater. Charact. 83, 198–211 (2013)
D. Ashkenazi, D. Cvikel, A. Stern, S. Klein, Y. Kahanov, Metallurgical characterization of brass objects from the Akko 1 shipwreck, Israel. Mater. Charact. 92, 49–63 (2014)
D. Cvikel, Y. Kahanov, The 19th-century Akko 1 shipwreck, Israel: hull-construction report. Int. J. Naut. Arch. 42(1), 167–187 (2013)
O. Barkai, Y. Kahanov, M. Avissar, The Tantura F shipwreck: the ceramic material. Levant 42(1), 88–101 (2010)
F.H. van Doorninck Jr., in: Yassi Ada Volume I: A Seventh-Century Byzantine Shipwreck, ed. by G.F. Bass, F.H. van Doorninck Jr. (Texas A&M University Press, College Station, 1982), pp. 121–141
D. Ashkenazi, E. Mentovich, Y. Kahanov, D., Cvikel, O. Barkai, A. Aronson, Archaeometallurgical investigation of iron artifacts from shipwrecks: a review, in Archaeology, New Approaches in Theory and Techniques, ed. by I. Ollich-Castanyer (InTech Publisher, Rijeka, 2012), pp. 169–186
G. Kapitän, Ancient anchors: technology and classification. Int. J. Naut. Arch. 13(1), 33–44 (1984)
E.D. Mentovich, D.S. Schreiber, Y. Goren, Y. Kahanov, H. Goren, D. Cvikel, D. Ashkenazi, New insights regarding the Akko 1 shipwreck: a metallurgic and petrographic investigation of the cannonballs. J. Archaeol. Sci. 37(10), 2520–2528 (2010)
D. McConnell, British Smooth-bore Artillery: A Technological Study to Support Identification, Acquisition, Restoration, Reproduction, and Interpretation of Artillery at National Historic Parks in Canada (National Historic Parks and Sites Branch, Parks Canada, Environment Canada, Ottawa, 1988)
N. North, M. Owens, C. Pearson, Thermal stability of cast and wrought marine iron. Stud. Conserv. 21(4), 192–197 (1976)
T. Herbert, J. Wallace, eds., The Cambridge Companion to Brass Instruments (Cambridge University Press, Cambridge, 1997)
W. Bolton, Engineering Materials Technology, 3rd edn. (Butterworth-Heinemann, Oxford, 1998)
Acknowledgments
The underwater excavations and research of the Akko 1 shipwreck were supported by the late Ron Marlar, the Yaacov Salomon Foundation, the late Reuven Sadnai—Coral Maritime Services Ltd., the Halpern Foundation, a Sir Maurice Hatter Fellowship, the Hecht Trust, a Jewish National Fund Fellowship, the President, Rector, Dean and Faculty of Humanities, University of Haifa, and anonymous donors, to whom the authors are grateful. The underwater excavations and research of the Tantura F shipwreck were supported by the late Lord Jacobs of London, the Israel Science Foundation, the Hecht Trust, a Sir Maurice Hatter Fellowship, and the University of Haifa. The authors are grateful to them all. Thanks are due to Mr. John Tresman for the English editing and to the anonymous reviewers for their constructive comments. The authors would also like to thank Y. Chen, Department of Mechanical Engineering, Afeka Academic College of Engineering, for the reconstruction simulation of anchor A (Fig. 8), and R. Ronen, Faculty of Engineering, Tel Aviv University, for the SolidWorks simulation of the 12-pdr cannonball (Fig. 10). All publication rights of the underwater objects in this article, including their original pictures, belong to the excavations licensee, the last author, Prof. Y. Kahanov.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashkenazi, D., Cvikel, D., Stern, A. et al. Archaeometallurgical Investigation of Joining Processes of Metal Objects from Shipwrecks: Three Test Cases. Metallogr. Microstruct. Anal. 3, 349–362 (2014). https://doi.org/10.1007/s13632-014-0153-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13632-014-0153-5