Abstract
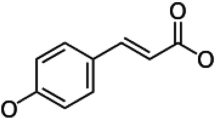
Caffeine (CA) and caffeic acid (CAF) are two bioactive phytochemicals found richly distributed in many plant foods such as coffee in different proportions. CA, an alkaloid, is adjudged world’s most consumed food bioactive substance, while CAF is a common phenolic acid in plants. With reports of potential cardiomodulatory properties of CA and CAF, we sought to investigate if proportional combinations of CA and CAF could influence the antihypertensive properties of each compounds by assessing their effect on activities of enzymes [angiotensin-1 converting enzyme (ACE), acetylcholinesterase, arginase, monoamine oxidase, phosphodiesterase-5 (PDE-5)] relevant to hypertension in vitro. Aqueous solutions of 1 mg/ml CAF and CA was prepared; thereafter, two combinations (C1 and C2) were prepared, where C1 is 5CA: 1CAF and C2 is 10CA: 1CAF. These samples were subsequently used for various enzyme assays. Results showed that C1 and C2 exhibited synergistic effects by eliciting significantly higher (P < 0.05) inhibition of enzyme activities assayed. Specifically, the inhibitory effects of C2 on ACE and PDE-5 were not significantly different (P > 0.05) from that of standard drugs Lisinopril and Sildenafil respectively. Also, both combinations exhibited higher antioxidant properties compared to CA and CAF. Hence, results presented in this paper support that combinations of CA and CAF as often found in plant foods exhibit improved antioxidant properties and enhanced inhibitory effects on critical enzymes relevant to hypertension, which, could be essential for the management of hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world burden of hypertension and other cardiovascular diseases is abruptly increasing, and African seems to be one of the most affected region in the world (WHO 2016; Adeloye et al. 2015). Hypertension is a critical cause of death associated with high blood pressure and comorbidities of several diseases such as heart failure, renal failure (Tian et al. 2011) coronary heart disease, atherosclerosis, myocardial infarction (Binda et al. 2001) and stroke (Chobanian et al. 2003). One of the major risk factor of cardiovascular diseases, hypertension results from an intricate interaction between genetic and environmental factors (Perk et al. 2013).
Inhibition of angiotensin-1-converting enzyme (ACE) leads to accumulation of kinins including bradykinin which promotes vasodilator activity (Tom et al. 2002). Angiotensin-1-converting enzyme is a zinc metallopeptidase enzyme responsible for formation of angiotensin II from its direct precursor, angiotensin I. The enzyme is found on the luminal surface of the small blood vessels of the lung and other tissues and in cells of the monocyte-macrophage series and is responsible for the mechanisms which increase the blood pressure (Lukic et al. 1999). Arginase has gained much interest in the as a regulator of endothelial function. The therapeutic potential of targeting arginase with arginase inhibitors has been reported in a number of experimental models of cardiovascular disease (Pernow and Jung 2013). Interestingly, pharmacological agents that target arginase indirectly through blockade of signaling transduction pathways which regulate arginase gene expression or activity, show beneficial effect on vascular functions (Yang and Ming 2013). PDE-5 activity was found to be increased in hypertension, thereby elevating the cGMP hydrolysis and thereby causing impairment of cGMP-mediated pulmonary vasodilation (Oyeleye et al. 2018). Several findings revealed that inhibition of PDE-5 have been shown to reverse the acute pulmonary vasoconstriction and thereby attenuate the development of hypertension in animal models (Oyeleye et al. 2018). Acetylcholinesterase (AChE) has been reported to play a crucial role in regulating number of vital functions such as learning and cerebral blood flow which reveals the level of importance of acetylcholine as a neurotransmitter target for examining cerebrovascular diseases (Cardoso et al. 2014).
Phenolic acids are a group of phenolic compounds that are widely distributed in foodstuffs, mostly in whole grains, fruits, vegetables, and beverages. Epidemiological studies have suggested an association between the consumption of phenolic acid-rich foods and beverages and the prevention of many diseases (Tsuda et al. 2004). These phenolic compounds exhibit good in vitro antioxidant and chemoprotective properties, which may have beneficial effects in vivo (Cos et al. 2002). Caffeic acid is the most abundant phenolic compound in coffee brew, and it is endowed with strong antioxidant activity in vitro and in vivo (Laranjinha 2001; Nardini et al. 1998). Oxidative stress occur when the reactive oxygen species overwhelms the antioxidant defense system. Studies have reported that increased production of reactive oxygen species play crucial role in the pathogenesis of hypertension (Demissie et al. 2006). The detection of caffeic acid in human plasma at micromolar concentrations after coffee drinking (Cheng et al. 2011) could infer that, at least in part, it is directly responsible for the increase in plasma antioxidant capacity. Caffeine is the most widely ingested psychoactive substance in the world and is largely unregulated (Lovett 2005). Caffeine can also bind directly to the vascular smooth muscle cell receptors and cause vasodilatation (Echeverri et al. 2010). Antihypertensive properties of caffeine and caffeic acid alone have been reported (Kolchin et al. 1991; Guessous et al. 2015). However, these two compounds are found in proportional combinations in plant sources (Ritchie et al. 2007; Santos et al. 2010), but there is dearth of information on the effect of proportional combinationa of caffeine and caffeic acid on their anti-hypertensive properties. Therefore, this study was conducted in two directions: (a) to determine the effect of caffeine, caffeic acid and various combinations on some key enzymes linked to hypertension (AChE, MAO, ACE, PDE-5 and arginase) in vitro; (b) to assess in vitro the antioxidant properties of caffeine, caffeic acid and their combinations.
Materials and methods
Chemicals and reagents
Caffeine and Caffeic acid were sourced from Sigma Al-drich Co. (St Louis, Missouri, USA). Other chemical reagents such as DPPH (2,2-diphenyl-1picrylhydrazyl), thiobarbituric acid (TBA), acetylthiocholine iodide and 1,10-phenanthroline were procured from Sigma Aldrich Co. (St Louis, Missouri, USA). Trichloroacetic acid (TCA) was sourced from Sigma Aldrich, Chemie GmbH (Steinheim, Germany). All other chemicals and reagents were of analytical grades and glass distilled water was used throughout the experiment.
Sample preparation
Aqueous solutions of 1 mg/ml CAF and CA was prepared. Thereafter, two combinations (C1 and C2) were prepared from the stock; where C1 is 5CA: 1CAF and C2 is 10CA: 1CAF. All samples were prepared to represent their predominant proportions in plant foods (Ritchie et al. 2007; Santos et al. 2010) and thereafter used for all assays. All samples were kept in the refrigerator at 4 °C for subsequent analysis.
Experimental animals
Wistar strain albino rats weighing 200–210 g were purchased from the breeding colony of the Department of Veterinary Medicine, University of Ibadan, Nigeria. Rats were maintained at 25 °C, on a 12-h light/12-h dark cycle, with free access to food and water. They were acclimatized under these conditions for 1 week before the experiment. All applicable institutional and national guild lines for the use of laboratory animals were followed.
Preparation of heart tissue homogenate
The rat was immobilized by cervical dislocation, and the heart tissue was rapidly isolated and placed on ice and weighed. This tissue was subsequently homogenized in 0.1 M phosphate buffer (pH 7.4; 1/10 w/v) with about ten up and down strokes at approximately 1200 rpm in a Teflon glass homogeniser (Mexxcare, mc14 362, Aayu-shi Design Pvt. Ltd. India). The homogenate was centrifuged for 10 min at 3000×g in a refrigerated centrifuge (KX3400C, KENXIN Intl. Co., Hong Kong) at 4 °C to yield a pellet that was discarded, and supernatant, that was used for all in vitro enzyme assays as well as for lipid peroxidation assay (Belle et al. 2004).
Angiotensin-1-converting enzyme inhibition assay (in vitro)
Appropriate dilution of the extracts (50 μl) and tissue homogenate (50 μl) was incubated at 37 °C for 15 min. The enzymatic reaction was initiated by adding 150 μl of 8.33 mM of the substrate Bz–Gly–His–Leu (Hippuryl–His–Leu free base) in 125 mM Tris–HCl buffer (pH 8.3) to the reaction mixture. After incubation for 30 min at 37 °C, the reaction was stopped by adding 250 μl of 1 M HCl. The Gly–His bond was then cleaved, and the Bz–Gly produced by the reaction was extracted with 1.5 ml ethyl acetate. Thereafter, the mixture was centrifuged to separate the ethylacetate layer and 1 ml of the ethyl acetate layer was transferred into a clean test tube and evaporated. The residue was redissolved in distilled water, and its absorbance was measured at 228 nm in a UV/visible spectrophotometer (Jenway 6305 model). The ACE inhibitory activity was expressed as percentage inhibition (Cushman and Cheung 1971).
Arginase Inhibition assay (in vitro)
The in vitro arginase activity was assayed according to previously reported method by Adefegha et al. (2015) in a reaction mixture containing Tris–HCl buffer (1.0 mM, pH 9.5, 1.0 mM MnCl2), 0.1 M arginine solution and extract. The mixture was made to a final volume of 1.0 ml. The mixture was incubated for 10 min at 37 °C. The reaction was terminated by the addition of 2.5 ml Ehrlich reagent [2.0 g of p-dimethylaminobenzaldehyde in 20 ml of absolute hydrochloric acid (37% purity) and made up to 100 ml with distilled water]. The absorbance was read after 20 min at 450 nm. The control experiment was performed without the test sample or standard and arginase inhibitory activity was calculated and expressed as % inhibition.
Acetylcholinesterase inhibition assay (in vitro)
The effect of the samples on acetylcholinesterase (AChE) activity was assessed by a modified colorimetric method (Perry et al. 2000). The cholinesterase activity was determined in a reaction mixture containing 200 μl of tissue homogenate in 0.1 M phosphate buffer (pH 8.0), solution of 5,5′-dithio-bis(2-nitrobenzoic) acid (DTNB 3.3 mM), sample extracts (0–100 μl), and phosphate buffer, pH 8.0. After incubation for 20 min at 25 °C, acetylthiocholine iodide for AChE activity assay was added as the substrate, and enzyme activity was determined in a UV/visible spectrophotometer (Jenway 6305 model) at 412 nm. The activity were thereafter expressed as percentage inhibition.
Phosphodiesterase-5 (PDE-5) inhibition assay (in vitro)
The ability of the extracts to inhibit PDE-5 activity was assessed (Oboh et al. 2017a). The reaction mixture containing 5 mM of the substrate (p-nitrophenyl phenylphosphonate), tissue homogenate, 20 mM Tris buffer (pH 8.0) and the extracts/sildenafil were incubated at 37 °C for 10 min. The intensity of p-nitrophenol produced was measured as a change in absorbance after 5 min at 400 nm. The control experiment was performed without the extracts/sildenafil. The PDE-5 inhibitory activity was expressed as percentage inhibition.
Monoamine oxidase inhibition assay (in vitro)
The effect of caffeine, caffeic acid and its various was measured according to a previously reported method (Green and Haughton 1961) with slight modification. In brief, the reaction mixture contained 0.025 M phosphate buffer (pH 7.0), 0.0125 M semicarbazide, 10 mM benzylamine, 75 µl of tissue homogenate and 0–100 μl of extracts. After 30 min incubation, acetic acid was added and incubated for 3 min in boiling water bath followed by centrifugation. The resultant supernatant (1 ml) was mixed with equal volume of 2, 4-DNPH, and 1.25 ml of benzene was added after 10 min incubation at room temperature. After separating the benzene layer, it was mixed with equal volume of 0.1 N NaOH. Alkaline layer was decanted and incubated at 80 °C for 10 min. The orange–yellow color developed was measured at 450 nm in a UV/visible spectrophotometer (Jenway 6305 model). The MAO activities were thereafter expressed as percentage inhibition.
Inhibition of lipid peroxidation and thiobarbituric acid reactions
The lipid peroxidation assay was carried out using the modified method of Ohkawa et al. (1979). Briefly, 100 μl tissue homogenate mixed with a reaction mixture containing 30 μl of 0.1 M pH 7.4 Tris–HCl buffer, extract (0–100 μl) and 30 μl of 250 μM freshly prepared FeSO4 (this procedure was also carried out using 5 μM sodium nitroprusside). The volume was made up to 300 μl by water before incubation at 37 °C for 1 h. The colour reaction was developed by adding 300 μl 8.1% sodium dodecyl sulphate (SDS) to the reaction mixture containing S1, and this was subsequently followed by the addition of 600 μl of acetic acid/HCl (pH 3.4) mixture and 600 μl 0.8% thiobarbituric acid (TBA). This mixture was incubated at 100 °C for 1 h. Thiobarbituric acid reactive species (TBARS) produced was measured at 532 nm in a UV/visible spectrophotometer (Jenway 6305 model) and expressed as malondialdehyde (MDA) equivalent.
Fe2+ chelation assay
The Fe2+ chelating ability of the extracts was determined using the method of Minotti and Aust (1987), with as light modification by Puntel et al. (2005). Freshly prepared 500 μM FeSO4 (150 μl) was added to a reaction mixture containing 168 μl 0.1 M Tris–HCl (pH 7.4), 218 μl saline and the extracts (0–25 μl). The reaction mixture was incubated for 5 min, before the addition of 13 μl 0.25% 1,10—phenanthroline (w/v). The absorbance was measured at 510 nm in a UV/visible spectrophotometer (Jenway 6305 model), and the percentage Fe2+ chelating ability was subsequently calculated as follows:
where Absref absorbance of reference and Abssam absorbance of sample.
Fenton reaction (degradation of deoxyribose) inhibition assay
The method of Halliwell and Gutteridge (1981) was used to determine the ability of the extracts to prevent Fe2+/H2O2 induced decomposition of deoxyribose. The extract (0–100 μl) was added to a reaction mixture containing 120 μl of 20 mM deoxyribose, 400 μl of 0.1 M phosphate buffer, 40 μl of 500 μM of FeSO4 and the volume was made up to 800 μl with distilled water. The reaction mixture was incubated at 37 °C for 30 min and stopped by the addition of 0.5 ml of 2.8% trichloroacetic acid. This was followed by addition of 0.4 ml of 0.6% thiobarbituric acid. The tubes were subsequently incubated in boiling water for 20 min, and the absorbance was measured at 532 nm in a UV/visible spectrophotometer (Jenway 6305 model).
Data analysis
Results were expressed as mean ± standard deviation (SD). Mean values were appropriately analysed and compared; one way analysis of variance (ANOVA) was used to compare the drug treatments, followed by Duncan’s post hoc test, while significance was accepted at P < 0.05. All statistical analysis were carried out using Statistical Package for Social Sciences (SPSS) program version 22.0.
Result
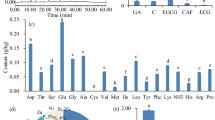
The effect of caffeic acid, caffeine and their combinations on angiotensin-1 converting enzyme (ACE) is presented in Fig. 1. The result revealed that combinations C1and C2 elicited higher significant (P < 0.05) inhibitory effect on ACE (C1 = 26.76 ± 0.06%; C2 = 37.38 ± 0.14%) than their individual effects. However, there was no significant difference (P > 0.05) between standard inhibitor drug-LIS (39.79 ± 0.01%) and C2.
The inhibition of phosphodiesterase-5 (PDE-5) activity as presented in Fig. 2 showed that there was no significant (P > 0.05) difference between standard inhibitor drug—sidenafil (22.35 ± 2.49%) and C2 (21.62 ± 2.53%); although caffeine possessed the highest inhibitory effect (26.36 ± 0.16%). Furthermore, the result of the inhibitory effect of samples on arginase activity as presented in Fig. 3 revealed that the combinations of caffeine and caffeic acid possessed highest inhibitory effects (C1 = 68.72 ± 0.13%; C2 = 79.66 ± 0.23%) when compared to their individual effects (CAF = 22.72 ± 0.04%; CA = 23.98 ± 0.15%).
The result of the inhibitory effect of monoamine oxidase (MAO) activity as shown in Fig. 4 revealed that both combinations elicited the highest significant (P < 0.05) inhibitory effect (C1 = 79.21 ± 0.58%; C2 = 86.85 ± 2.00%) compared to their individual effects. In addition, Fig. 5 shows that caffeine, caffeic acid and their combinations inhibited acetylcholinesterase activity. However, there is no significant difference between acetylcholinesterase inhibitory activities of caffeine and the two combinations used in the study.
Figure 6 represents the Fe2+ chelating ability of the combinations of CA and CAF. The results revealed that CA (97.48 ± 0.06%) elicited the highest inhibitory effect and also had significantly (P < 0.05) the highest Fe2+ chelating ability.
The OH* scavenging ability of the combinations of caffeine and caffeic acid is also represented in Fig. 7. The results showed that combinations C2 (53.88 ± 0.78%) had higher scavenging ability but was significantly lower than the individual effect of CA (77.22 ± 0.78%).
The inhibition of Fe2+-induced lipid peroxidation in isolated rat heart homogenates by the samples is presented in Fig. 8. Incubation of heart tissue homogenates in the presence of iron caused significant increase in MDA level. However, addition of caffeine, caffeic acid and their various combinations to the reaction mixture lowered MDA level in rats’ heart tissue homogenate.
Inhibitory Effects of Caffeine, Caffeic acid and their Combinations on Iron (Fe 2+)-induced Lipid Peroxidation in Rat Heart Tissue Homogenate. Values represent mean ± standard deviation of triplicate readings. Where C1 represents 5CA: 1CAF, C2 represents 10CA: 1CAF, CA represents Caffeic acid, CAF represents Caffeine
The inhibition of SNP-induced lipid peroxidation in isolated rat heart homogenates by the samples is presented in Fig. 9. Incubation of heart tissue homogenates in the presence of sodium niroprusside (SNP) caused significant increase in MDA level. However, addition of caffeine, caffeic acid and their various combination to the reaction mixture lowered MDA level in rats’ heart tissue homogenate.
Inhibitory Effects of Caffeine, Caffeic acid and their Combinations on Sodium nitropruside (SNP)-induced Lipid Peroxidation in Rat Heart Tissue Homogenate. Values represent mean ± standard deviation of triplicate readings. Where C1 represents 5CA: 1CAF, C2 represents 10CA: 1CAF, CA represents Caffeic acid, CAF represents Caffeine
Discussion
In this study, we evaluated the antioxidant properties and the effect of combinations of caffeine (CAF) and caffeic acid (CA) on activities of critical enzymes (arginase, angiotensin-1-converting enzyme acetylcholinesterase and monoamine oxidase) relevant to hypertension in vitro. CAF and CA are two bioactive phytochemicals found richly distributed in many plant foods. Caffeic acid is a major dietary polyphenol present in various foods and beverages and the most abundant hydroxycinnamic acids in the human diet (Bravo 1998). Caffeine is the most routinely consumed bioactive substance throughout the world and it’s a naturally occurring alkaloid found in numerous foods and beverages such as coffee, tea and cocoa (Nehlig 1999; Clifford 1999). Caffeine and caffeic acid exist in different proportions in food sources and the plausible effect of such proportional interactions are not often taken into consideration. In coffee, caffeine is the most abundant alkaloid while caffeic acid is one of the predominant phenolic acid (Ritchie et al. 2007; Santos et al. 2010). Due to the biological effect of caffeine and caffeic acid, studies have reported their potential in the prevention of several cardiovascular diseases (Beyer and Melzig 2003; Suzuki et al. 2008; Echeverri et al. 2010) but not their proportional combinations.
Phosphodiesterase-5 (PDE5) inhibitors have demonstrated various pharmacological properties including vasodilatory, antithrombotic, bronchodilation, anti-inflammatory, and antioxidant properties (Milani et al. 2005). PDE-5 is an enzyme responsible for the hydrolysis of cyclic 3,5 cyclic guanosine monophosphate (cGMP) to linear GMP, thus preventing the accumulation cGMP for its NO-mediated cGMP vasodilatory role (Kukreja et al. 2004). Therefore, PDE-5 inhibition is a good mechanism by which the vasodilatory role of NO can be preserved. Results from this study revealed that caffeine at low concentration possessed the highest inhibitory effect on PDE5. This is in agreement with previous study where caffeine had been reported to inhibit PDE5 (Goyarts et al. 2000; Boswell-Smith et al. 2006). The therapeutic drug-Sildenafil, is an inhibitor of PDE5 which acts as a potent pulmonary vasorelaxant in the pulmonary artery wall and is essentially cytosolic but might possess some adverse reactions (Ghofrani et al. 2002). However, in this study, since C2 produced no significantly different PDE5 inhibition compared to sildenafil, hence the combination of caffeic acid and caffeine at this proportion could offer a possible alternative and help reduce the attendant side effects associated with using this drug.
Angiotensin II produced in renin-angiotensin system from angiotensin I by the action of ACE, a vasoconstrictor in renal vessels and has been implicated in hypertension (Satoh et al. 2008). However, its effect under pathological conditions is counteracted by nitric oxide which serves as a potent vasodilator and plays an important role in maintaining vascular tone (Satoh et al. 2008). Phenolic compounds such as curcumin, quercetin, gallic acid and caffeic acids have been reported to inhibit ACE activity either as a single compound or in synergy with other compounds (Pang et al. 2015; Kang et al. 2015; Bhullar et al. 2014). This approach has been used in various practices of traditional medicine where mixture of plant constituents is commonly prescribed for the management of hypertension. They have also been reported to exhibit blood pressure lowering effect in hypertensive rats (Pang et al. 2015; Kang et al. 2015; Butcher et al. 1968). Nevertheless, our findings reveal that the synergistic effect of caffeic acid and caffeine brought about a significant decrease in the inhibitory activity of angiotensin-1-converting enzyme in vitro compared to their individual effects. Interestingly, there was no significant difference between the ACE inhibitory effect of the standard therapeutic drug-Lisonopril and C2 (10CA: 1CAF). Therefore, inhibition of ACE activity could be attributed to the combinatory effect caffeic acid and caffeine.
This study revealed that synergistic effect of the combinations of caffeine and caffeic acid inhibited the activity of arginase in cardiac tissue homogenate. Upregulation of vascular arginase has been identified in hypertension (Zhang et al. 2004). It was revealed in this study the combinations of caffeine and caffeic acid had higher arginase inhibitory effect compared to their individual effects. Hypertension is often associated with decreased nitric oxide bioavailability (Chowdhary and Townend 2001). The inhibition of arginase activity is a crucial factor by which endothelial function can be restored through increased bioavailability of NO. NO is synthesized by nitric oxide synthase (NOS) from l-arginine. However, l-arginine is a common substrate for both NOS and arginase which plays the role of the hydrolysis of l-arginine to urea and l-ornithine (Durante et al. 2007).
Therefore, inhibition of arginase activity has been suggested as a pragmatic therapeutic approach of elevating l-arginine as a substrate for NOS, and allowing the bioavailability of NO. Hence, the observed arginase inhibitory effects of the combinations of caffeine and caffeic acid in this study suggest their possible role in the management of hypertension by ensuring availability of l-arginine for NO synthesis.
Acetylcholinesterase (AChE) is an enzyme responsible for the hydrolysis of ACh into inactive choline and acetate in the neuromuscular junctions and the synaptic cleft of cholinergic synapses (Lendvai and Vizi 2008). The regulation of acetylcholinesterase as a therapeutic strategy for the management of cardiovascular diseases and hypertension has been established (Cardoso et al. 2014). In this study, caffeine, caffeic acid and their combinations exhibited inhibitory action on acetylcholinesterase in cardiac tissue homogenate. This is in agreement with an earlier report on the in vitro acetylcholinesterase inhibitory properties of some bioactive compounds including alkaloid and phenolic compounds (Uddin et al. 2016; Oboh et al. 2017b). Therefore, inhibition of AChE activity by caffeine, caffeic acid and their combinations in rat cardiac tissue homogenate could induce the bioavailability of acetylcholine which could generates vasodilatory effects that can help regulate vascular blood flow. This could also contribute significantly to the therapeutic potentials of the combination of caffeine and caffeic acid for the prevention and management of hypertension.
Lipid peroxidation in biological membranes is one of the important mechanisms underlying cell injury in oxygen-utilizing organisms subjected to oxidative stress (Oboh et al. 2007). It is related with the loss of rigidity and increased permeability ultimately resulting in loss of membrane function (Oboh et al. 2007). As such, the inhibition of lipid peroxidation is considered an antioxidant mechanism. The level of malondialdehyde (MDA) produced is used as a biomarker to measure the level of oxidative stress in an organism (Devasagayam et al. 1996). Malondialdehyde is the end-product of lipid peroxidation, which is a process where reactive oxygen species (ROS) degrade polyunsaturated fatty acids (Devasagayam et al. 1996). This compound is a reactive aldehyde and is one of the many reactive electrophile species that cause toxic stress in cells and form advanced glycation end-products (Devasagayam et al. 1996). The level of these products has been implicated in several pathological conditions such as hypertension, atherosclerosis and erectile dysfunction among others (Halliwell and Chirico 1993).
The inhibition of Fe2+ induced lipid peroxidation in rat’s heart homogenate by caffeine, caffeic acid and their combination could be related to the abilities of the compounds to form complexes with the Fe2+, thereby preventing them from catalyzing the initiation of lipid peroxidation, or perhaps scavenged the free radicals produced by the Fe2+-catalyzed reaction (Oboh et al. 2007). This result agrees with previous work reported by Oboh et al. (2012) where Fe2+ was shown to be a very potent initiator of lipid peroxidation. The increased lipid peroxidation in the presence of Fe2+ could be attributed to the fact that Fe2+ is capable of catalysing one-electron transfer reactions that generate ROS, such as the reactive hydroxyl radical (·OH), which is formed from hydrogen peroxide (H2O2) through the Fenton reaction. Iron also decomposes lipid peroxides, thus generating peroxyl and alkoxyl radicals, which favours the propagation of lipid oxidation (Kizil et al. 2010). Furthermore, caffeine, caffeic acid and their combination inhibited sodium nitroprusside (SNP)-induced lipid peroxidation in rat’s heart homogenate. Sodium nitroprusside is a component of antihypertensive drugs which causes cytotoxicity through the release of cyanide and nitric oxide (NO) (Oboh and Rocha 2008). The protective properties of these compounds against SNP-induced lipid peroxidation in the heart could be because of their abilities to scavenge the nitrous radicals produced from the decomposition of SNP.
Conclusion
This present study has revealed that caffeine, caffeic acid and their combinations exhibited inhibitory effects on some key enzymes’ activities linked to hypertension (in vitro), with its proportional combinations mostly exhibiting stronger inhibitory activities than the individual compounds. Therefore, the results of this study suggest that combinations of these two bioactive phtyochemicals could offer more antihypertensive effects via higher antioxidant properties and inhibitory effects on key enzymes linked to hypertension.
References
Adefegha SA, Oboh G, Ejakpovi II, Oyeleye SI (2015) Antioxidant and antidiabetic effects of gallic and protocatechuic acids: a structure-function perspective. Comp Clin Pathol 24:1579–1585
Adeloye D, Basquill C, Aderemi AV, Thompson JY, Obi FA (2015) An estimate of the prevalence of hypertension in Nigeria: a systematic review and meta-analysis. J Hyper 33:230–242
Belle NAV, Dalmolin GD, Fonini G, Rubim MA, Rocha JBT (2004) Polyamines reduce lipid peroxidation induced by different prooxidant agents. Brain Res 1008:245–251
Beyer G, Melzig MF (2003) Effects of selected flavonoids and caffeic acid derivatives on hypoxanthine-xanthine oxidase induced toxicity in cultivated human cells. Planta Med 69:1125–1129
Bhullar KS, Lassalle-Claux G, Touaibi M, Rupasinghe HPV (2014) Antihypertensive effect of caffeic acid and its analogs through dual rennin-angiotensin-aldosterone system inhibition. Eur J Pharmacol 730:125–132
Binda D, Nicod L, Viollon-Abadie C, Rodriguez S, Berthelot A, Coassolo P, Richert L (2001) Strain difference (WKY, SPRD) in the hepatic antioxidant status in rat and effect of hypertension (SHR, DOCA). Ex vivo and in vitro data. Mol Cell Biochem 218:139–146
Boswell-Smith V, Spina D, Page CP (2006) Phosphodiesterase inhibitors. Br J Pharmacol 147:S252–S257
Bravo L (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 56(11):317–333
Butcher RW, Baird CE, Sutherland EW (1968) Effects of lipolytic and antilipolytic substances on adenosine 3′,5′-monophosphate levels in isolated fat cells. J Biol Chem 243:1705–1712
Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, Barrios CH, Bergh J, Biganzoli L, Blackwell KL, Cardoso MJ (2014) ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Ann Oncol 25(10):1871–1888
Cheng B, Liu X, Gong H, Huang L, Chen H, Zhang X, Li C, Yang M, Ma B, Jiao L, Zheng L (2011) Coffee components inhibit amyloid formation of human islet amyloid polypeptide in vitro: possible link between coffee consumption and diabetes mellitus. J Agric Food Chem 59(24):13147–13155
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT Jr et al (2003) The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 289:2560–2572
Chowdhary S, Townend JN (2001) Nitric oxide and hypertension: not just an endothelium derived relaxing factor! Department of Cardiovascular Medicine, University of Birmingham, Birmingham, UK. J Hum Hyper 15:219–227
Clifford MN (1999) Chlorogenic acids and other cinnamates-nature, occurrence and dietary burden. J Sci Food Agric 79:362–372
Cos P, Rajan P, Vedernikova I, Calomme M, Pieters L, Vlietinck AJ, Augustyns K, Haemers A, VandenBerghe D (2002) In vitro antioxidant profile of phenolic acid derivatives. Free Rad Res 36:711–716
Cushman DW, Cheung HS (1971) Spectrophotometric assay and properties of the Angiotensin 1-converting enzyme of rabbit lung. Biochem Pharm 20:1637–1648
Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A (2006) Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell 15(4):325–330
Devasagayam T, Kamat J, Mohan H, Kesavan P (1996) Caffeine as an antioxidant: inhibition of lipid peroxidation induced by reactive oxygen species. Biochim Biophys Acta 1282:63–70
Durante W, Johnson FK, Johnso RA (2007) Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34(9):906–911
Echeverri D, Montes FR, Cabrera M, Galán A, Prieto A (2010) Caffeine’s vascular mechanisms of action. Intl J Vas Med Int 2010:10
Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, Gunther A, Walmrath D, Seeger W, Grimminger F (2002) Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet 360(9337):895–900
Goyarts E, Mammone T, Muizzuddin N, Marenus K, Maes D (2000) Correlation between in vitro cyclic adenosine monophosphate phosphodiesterase inhibition and in vivo anti-inflammatory effect. Skin Pharmacol Physiol 13(2):86–92
Green AL, Haughton TM (1961) A colorimetric method for the estimation of monoamine oxidase. Biochem J 78:172
Guessous I, Pruijm M, Ponte B, Ackermann D, Ehret G, Ansermot N, Vuistiner P, Staessen J, Gu Y, Paccaud F, Mohaupt M (2015) Associations of ambulatory blood pressure with urinary caffeine and caffeine metabolite excretions. Hypertension 65(3):691–696
Halliwell B, Chirico S (1993) Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 57(5):715S–724S
Halliwell B, Gutteridge JMC (1981) Formation of a thiobarbituric acid reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett 128:347–352
Kang N, Lee JH, Lee WW, Ko JY, Kim EA, Kim JS et al (2015) Gallic acid isolated from Spirogyra sp. Improves cardiovascular disease through a vasorelaxant and antihypertensive effect. Environ Toxicol Pharmacol 39:764–772
Kizil M, Kizil G, Yavuz M, Ceken B (2010) Protective activity of ethanol extract of three Achillea species against lipid peroxidation, protein oxidation and DNA damage in vitro. Acta Aliment 39(4):447–457
Kolchin I, Maksiutina NP, Balanda PP, Luĭk AI, Bulakh VN, Moĭbenko AA (1991) The cardioprotective action of quercetin in experimental occlusion and reperfusion of the coronary artery in dogs. Farmakol i toksikologiia 54(6):20–23
Kukreja RC, Ockailli R, Salloum F, Yin C, Hawkins J, Das A, Xi L (2004) Cardiprotection with phosphodiesterase-5 inhibition - a novel preconditioning strategy. J Mol Cell Cardiol 36(2):165–173
Laranjinha J (2001) Redox cycles of caffeic acid with α-tocopherol and ascorbate. Methods Enzymol 335:282–295
Lendvai B, Vizi ES (2008) Nonsynaptic chemical transmission through nicotinic acetylcholine receptors. Physiol Rev 88(2):333–349
Lovett R (2005) Demon drink. N Sci 187:38–41
Lukic ML (1999) Immunopathological correlates of hypertension. In: Frossard PM, Parvez SH, Minami M, Parvez S, Saito H (eds) Genetic, immune and molecular predisposition to hypertension. VSP Press Zeist, The Netherlands, pp 175–195
Milani E, Nikfar S, Khorasani R, Zamani MJ, Abdollahi M (2005) Reduction of diabetes-induced oxidative stress by phosphodiesterase inhibitors in rats. Comp Biochem Physiol C Toxicol Pharmacol 140:251–255
Minotti G, Aust SD (1987) The requirement for iron(III) in the initiation of lipid peroxidation by iron(II) and hydrogen peroxide. J Biol Chem 262(3):1098–1104
Nardini M, Pisu P, Gentili V, Natella F, Di M, Piccolella F, Scaccini C (1998) Effect of caffeic acid on tert-butyl hydroperoxide-induced oxidative stress in U937. Free Rad Biol Med 25(9):1098–1105
Nehlig A (1999) Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci Biobehav Rev 23:563–576
Oboh G, Rocha JBT (2008) Antioxidant and neuroprotective properties of sour tea (Hibiscus sabdariffa, calyx) and green tea (Camellia sinensis) on some pro-oxidant-induced lipid peroxidation in brain in vitro. Food Biophys 3(4):382
Oboh G, Puntel RL, Rocha JBT (2007) Hot pepper (Capsicum annuum, Tepin and Capsicum chinese, Habanero) prevents Fe2+-induced lipid peroxidation in brain-in vitro. Food Chem 102(1):178–185
Oboh G, Ademiluyi AO, Akinyemi AJ (2012) Inhibition of acetylcholinesterase activities and some pro-oxidant induced lipid peroxidation in rat brain by two varieties of ginger (Zingiber officinale). Exp Toxicol Pathol 64:315–319
Oboh G, Adebayo AA, Ademosun AO, Boligon AA (2017a) In vitro inhibition of phosphodiesterase-5 and arginase activities from rat penile tissue by two Nigerian herbs (Hunteria umbellata and Anogeissus leiocarpus). J Basic Clin Physiol Pharmacol 28:393. https://doi.org/10.1515/jbcpp2016-0143c
Oboh G, Ogunruku OO, Oyeleye SI, Olasehinde TA, Ademosun AO, Boligon AA (2017b) Phenolic extracts from clerodendrum volubile leaves inhibit cholinergic and monoaminergic enzymes relevant to the management of some neurodegenerative diseases. J Dietary Suppl 14:358–371
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Oyeleye SI, Adebayo AA, Ogunsuyi OB, Dada FA, Oboh G (2018) Phenolic profile and enzyme inhibitory activities of almond (Terminalia catappa) leaf and stem bark. Intl J Food Prop 20(3):S2810–S2821
Pang XF, Zhang LH, Bai F, Wang NP, Shah A, Garner R et al (2015) Dual ACE-inhibition and angiotensin II AT1 receptor antagonism with curcumin attenuate maladaptive cardiac repair and improve ventricular systolic function after myocardial infarction in rat heart. Eur J Pharmacol 746:22–30
Perk J, De Backer G, Gohlke H et al (2013) Comitato per Linee Guida Pratiche (CPG) dell’ESC. [European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts)]. G Ital Cardiol (Rome) 14:328–392
Pernow J, Jung C (2013) Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal. Cardio Res 98:334–343
Perry NS, Houghton PJ, Theobald A, Jenner P, Perry EK (2000) In vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J Pharm Pharmacol 52(7):895–902
Puntel RL, Nogueira CW, Rocha JBT (2005) Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem Res 30:225–235
Ritchie K, Carriere I, De Mendonca A, Portet F, Dartigues JF, Rouaud O (2007) The neuroprotective effects of caffeine: a prospective population study (the Three City Study). Neurology 69:536–545
Santos C, Lunet N, Azevedo A, De Mendonça A, Ritchie K, Barros H (2010) Caffeine intake is associated with a lower risk of cognitive decline: a cohort study from Portugal. J Alzheimers Dis 20:175–185
Satoh M, Fujimoto S, Arakawa S, Yada T, Namikoshi T, Haruna Y et al (2008) Angiotensin II type 1 receptor blocker ameliorates uncoupled endothelial nitric oxide synthase in rats with experimental diabetic nephropathy. Nephrol Dial Transpl 23:3806–3813
Suzuki A, Fujii A, Jokura H, Tokimitsu I, Hase T, Saito I (2008) Hydroxyhydroquinone interferes with the chlorogenic acid-induced restoration of endothelial function in spontaneously hypertensive rats. Am J Hypertens 21(1):23–27
Tian D, Ling S, Chen G, Li Y, Liu J, Ferid M, Bian K (2011) Hypertensive nephropathy treatment by heart-protecting musk pill. A study of anti-inflammatory therapy for target organ damage of hypertension. Intl J Gen Med 4:131–139
Tom B, Dendorfer A, Vries RD, Saxena PR, Jan Danser AH (2002) Bradykinin potentiation by ACE inhibitors: a matter of metabolism. Br J Pharm 137(2):276–284
Tsuda H, Ohshima Y, Nomoto H, Fujita K, Matsuda E, Iigo M, Takasuka N, Moore MA (2004) Cancer prevention by natural compounds. Drug Metab Pharmacokinet 19:245–263
Uddin MJ, Alam MN, Biswas K, Rahman MA (2016) In vitro antioxidative and cholinesterase inhibitory properties of Thunbergia grandiflora leaf extract. Cogent Food Agric 2(1):1256929
World Health Organization (2009) Cardiovascular diseases, 16 July 2012. http://www.who/cardiovascular_diseases/priorities/em/index.html
World Health Organization (WHO) Updates on cardiovascular disease. http://www.who.int/cardiovascular_diseases/about_cvd/en/. Last Accessed 17 May 2016
Yang Z, Ming XF (2013) Arginase: the emerging therapeutic target for vascular oxidative stress and inflammation. Front Immunol 4:149
Zhang C, Hein TW, Wang W, Miller MW, Fossum TW, McDonald MM, Kuo L (2004) Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension 44(6):935–943
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
Both international and institutional ethical standards were observed in this study. All animal experiments were approved by the Department of Veterinary Medicine, University of Ibadan, Nigeria.
Conflict of interest
Authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Oboh, G., Adeoyo, O.O., Ademosun, A.O. et al. Effect of combinations of caffeine and caffeic acid on key enzymes linked to hypertension (in vitro). Orient Pharm Exp Med 18, 247–255 (2018). https://doi.org/10.1007/s13596-018-0313-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-018-0313-2