Abstract
This study sought to investigate antioxidant and antidiabetic effects of gallic acid (GA) and protocatechuic acids (PCAs) based on their structure–function relationship. Twenty micromolar of phenolic acid (GA and PCA) solutions was prepared and their antioxidant properties determined. Then, interaction of the phenolic acids with key enzymes linked to type 2 diabetes (α-amylase, α-glucosidase) was subsequently assessed. The results showed that both phenolic acids significantly (P < 0.05) decreased Fe2+-elevated pancreas malondialdehyde (MDA) contents, chelated Fe2+, reduced Fe3+ to Fe2+, and scavenged 1,1-diphenyl-2-picrylhydrazyl, 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonate), and hydroxyl radicals and furthermore inhibit α-amylase and α-glucosidase activities in a dose-dependent manner. However, GA (IC50 = 1.22 μM) had significantly (P < 0.05) higher inhibitory effect on the α-glucosidase activity than PCA (IC50 = 1.76 μM). Conclusively, both GA and PCA are rich sources of antioxidant and antidiabetic molecules. However, GA showed better antioxidant and antidiabetic effects than PCA. These effects may be due to additional hydroxyl group on its aromatic ring structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both epidemiological and experimental evidences have shown an inverse relationship between the prevalence of several degenerative diseases such as diabetes and cardiovascular diseases and consumption of dietary phenolic acids (Pandey and Rizvi 2009). Phenolic acids possess different mechanisms for their action as antioxidants: the ability to scavenge free radicals, delocalize/stabilize the resulting phenoxyl radical within the structure, reduce oxygen concentration, decompose primary products of oxidation to nonradical species, prevent continued hydrogen abstraction from substrate, and chelate metal ions such as iron that participates in Fenton reaction and are capable of inducing lipid peroxidation in tissues (Shahidi and Naczk 2004). They are the antioxidant components of most fruits and vegetables and have been employed in clinical and epidemiological research (Kelsey et al. 2010).
Type 2 diabetes is a metabolic disorder that results from the inability of the body to either produce enough or properly utilize insulin (World Health Organization Consultation 1999). It is characterized by high blood glucose level, which can cause various types of secondary complication associated with morbidity and mortality. α-Amylase and α-Glucosidase degrade complex dietary carbohydrates into glucose. Absorption of this glucose results in postprandial hyperglycemia (Kim et al. 2000; Shim et al. 2003). Thus, inhibition of these two key enzymes can be a useful point to regulate postprandial hyperglycemia in type 2 diabetes and checking associated chronic complications (Kim et al. 2000; Ali et al. 2009). Oxidative stress can be an underlying cause of type 2 diabetes (Oberley 1988). It can act through development of insulin resistance, β-cell dysfunction, impaired glucose tolerance, and mitochondrial dysfunction resulting in diabetic mellitus (Marc et al. 2005; Kenneth et al. 2007).
Gallic acid (GA) is also known as 3,4,5-trihydroxybenzoic acid. It is a commonly found phenolic acid in spices, fruits, vegetables, tea, grapes, and wine (Dai and Russell 2010; Keith and Christy 2010). It exerts antioxidant effects, anticancer, antidiabetic, antihypertensive, antiinflammatory, and antimicrobial effects (Satish et al. 2011; Dongyan et al. 2014). Protocatechuic acid (PCA), otherwise known as 3,4-dihydroxybenzoic acid, is widely distributed in virtually all plants and main component of human diet. They are present in brown rice (bran and grain), onion, fruits, spices, vegetables, gooseberries, grapes, nuts, olive oil, and wine (Ali et al. 2009; Tanaka et al. 2011). Several studies have also indicated that PCA exhibits antioxidant, antidiabetic, anticancer, antifungal, antiviral, and antiinflammatory effects which may be linked to its antioxidant properties (Kunle and Egharevba 2013; Sahil and Souravh 2014).
The antioxidant capacity of a phenolic compound is dependent on some factors which are structure of the phenolic compound, number of aromatic and hydroxyl groups in their structure, and the distribution of these groups in the structure (Balasundram et al. 2006; Heo et al. 2007). Both phenolic acids (GA and PCA) have their hydroxyl groups in meta position with respect to the carboxylic group, and this arrangement may influence their biological activities. Information about the possible mechanism of action of some phenolic acids in relation to their antioxidant capacity and their health benefits remain limited. For better understanding of these two phenolic acids: GA and PCA, the structure–function relationship of the phenolic acids needs to be assessed. Therefore, we sought to investigate the antioxidant activities and antidiabetic effects of these two phenolic acids found in food: gallic acid and protocatechuic acid.
Materials and methods
Chemical and reagents
Chemicals such as gallic acid, protocatechuic acid, thiobarbituric acid (TBA), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azinobis(3-ethylbenzthiazoline-6-sulphonic acid (ABTS), and pancreatic α-amylase were purchase from Sigma-Aldrich, Chemie GmH (Steinheim, Germany); acetic acid was procured from BDH Chemical Ltd., (Poole, England). Except otherwise stated, all other chemicals and reagents are of analytical grade while the water was glass distilled. A JENWAY UV–visible spectrophotometer (Model 6305; Jenway, Barloworld Scientific, Dunmow, UK) was used to measure absorbance throughout the experiment.
α-Amylase inhibition assay
Appropriate dilution of the phenolic acid (gallic acid and protocatechuic acid) solution was mixed with 500 μL of 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) containing Hog pancreatic α-amylase (EC 3.2.1.1) (0.5 mg/mL) and then incubated at 25 °C for 10 min. Then, 500 μL of 1 % starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) was added to each tube. The reaction mixtures were incubated at 25 °C for 10 min and stopped with 1.0 mL of dinitrosalicylic acid color reagent followed by incubation in a boiling water bath for 5 min and cooled to room temperature. The reaction mixture was diluted by adding 10 mL of distilled water and absorbance measured at 540 nm (Worthington Biochemical Corp 1978).
α-Glucosidase inhibition assay
Appropriate dilutions of phenolic acid solution and 100 μL of α-glucosidase solution (1.0 U/mL) in 0.1 M phosphate buffer (pH 6.9) were incubated at 25 °C for 10 min. Then, 50 μL of 5 mM p-nitrophenyl-α-D-glucopyranoside solution in 0.1 M phosphate buffer (pH 6.9) was added. The mixtures were incubated at 25 °C for 5 min, before reading the absorbance at 405 nm in the spectrophotometer. The α-glucosidase inhibitory activity was expressed as percentage inhibition (Apostolidis et al. 2007).
Lipid peroxidation and thiobarbituric acid reactions
The rats were decapitated via cervical dislocation, and the pancreatic tissue was rapidly dissected, placed on ice, and weighed. This tissue was subsequently rinsed in cold saline solution and later homogenized in phosphate buffer pH 7.4 (1:5 w/v) with about 10 up and down strokes at approximately 1200 rev/min in a Teflon-glass homogenizer. The homogenate was centrifuged for 10 min at 3000×g to yield a pellet that was discarded, and the supernatant was used for lipid peroxidation assay (Belle et al. 2004). The lipid peroxidation assay was carried out using a modified method of Ohkawa et al. (1979). Briefly, 100 μL of the tissue supernatant was mixed with a reaction mixture containing 30 μL of 0.1 M Tris–HCl buffer (pH 7.4), gallic acid or protocatechuic acid solution, and 30 μL of 250 μM freshly prepared FeSO4. The volume was made up to 300 μL with distilled water before incubation at 37 °C for 1 h. Subsequently, 300 μL of 8.1 % sodium dodecyl sulphate (SDS), 500 μL of acetic acid/HCl buffer (pH 3.4), and 500 μL of 0.8 % TBA were added to the reacting mixture. This mixture was incubated at 100 °C for 1 h, and thiobarbituric acid reactive species (TBARS) produced were measured at 532 nm using a spectrophotometer. Malondialdehyde (MDA) was used as standard, and TBARS produced was reported as MDA equivalent.
Reducing power determination
The reducing power of gallic acid and protocatechuic acid solutions was determined by assessing the ability of the extracts to reduce a FeCl3 solution as described by Oyaizu (1986). A 2.5 mL aliquot was mixed with 2.5 mL, 200 mM sodium phosphate buffer (pH 6.6), and 2.5 mL, 1 % potassium ferricyanide. The mixture was incubated at 50 °C for 20 min, and then, 2.5 mL, 10 % TCA was added. This was then centrifuged at 650×g for 10 min; 5 mL of the supernatant was mixed with an equal volume of water and 1 mL, 0.1 % ferric chloride. The absorbance was measured at 700 nm in the UV–visible spectrophotometer (Model 6305; Jenway, Barloworld Scientific, Dunmow, UK), and reducing power was thereafter calculated as ascorbic acid equivalent (AAE).
Total antioxidant capacity
The total antioxidant capacity of the phenolic acids was determined as their scavenging ability on 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) radical (ABTS+) as described by Re et al. (1999). The ABTS+ was generated by reacting an ABTS aqueous solution (7 mM) with K2S2O8 (2.45 mM, final concentration) in the dark for 16 h and adjusting the absorbance at 734 nm to 0.700 with ethanol. Appropriate dilution of phenolic acids (0.2 mL) was added to 2.0 mL ABTS+ cation solution, and the absorbance was measured at 734 nm after 15 min. The trolox equivalent antioxidant capacity (TEAC) was subsequently calculated.
DPPH free radical scavenging ability
The free radical scavenging ability of the phenolic acids against DPPH free radical was evaluated as described by Gyamfi et al. (1996). Briefly, appropriate dilution of the extracts (1 mL) was mixed with 1 mL, 0.4 mM methanolic solution containing DPPH radicals; the mixture was left in the dark for 30 min, and the absorbance was taken at 516 nm. The DPPH free radical scavenging ability was subsequently calculated as percentage of the control.
Fenton reaction (inhibition of degradation of deoxyribose)
The method of Halliwell and Gutteridge (1981) was used to determine the preventive ability of the phenolic acids against Fe2+/H2O2 induced decomposition of deoxyribose. The phenolic acids (0–100 μL) were added to a reaction mixture containing 120 μL of 20 mM deoxyribose, 400 μL of 0.1 M phosphate buffer, 40 μL of 500 μm of FeSO4, and the volume were made up to 800 μL with distilled water. The reaction mixture was incubated at 37 °C for 30 min, and the reaction was then stopped by the addition of 0.5 mL of 2.8 % trichloroacetic acid. This was followed by addition of 0.4 mL of 0.6 % thiobarbituric acid solution. The tubes were subsequently incubated in boiling water for 20 min. The absorbance was measured at 532 nm in a spectrophotometer. The percentage (%) OH radical scavenging ability was subsequently calculated.
Determination of Fe2+ chelating ability
The Fe2+ chelating ability of the phenolic acids was determined using a modified method of Minotti and Aust (1987) with a slight modification by Puntel et al. 2005. Freshly prepared 500 μM FeSO4 (150 μL) was added to a reaction mixture containing 168 μL 0.1 M Tris–HCl (pH 7.4), 218 μL saline, and the phenolic acids. The reaction mixture was incubated for 5 min, before the addition of 13 μL of 0.25 % 1,10-phenanthroline (w/v). The absorbance was subsequently measured at 510 nm in a spectrophotometer. The Fe(II) chelating ability was subsequently calculated.
Data analysis
The results of replicate experiments (n = 6) were pooled and expressed as mean ± standard deviation (SD). The means were analyzed using one-way analysis of variance (ANOVA), and Duncan test was used for the post hoc treatment. Significance was accepted at P ≤ 0.05.
Results
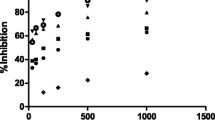
The ability of the phenolic acids (gallic and protocatechuic acids) to inhibit α-amylase and α-glucosidase activity in vitro was investigated, and the result is presented in Figs. 1 and 2, respectively. The result revealed that both phenolic acids inhibited α-amylase and α-glucosidase activities in a dose-dependent manner. As shown in Table 1, the IC50 (concentration of sample causing 50 % enzyme inhibition) value revealed that gallic acid (IC50 = 1.22 μM) had significantly (P < 0.05) higher inhibitory effect on α-glucosidase activities than protocatechuic acid (IC50 = 1.76 μM), however, no significant (P > 0.05) difference when observed in the α-amylase inhibitory activities of gallic acid (IC50 = 1.09 μM) and protocatechuic acid (IC50 = 1.12 μM). Furthermore, the protective ability of the phenolic acids (gallic and protocatechuic acids) against Fe2+-induced lipid peroxidation in rat pancreas is presented in Fig. 3. The result revealed that incubation of the rat pancreas tissue homogenate in the presence of 250 μM Fe2+ caused a significant (P < 0.05) increase in MDA content (116.67 %). The phenolic acids were able to significantly (P < 0.05) decrease the elevated MDA content in a dose-dependent manner (0–1.25 μM). However, the EC50 (concentration of sample that caused decrease in 50 % MDA production) value revealed that gallic acid (IC50 = 0.30 μM) had significantly (P < 0.05) higher inhibition of Fe2+-induced MDA production in the rat pancreas homogenates than protocatechuic acid (IC50 = 0.98 μM) in a dose-dependent manner. In addition, the total antioxidant capacity of the phenolic acids reported as TEAC was presented in Table 2. The result showed that both phenolic acids (GA and PCA) are strong antioxidant molecules capable of scavenging ABTS radicals in vitro. However, gallic acid (0.12 mmol TEAC/100 g) had significantly (P < 0.05) higher total antioxidant capacity than protocatechuic acid (0.09 mmol TEAC/100 g). In a similar manner, the DPPH radical scavenging ability of the phenolic acids was presented in Fig. 4. The DPPH radical scavenging capacity is expressed as 50 % radical quenching concentration of the samples (median effective dose, ED50).
Gallic acid (ED50 = 5.73 μM) had significantly higher (P < 0.05) DPPH radical scavenging ability when compared with protocatechuic acid (ED50 = 8.29 μM) (Table 2). Furthermore, the hydroxyl radical (OH*) scavenging ability of the phenolic acids was also determined, and the result presented in Fig. 5. Both phenolic acids scavenged OH* concentration dependently (0–1.74 μM); however, gallic acid (ED50 = 0.27 μM) had a significantly (P < 0.05) higher hydroxyl radical (OH*) scavenging than protocatechuic acid (ED50 = 0.35 μM) (Table 2). The result of the Fe2+ chelating ability of the phenolic acids was reported in Fig. 6. Judging by the EC50 (effective concentration that will chelate 50 % of Fe2+). Both phenolic acids chelated Fe2+ in a dose-dependent manner (0–2.00 μM). Moreover, gallic acid (EC50 = 0.75 μM) showed significantly (P < 0.05) higher Fe2+ chelating ability than protocatechuic acid (EC50 = 1.33 μM).
Discussion
This present study was convened to assess and compare the antioxidant and antidiabetic effects of GA and PCA through the inhibition of key enzymes relevant to type 2 diabetes (α-amylase and α-glucosidase) as well as FeSO4-induced oxidative stress in isolated rat pancreas homogenates in vitro. Inhibition of these starch-hydrolyzing enzymes (α-amylase and α-glucosidase) has been accepted as an effective modern therapeutic toward the management of diabetes and its related complications (Shim et al. 2003) as this moderates the blood glucose level by slowing down catabolism of glucose into starch. From this study, both phenolic acids (GA and PCA) showed concentration-dependent inhibition of α-amylase and α-glucosidase activities in vitro. However, GA exhibited a stronger inhibition of α-glucosidase activity than PCA. This can be attributed to the additional hydroxyl (OH) group on the structure of GA. GA is a trihydroxylated phenol containing a pyrogallol moiety while PCA is a dihydroxylated phenol molecule containing a catechol moiety (Tanaka et al. 2011; Amorati and Valgimigli 2012). The inhibition of α-amylase and α-glucosidase activities by GA and PCA may therefore point the attention of the phenolic acids as cheap dietary or nutraceutical sources in the management of type 2 diabetes and its complication. The observed inhibition of α-amylase and α-glucosidase by these phenolic acids (GA and PCA) is consistent with earlier study on some phenolic compounds (Lee et al. 2002). Earlier in vivo animal models have also indicated that phenolic may be employable in preventing diabetes (Oberley 1988).
The inhibition of MDA production in rat pancreas tissue homogenate under the influence of Fe2+ as pro-oxidant shown by these phenolic acids (GA and PCA) supports the fact that the phenolic acids are strong antioxidant compounds (Table 1). Fe2+ may participate in Fenton reaction thereby leading to the production of reactive oxygen species (ROS) and subsequently causing damage to membrane biomolecules (lipids and proteins) and ultimately cell death (Puntel et al. 2005). It has been established that the phenolic groups in polyphenols can accept electron thereby forming a phenoxyl radical which, in turn, disrupts chain oxidation reaction in cellular components (Rice-Evans et al. 1997). Phenolic compounds have the ability to delocalize phenoxide ion thereby making them good radical scavengers and antioxidant compounds (Waterman and Mole 1994). The antioxidant capacity of a phenolic compound may depend on some factors including the structure of the phenolic compounds, number of aromatic and hydroxyl groups in their structure, and the distribution of these groups in the structure (Balasundram et al. 2006; Heo et al. 2007; Lindsay 1996; Yuan et al. 2005).
In order to compare the antioxidant properties of GA and PCA, the radicals (DPPH, ABTS, and OH) scavenging and Fe2+ chelating abilities were evaluated. DPPH is a free radical, and it accepts hydrogen or electron to become a stable molecule (Osawa et al. 1995). Activity involving radical scavenging takes into consideration the tendency of hydrogen or electron donation (Hu et al. 2000). Higher ABTS and DPPH radical scavenging abilities shown by GA in comparison to PCA can be attributed to the dihydroxylation and trihydroxylation of PCA and GA, respectively, with the presence of a methylene group (–CH2–) on the para, ortho, and meta positions in their structure which may enhance their radical scavenging properties against ABTS and DPPH (Rice-Evans et al. 1996; Sroka and Cisowski 2003).
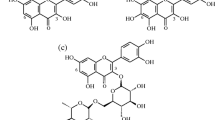
The possible mechanism through which the phenolic acids (GA and PCA) protect the pancreas could be by Fe2+ chelation (Oboh and Rocha 2007) and the scavenging of OH* (Puntel et al. 2005; Oboh and Rocha 2007). In a weakly polar environment, antioxidative properties of phenolic acids are reciprocally proportional to the size of dissociation of the O–H bond (Oboh and Rocha 2007; Gulcin 2012). The mechanism of O–H bond breaking in a phenolic ring involves hydrogen atom transfer to a superoxide radical (Gulcin 2012). The presence of an additional “OH” group and presence of a methylene group (–CH2–) on the GA structure could be an explanation for the higher OH radical scavenging ability shown by GA when compared with PCA (Fig. 7).
The presence of the functional group “COOH” and the arrangement of the OH groups in the meta, para, and ortho positions on structure of these phenolic acids (GA and PCA) may have contributed to their antioxidant and antidiabetic activities (Lindsay 1996; Yuan et al. 2005). This structure–function relationship may therefore account for the observed higher antioxidant and antidiabetic effects of GA when compared with PCA.
Conclusion
This study revealed the structural components of GA and PCA linked to their antioxidant and antidiabetic effects. The antioxidant activities of GA and PCA as well as their inhibition on key enzymes linked to diabetes (α-amylase and α-glucosidase activities) could be part of the mechanism by which the phenolic acids manage and/or prevent type 2 diabetes. However, gallic acid showed higher antioxidant and antidiabetic potentials than protocatechuic acid. Further in vivo experiments and clinical trials are recommended.
References
Ali M, Fauzia BF, Jamal M (2009) Edible compounds as antitumor agent. Indian J Sci Technol 2(5) ISSN: 0974- 6846
Amorati R, Valgimigli L (2012) Modulation of the antioxidant activity of phenols by non-covalent interactions. Org Biomol Chem 10(21):4147–4158
Apostolidis E, Kwon YI, Shetty K (2007) Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov Food Sci Emerg Technol 8:46–54
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 99:191–203
Belle NAV, Dalmolin GD, Fonini G, Rubim MA, Rocha JBT (2004) Polyamines reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res 1008:245–251
Dai J, Russell JM (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–7352
Dongyan T, Yinmao D, Hankun R, Li L, Congfen H (2014) A review of phytochemistry, metabolite changes, and medicinal uses of the common food mung bean and its sprouts (Vigna radiata). Chem Cent J 8(4)
Gulcin I (2012) Antioxidant activity of food constituents: an overview. Arch Toxicol 86:345–391
Gyamfi MA, Yonamine M, Aniya Y (1996) Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. Gen Pharmacol 32:661–667
Halliwell B, Gutteridge JMC (1981) Formation of thiobarbituric acid-reactive substance from deoxyribose in the presence of iron salts. FEBS Lett 128:347–352
Heo H, Kim Y, Chung D, Kim D (2007) Antioxidant capacities of individual and combined phenolics in a model system. Food Chem 104:87–92
Hu C, Zhang Y, Kitts DD (2000) Evaluation of antioxidant and prooxidant activities of bamboo Phyllostachys niger Var. Henonis leaf extract in vitro. J Agric Food Chem 48:3170–3176
Keith RM, Christy LA (2010) Polyphenols as dietary supplements: a double edged sword. Nutr Diet Suppl 2:1–12
Kelsey NA, Wilkins HM, Linseman DA (2010) Nutraceutical antioxidants as novel neuroprotective agents. Molecules 15:7792–7814
Kenneth M, Simona DM, Zhao Z (2007) Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res 4(1):63–71
Kim JS, Kwon CS, Son KH (2000) Inhibition of alpha glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem 64(11):2458–2461
Kunle OF, Egharevba HO (2013) Chemical constituents and biological activity of medicinal plants used for the management of sickle cell disease—a review. J Med Plants Res 7(48):3452–3476
Lee MJ, Chou FP, Tseng TH, Hsieh MH, Lin MC, Wang CJ (2002) Hibiscus protocatechuic acid or esculetin can inhibit oxidative LDL induced by either copper ion or nitric oxide donor. J Agric Food Chem 50(7):2130–2136
Lindsay RC (1996) Food additives. In: Fennema OR (ed) Food chemistry. Marcel Dekker Inc., New York, pp 778–780
Marc YD, Jan AE, Kathrin M, Desiree MS, Helga E, Elisabeth E, Manfred R (2005) Mechanisms of β-cell death in type 2 diabetes. Diabetes 54(2):108–113
Minotti G, Aust S (1987) An investigation into the mechanism of citrate-Fe2+ dependent lipid peroxidation. Free Radic Biol Med 3:379–387
Oberley LW (1988) Free radicals and diabetes. Free Radic Biol Med 5:113–124
Oboh G, Rocha JBT (2007) Polyphenols in red pepper [Capsicum annuum var. aviculare (Tepin)] and their protective effect on some pro-oxidants induced lipid peroxidation in brain and liver. Eur Food Res Technol 225:239–247
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S (1995) Antioxidative activity of tetrahydrocurcuminoids. Biosci Biotechnol Biochem 59: 1609–1612
Oyaizu M (1986) Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Jpn J Nutr 44:307–315
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev 2(5):270–278
Puntel RL, Nogueira CW, Rocha JBT (2005) Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in Rat Brain in-vitro. Neurochem Res 30:225–235
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Riceevans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radic Biol Med 26:1231–1237
Rice-Evans CA, Miller JM, Paganga G (1996) Structure-antioxidant activity relationship of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Rice-Evans CA, Miller NJ, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Sahil K, Souravh B (2014) A review on protocatechuic acid and its pharmacological potential. Hindawi Publishing Corporation ISRN Pharmacology Volume 2014, Article ID 952943,9 pages
Satish P, Sneha A, Ram JS (2011) Evaluation of anti inflammatory activity and in vitro antioxidant activity of Indian Mistletoe, the Hemiparasite Dendrophthoe falcate L. F. (Loranthaceae). Iran J Pharm Res 10(2):253–259
Shahidi F, Naczk M (2004) Phenolic in food and nutraceutical. CRC Press, Boca Raton, pp 1–558
Shim YA, Doo HB, Ahn SB, Kim YC (2003) Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J Ethnopharmacol 85:283–287
Sroka Z, Cisowski W (2003) Hydrogen peroxide scavenging, antioxidant and anti radical activity of some phenolic acids. Food Chem Toxicol 41:753–758
Tanaka T, Tanaka T, Tanaka M (2011) Potential cancer chemopreventive activity of protocatechuic acid. J Exp Clin Med 3(1):27–33
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites. Blackwell Scientific Publications, Oxford
World Health Organization Consultation (1999) Definition, diagnosis and classification of diabetes mellitus and its complications, part 1: diagnosis and classification of diabetes mellitus. Report of a WHO Consultation. World Health Organization, Geneva
Worthington Biochemical Corp (1978) Worthington, enzyme and related biochemicals. Worthington Biochemmical Corp, Freehold
Yuan YV, Bone DE, Carrington MF (2005) Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem 91:485–494
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adefegha, S.A., Oboh, G., Ejakpovi, I.I. et al. Antioxidant and antidiabetic effects of gallic and protocatechuic acids: a structure–function perspective. Comp Clin Pathol 24, 1579–1585 (2015). https://doi.org/10.1007/s00580-015-2119-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-015-2119-7