Abstract
Salt inundation leads to increased salinization of arable land in many arid and semi-arid regions. Until genetic solutions are found farmers and growers must either abandon salt-affected fields or use agronomic treatments that alleviate salt stress symptoms. Here, field experiments were carried out to study the effect of the osmoregulators proline at 200 mg L−1 and glycine betaine at 400 mg L−1 in counteracting the harmful effect of soil salinity stress on canola plants grown in Egypt. We assessed growth characteristics, yield and biochemical constituents. Results show first that all growth characters decreased with increasing salinity stress but applied osmoregulators alleviated these negative effects. Second, salinity stress decreased photosynthetic pigments, K and P contents, whilst increasing proline, soluble sugars, ascorbic acid, Na and Cl contents. Third, application of osmoregulators without salt stress increased photosynthetic pigments, proline, soluble sugars, N, K and P contents whilst decreasing Na and Cl contents. It is concluded that the exogenously applied osmoregulators glycine betaine and proline can fully or partially counteract the harmful effect of salinity stress on growth and yield of canola.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Salinity is one of the major abiotic stresses in arid and semi-arid regions and substantially reduces the average yield of major crops by more than 50% (Bray et al. 2000). Salinity has been estimated to affect over 77 million hectares or 5% of the arable land worldwide. It has been predicted that this will rise substantially and could reach as much as 50% of the irrigated arable area in the next 50 years (Munns and Rawson 1999; Wang et al. 2001). Crop losses due to salinity are of great concern for many countries like Egypt, which rely heavily on agriculture and where it has been estimated that over 1 million hectares is already salt affected (Athar and Ashraf 2009).
Canola or oil-seed rape (Brassica napus L.) is grown mainly for the purpose of the production of edible oil and it is a moderately salt tolerant crop species (Francois 1994). Canola is now the second largest oilseed crop after soybean in the world providing 13% of the world’s supply. Seeds of canola have an oil content of more than 40% and produce post-crushing meals with 35% to 40% protein which is used mainly for animal feed (Snowdon et al. 2007). Canola is important due to the low Erucic acid in its oil which makes it a good quality edible oil but it also has high Erucic acid containing varieties which are used for manufacturing. Seed oils are an important source of fatty acids for human nutrition and hydrocarbon chains for industrial products such as oleochemistry or as a replacement for petroleum products for combustion engines (Friedt and Lühs 1998). Canola is the preferred oil seed crop under Egyptian conditions, especially where salinity and drought are commonplace and in newly reclaimed soil (Weiss 1983).
Several authors who have studied the effects of salinity on Brassicas report reductions in plant height, shoot and root dry weight, leaf number, leaf area, pod number/plant, seed number/pod, 100 seed weight, seed yield/plant, oil and protein content in the seeds Redmann et al. (1994) on canola, Ashraf and Sharif (1998) on Brassica carinata, El-Ghamry et al. (1992) on rapeseed, Francois (1994) on B. napus and Brassica campestris, Hashem et al. (1998) on B. napus and Wright et al. (1995) on canola and Indian mustard.
Currently there is intensive work by many researchers to study the responses of plants to salt stress in order to try to overcome salt injury. One approach is the exogenous application of substances which have been identified at a cellular level to be involved in resistance to stresses such as betaines, proline and antioxidants (Lopez and Satti 1996). Glycine betaine and proline are two major organic osmolytes that accumulate in a variety of plant species in response to environmental stresses such as drought, cold and salinity (Ashraf and Foolad 2007). Proline is an amino acid and is one of the most commonly occurring compatible solutes and plays a crucial major role in osmoregulation and osmotolerance (Rhodes and Hanson 1993; Hasegawa et al. 2000). It protects membranes and proteins against the destabilizing effects of dehydration during abiotic stress. In addition, it has some ability to scavenge free radicals generated under stress conditions (Ashraf and Foolad 2007).
Glycine betaine is an amino acid derivative known for its protective effects in higher plants against salt/osmotic stresses, not only by maintaining osmotic adjustment (Ashraf and Foolad 2007), but by stabilizing many functional units, like the oxygen-evolving PS-II complex (Harinasut et al. 1996), membranes, quaternary structures of complex proteins (Murata et al. 1992) and enzymes such as rubisco. Some plants therefore can protect themselves against abiotic stresses by enhanced synthesis and accumulation of glycine betaine. Exogenous foliar application of glycine betaine has been suggested as an approach to induce stress tolerance in crops with poor or no solute accumulating ability (Ashraf and Foolad 2007). Foliar application of glycine betaine on tobacco and lupin (Agboma et al. 1996) and on wheat and barley (Makela et al. 1996) enhanced leaf area, dry matter accumulation and plant growth as well as increasing soluble sugars. This osmo-induced accumulation of soluble sugars was almost maximal at 1 MPa and did not change significantly at higher osmolarities.
Ashraf and Sharif (1998) on Raphanus sativa and El-Tayeb (1996) on sorghum and peas showed that foliar application of proline increased transpiration rate, leaf area, dry matter accumulation, enhanced growth, increased the germination rate and final germination percentage, water content and amounts of photosynthetic pigments and strongly stimulated the production of polysaccharides, total nitrogen and soluble protein at all salinities and showed that application of proline alleviated the harmful effect of salinity stress. Exogenous application of proline counteracted the adverse effects of salt stress by stimulating growth of cells and plants (Ali et al. 2008) improving metabolism (Alia et al. 1991; Rana and Rana 1996), and reducing oxidation of membrane lipids (Okuma et al. 2004; Yazici et al. 2007) under stress conditions.
Athar et al. (2009) also showed that exogenously applied glycine betaine and proline at the germination and seedling stages alleviated the adverse effects of salt stress on canola cultivars and Okuma et al. (2004) reported that proline-induced alleviation of the adverse effects of salt stress on growth was more pronounced than those of glycine betaine.
The work reported in this paper is the result of trying to determine whether glycine betaine and proline can be applied exogenously in the field and alleviate field induced salt stress under Egyptian conditions. This may then provide an agronomic option for the alleviation of stress which could be used whilst plant breeders and biotechnologists search for genetic and physiological solutions to this problem.
2 Materials and methods
Two field experiments during successive seasons 2006/2007 and 2007/2008 were carried out on the El-Serw farm site, Field Crops Research Department, National Research Centre, Dakahlia governerate, Egypt.
Within a single field of uniform silty-loam soil type with known salt inundation three locations which differed in their soil salinity as measured by a standard sodicity test (Rhoades et al. 1999) were used as follows:
-
Area 1. Low salinity (control) 1.5 dS m−1
-
Area 2. Moderate salinity 9.1 dS m−1
-
Area 3. Severe salinity 13.5 dS m−1
Each area was sub-divided into a randomized block design with three replicates and three treatments randomly allocated to plots within a block.
Phosphorous as calcium superphosphate (350 kg ha−1 P) and potassium as potassium sulphate (120 kg ha−1 K) were added before planting according to local agronomic practice. Canola (B. napus L. cv. Pactol seed (courtesy of the Ministry of Agriculture “Oil Crop Research Center” Giza, Egypt) was sown on 25th November in both experimental seasons. After 20 days from sowing, plants were thinned to a plant population of 50 plants per m2 (interplant spacings of 20 cm). Nitrogen fertilizer was added in three equal doses at 20, 30 and 60 days after sowing as ammonium nitrate (143 kg ha−1 N total).
The plants were treated with distilled water or the osmoregulators, glycine betaine at 400 mg L−1 or proline at 200 mg L−1 plus 0.05% Tween 20 as a wetting agent. Seed was presoaked for 6 h before planting and subsequently plants were sprayed with the same applied osmoregulators at 40 and 70 days post-sowing corresponding to mid-vegetative and flowering stages respectively. Automatic atomizers were used for spraying solutions and plants were sprayed until run-off.
Five plants were taken randomly from each treatment after 60 days from sowing and growth characters, stem and main root length, leaf area and number of leaves per plant and dry weight of shoot and roots were recorded. At harvest after154 days from sowing, the number of flowering branches/plant, seeds/siliqua, weight of 100 seeds, seed yield/plant and seed oil content were also determined. Photosynthetic pigments, proline, sugars, ascorbic acid, N, P, K, Na and Cl contents were determined in the second season.
Seed oil content was determined according to Folch et al. (1957). A 50-g sample of seeds harvested from each plot was finely ground in a household electric grinder. An aliquot (10 g) of ground seed was extracted by homogenization (Polytron homogenizer) in 80 mL chloroform–methanol (2:1 v/v) containing 0.01% butylated hydroxytoluene as an antioxidant for 3 h at laboratory temperature with occasional stirring. The mixture was then filtered through Whatmann No. 1 filter paper. The residual was re-extracted three times with chloroform–methanol for 1 h, then filtered and the three filtrates combined together. The filtrates were shaken (Eberbach Corp. horizontal shaker) and centrifuged for 3 min at 3,000 rpm (1,380×g) to separate the aqueous and organic layers. Upon completion, the aqueous layer was siphoned off, leaving the fatty acid dissolved in the lower, chloroform layer. The chloroform was then evaporated (Organomation Meyer N-evap analytical evaporator model no. 112) under a N stream at 60 C. The resulting product was then transferred to a 15 ml glass screw-top tub to await methylation and analysis.
Photosynthetic pigments were determined in fully expanded young leaves (4th leaf down from the apex) 60 days after sowing. Leaves were cut into pieces and a representative 0.05 g sample extracted in 10 mL methanol 90% for 24 h then both chlorophylls and carotenoids were determined by spectrophotometer at wave lengths 452.5, 650 and 665 nm according to the methods of Mackinny (1941).
Proline content was determined from shoots harvested 60 days after sowing by the modified ninhydrin method (Troll and Lindsley 1955). A 2-g representative sample of green leaf and stem material was placed into a test tube containing 10 mL of distilled water and the tubes were kept in a boiling water bath for 30 min, and then cooled to room temperature. An aliquot from water extract, added 2 mL of ninhydrin reagent and the mixture were maintained in the boiling water bath for 20 min, and then was cooled in an ice-water bath. The reaction mixture was extracted with 2 mL toluene mixed vigorously shaking and left at room temperature for 30 min until separation of the two phases. The chromophore containing toluene (1 mL, upper phase) was wormed to room temperature and its optical density was measured at 520 nm by Spectrophotometer using toluene for a blank.
Shoot and root dry matter samples per plot were macerated to provide homogeneous samples and total soluble carbohydrates were extracted from duplicate 0.1 g dm representative samples of shoots and roots using 80% ethanol overnight at laboratory temperature then filtered through Whatman no. 1 filter paper. Total soluble sugars were determined by the anthron method (Sadasivam and Manickam 1996) by adding 3 mL anthron reagent to 0.1 mL filtrate, heated for 10 min in a boiling water bath, cooled rapidly and the developed green color was read at 630 nm by Spectrophotometer.
Ascorbic acid was extracted from 2 g shoot fresh material by 4% oxalic acid, then made up to 100 mL and centrifuged at 2,000 rpm for 5 min, then 10 mL of 4% oxalic acid was added and titrated with 2,6-dichlorphenol-indophenol as described by Sadasivam and Manickam (1996), the amount of ascorbic acid mg/100 g sample was calculated using the equation
where: V1 = amount of dye reacted with 10 ml of oxalic acid and V2 = amount of dye reacted with 10 mL supernatant.
Total nitrogen content estimated using a micro-Kjeldahl method (Jackson 1967). A sample of 0.2 g of homogenized dry material was digested by sulphuric and perchloric acids and distillation was carried out with 40% NaOH and ammonia was received in 4% boric acid solution.
Sodium, potassium and chloride contents were measured using 0.2 g of homogenized shoot dry matter extracted for 1 h in a boiling-tube of distilled water in a boiling water bath and the extract filtered. Sodium and potassium contents in the aqueous extracts were measured with a Flame Photometer. Meanwhile, chloride was determined by titration with 0.001 N AgNO3 using potassium dichromate as an indicator (Chaudhary et al. 1996).
Phosphorous content was determined colorimetrically at wave length 725 nm using chlorostannous-reduced molybdophosphoric blue colour method, as described by Jackson (1967).
Data were analyzed by analysis of variance according to Gomez and Gomez (1984). Least significant difference values were used for the comparison of means. Data are presented to show the effects of salinity in the first instance (water control) and then the effects of the applied osmoregulators for each measured parameter.
3 Results and discussion
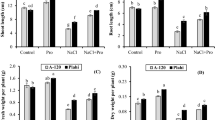
Salinity affected all stages of canola growth and development and resulted in a depression of seed yield (Fig. 1). Seed yield declined proportionately more than the vegetative characters measured and this may be attributed to a decrease in the viability of pollen grains or in the receptivity of the stigmatic surface or both as suggested by Sakr et al. (2007) and caused by decreased calcium mobilization as a result of high sodium levels in leaves. Abscission of flowers or young fruit due to ethylene induction by salinity could explain the reduced pod set observed. Chrominski et al. (1989) also noted a decrease in fruit abscission which led to increasing fruit number and seed number per fruit and consequently increased seed yield per plant and this was attributed to a decrease in ethylene production in the plants. A reduction in the supply of carbon assimilate due to decreased leaf area is also probably responsible for the reductions in the yield components (Sakr et al. 2007). Ozdemir et al. (2004) proposed that the relatively greater uptake of Cl− than Na+ in salt-stressed plants could be responsible for growth reduction by depressing the uptake of other anions (Sakr et al. 2007).
Growth characters including stem and root length, leaf number and leaf area per plant, shoot and root dry weights and flowering branches per plant were all significantly decreased with increasing salinity stress levels and the greatest reduction in these parameters were observed under the highest salinity level (Table 1). These growth parameters reduced the components of yield (pods per plant, seed number per fruit, 100 seed weight per plant) which combined reduced the seed yield by up to 25% at the highest salinity level. This inhibitory effect of salinity may be due to a number of physiological processes such as a decrease in meristematic activity and/or cell enlargement (Khadr et al. 1994; Sakr et al. 2007) or a perturbation of the functioning of vital components of photosynthesis (Yang and Britton 1990) and there was some evidence for this in the decreased levels of chlorophyll measured.
The results of this investigation clearly demonstrated that the adverse effects of salinity could be partially or fully offset by the exogenous application of proline and glycine betaine (Fig. 1 and Table 1). Proline was generally more effective than glycine betaine and proline led to an enhanced seed yield in the absence of salt stress and this effect was maintained as salt stress increased. The exact mechanism of the effect of these osmoregulators is not known but may be due to osmotic protection (Arteca 1996) or promotion of the uptake of essential macro-nutrients which then facilitated normal growth and development (Foyer and Spencer 1986). Whatever the mechanism of action the application of exogenous proline or glycine betaine revealed partial counteracting effects in dry matter reduction caused by salinity stress and this is attributed to a combination of an increase in leaf area and improvement in the components of yield.
Oil content was decreased by increased salinity but the Erucic acid content increased. This increases the danger of such crops for human consumption. All measured growth characters improved with both osmoregulator treatments and improvements were recorded even in the low salinity (control) treatment. Proline was the most effective osmoregulator in enhancing growth characters and led to a significant increase in yield under low salinity. Both osmoregulators partially counteracted the adverse growth effects of salinity stress. The osmoregulators were able to re-establish the oil content of the seed. Ross and Murphy (1993) suggested that increasing sucrose content or the carbon/nitrogen ratio increases oil content due to their effect on the specific and total activity of diacylglycerol acyltransferase and therefore the re-establishment of good growth caused by the osmoregulator was probably responsible for the improved oil content of the seeds. However osmoregulators could not fully reduce the Erucic acid content of the salt affected seed and were therefore not capable of guaranteeing the human consumption safety of the harvested seed.
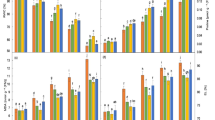
Increasing salinity reduced total chlorophyll and carotenoid levels but increased proline, soluble sugars and ascorbic acid content (Table 2). The nutritional balance of plants was affected by increasing salinity with nitrogen, phosphorus and potassium contents all being depressed whilst sodium and chloride contents increased. This is a commonly found observation under increasing salinity and reflects the limited ability of non-adapted terrestrial plants to control sodium and chloride uptake. Chloride is a more sensitive indicator of salt damage than sodium, since it is stored by the plant. Accumulation of Cl− may cause leaf injury, thereby decreasing photosynthesis and productivity. Ozdemir et al. (2004) proposed that the relatively greater uptake of Cl− than Na+ in salt-stressed plants could be responsible for growth reduction by depressing the uptake of other anions (Sakr et al. 2007). Sodium frequently replaces potassium as cell channels cannot distinguish between these elements and the antagonism between K+ and Na+ cations increases considerably as salinity increases (Sairam and Srivastava 2002). However, the sodium and chloride levels recorded in plants under high salinity in these trials were not extremely different from the low salt condition reflecting the reasonable salt resistance of canola.
When plants are challenged by abiotic stress they frequently produce a stress response to try to mitigate the effects of the stressor and cellular compatible solutes such as proline and sugars rise and confer desiccation tolerance (Ashraf and MacNeilly 2004; Thakur and Rai 1985; Munns and Termaat 1986; Stewart and Lee 1974). Proline accumulation is one of the most frequently reported modifications induced by water deficit and salt stress in plants and it is involved in stress resistance mechanisms (Sakr et al. 2007; Ashraf and Harris 2004). Proline has been associated with the relief of cellular osmotic stress, detoxification of excess ammonia, stabilization of proteins and/or membranes and improving the stability of some cytoplasmic and mitochondrial enzymes (Ozdemir et al. 2004). In this experiment however proline did not rise substantially but soluble sugars did increase and the anti-oxidant ascorbic acid rose significantly under salt stress. With increasing salinity there is an associated soil osmotic effect which limits macro-nutrient uptake such as nitrogen and phosphorous which could account for the depressed macro-nutrient contents recorded. Also an increase in chloride uptake and accumulation is reported to be accompanied by a decrease in shoot nitrate concentrations of plants due to the competition between chloride and nitrate which decreases nitrate content (Sakr et al. 2007).
In this study, osmoregulators were shown to offset the effects of increasing salinity and had some positive stimulatory effects in the absence of salinity stress supporting the reports of others (Ali et al. 2008; Athar et al. 2009; Okuma et al. 2004) and exogenous application of proline was the most effective in this respect. This clearly demonstrated that exogenous applications in the field can be a useful method to alleviate salt induced yield reductions in canola and opens the possibility of using exogenous applications to alleviate stress in the agronomic situation. This study did not attempt to assess the economic aspects of these treatments but such an assessment would clearly be necessary to assess the efficacy of applications in an agronomic situation, however both osmoregulators are readily available. Further work is necessary to investigate whether these compounds could be combined to work synergistically as they have different cellular modes of action. The results indicate that there are exciting opportunities for the alleviation of moderate or severe salt stress in the field.
4 Conclusions
These results clearly demonstrate that exogenous applications of proline or glycine betaine could be used to reduce the harmful effect of salinity on both physiological aspects and growth parameters of canola. They are capable of restoring yield potential and oil content of seed and may be useful useful in agronomic situations where soil sodicity is diagnosed as a problem.
References
Agboma PC, Peltonen-Sainio P, Hinkkanen R, Pehu E (1996) Effect of foliar applied glycinebetaine on yield components of drought-stressed tobacco (Nicotiana tabacum L). Exp Agric 33:345–352
Ali Q, Ashraf M, Shahbaz M, Humera H (2008) Ameliorating effect of foliar applied proline on nutrient uptake in water stressed maize (Zea mays L.) plants. Pak J Bot 40(1):211–219
Alia P, Saradhi PP, Mohanthy P (1991) Proline enhances primary photochemical activities in isolated thylakoid membranes of Brassica juncea by arresting photoinhibitory damage. Biochem Biophys Res Commun 181:1238–44
Arteca RN (1996) Plant growth substances; principles and application. Chapman and Hall, New York, ISBN-13: 9780412039119
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Ashraf M, Harris P (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Ashraf M, MacNeilly T (2004) Salinity tolerance in Brassica oilseeds. Crit Rev Plant Sci 23:157–174
Ashraf M, Sharif R (1998) Does salt tolerance vary in a potential oilseed crop Brassica carinata at different growth stages? J Agron Crop Sci 181:103–115
Athar HR, Ashraf M (2009) Strategies for crop improvement against salt and water stress: an overview. In: Ashraf M, Ozturk M, Athar HR (eds) Salinity and water stress: improving crop efficiency. Springer, The Netherlands, pp 1–16
Athar HR, Ashraf M, Wahid A, Jamil A (2009) Inducing salt tolerance in Canola (Brassica napus L.) by exogenous application of Glycine beanie and proline: response at the initial growth stages. Pak J Bot 41(3):1311–1319
Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to abiotic stress. In: Buchanan B, Gruissem W, Jones R (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiology. Rockville, pp 1158–1203
Chaudhary MT, Wainwright SJ, Merrett MJ (1996) Comparative NaCL tolerance of Lucerne plants regenerated from salt-selected suspension cultures. Plant Sci 114:221–232
Chrominski A, Halls S, Weber DJ, Smith N (1989) Proline affects ACC to ethylene conversion under salt and water stresses in the Halophyte, Allenrolfea occidentalis. Environ Exp Bot 29(3):359–363
El-Ghamry WM, Al-Ahmar BA, El-Kafoury AA, Habib MS (1992) Salt tolerance of six varieties of rapeseed. Proceedings 5th Conference of Agronomy, Zagazig, 13–15 Sept., 1992, vol. (2):908–917
El-Tayeb M (1996) Effect of proline application on two salinity stressed crop plants 2-Growth and some metabolic activities. Bull Fac Sci 25(1-D):21–30, Assiut Univ
Folch J, Lee M, Sloane-Stanley GH (1957) A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509, In: Food Agriculture Organization (FAO) Production year Book, 1999
Foyer C, Spencer C (1986) The relationship between phosphate status and photosynthesis in leaves. Effects on intracellular orthophosphate distribution, photosynthesis and assimilate partitioning. Planta 167:369–375
Francois LE (1994) Growth, seed yield, and oil content of canola grown under saline conditions. Agron J 86(2):233–237
Friedt W, Lühs W (1998) Recent developments and perspectives of industrial rapeseed breeding. Fett-Lipid 100:219–226
Gomez KA, Gomez AA (1984) Statistical procedures for Agricultural Research, 2nd edn. Wiley, New York, p 680
Harinasut P, Tsutsui K, Takabe T, Nomura M, Kishitani S (1996) Exogenous glycine betaine accumulation and increased salt tolerance in rice seedlings. Biosci Biotechnol Biochem 60:366–368
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Hashem MN, Majumdar A, Hamid A, Hossain MM (1998) Drought stress effects on seed yield, yield attributes, growth, cell membrane stability and gas exchange of synthesized Brassica napus L. J Agron Crop Sci 180:129–136
Jackson ML (1967) Soil chemical analysis, 1st edn. Prentice Hall of India Pvt. Ltd, New Delhi, pp 144–197
Khadr I, Nyireds F, Shanahan F, Nielsen C, Andria R (1994) Ethephon alters corn growth under drought stress. Agron J 86:283–288
Lopez ML, Satti SME (1996) Calcium and potassium enhanced growth and yield of tomato under sodium chloride stress. Plant Sci 114:19–27
Mackinny G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Makela P, Peltonen-Sainio P, Jokinen K, Pehu E, Setaia H, Hinkkanen R, Somersalo S (1996) Uptake and translocation of foliar-applied glycine betaine in crop plants. Plant Sci 121:221–230
Munns R, Rawson HM (1999) Effect of salinity on salt accumulation and reproductive development of the apical meristem of wheat and barley. Aust J Plant Physiol 26:459–465
Munns R, Termaat A (1986) Whole plant responses to salinity. Aust J Plant Physiol 13:143–160
Murata N, Mohanthy PS, Hayashi H, Papageorgiou GC (1992) Glycinebetaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen-evolving PS-II complex against the inhibitory effects of NaCl. FEBS Lett 296:187–9
Okuma E, Murakami Y, Shimoishi Y, Tada M, Murata Y (2004) Effects of exogenous application of proline and betaine on the growth of tobacco cultured cells under saline conditions. Soil Sci Plant Nutr 50(8):1301–1305
Ozdemir O, Melike B, Tijen D, Ismail T (2004) Effects of 2,4-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regul 42:203–211
Rana VK, Rana U (1996) Modulation of calcium uptake by exogenous amino acids in Phaseolus vulgaris seedlings. Acta Physiol Plant 18:117–20
Redmann RE, Qi MQ, Belyk M (1994) Growth of transgenic and standard canola (Brassica napus) varieties in response to soil-salinity. Can J Plant Sci 74(4):797–799
Rhoades JD, Chanduvi F, Lesch S (eds) (1999) Soil salinity assessment. Methods and interpretation of electrical conductivity measurements. Irrigation and Drainage Paper No 57. FAO, Rome, p 153
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol 44:357–384
Ross JHE, Murphy DJ (1993) Differential accumulation of storage products in developing seeds and somatic cell cultures of Daucus carota L. Plant Sci 88:1–11
Sadasivam S, Manickam A (1996) Biochemical methods, 2nd edn. New Age International (P)Ltd., Publishers, New Delhi, ISBN 81-224-0976-8
Sairam RK, Srivastava GC (2002) Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162:897–904
Sakr MT, El-Emery ME, Fouda RA, Mowafy MA (2007) Role of some antioxidants in alleviating soil salinity stress. J Agric Sci Mansoura Univ 32:9751–9763
Snowdon R, Lühs W, Friedt W (2007) Oilseed Rape. In: Kole C (ed) Genome mapping and molecular breeding in plants. Springer, Berlin, pp 55–114
Stewart CK, Lee JA (1974) The role of proline accumulation in halophytes. Planta 120:279–289
Thakur PS, Rai VK (1985) Exogenously supplied amino acids and water deficits in Zea mayz cultivars. Biol Plant 27:458–461
Troll W, Lindsley J (1955) A Photometric method for the determination of proline. J Biol Chem 215:655–660
Wang W, Vinocur B, Shoseyour O, Altman A (2001) Biotechnology of plant osmotic stress tolerance: physiological and molecular considerations. Acta Horticult 590:286–292
Weiss EW (1983) Oilseed crops. Longman, London, 660
Wright PR, Morgan JM, Jessop RS, Cass A (1995) Comparative adaptation of canola (Brassica napus) and Indian mustard (B. juncea) to soil water deficits: yield and yield components. Field Crop Res 42:1–13
Yang A, Britton G (1990) Carotenoids and Stress. In: Alscher RG, Cummings JR (eds) Stress responses in plant adaptation and acclimation mechanisms. Wiley-Liss, New York, pp 87–112
Yazici I, Turkan F, Sekmen AH, Demiral T (2007) Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environ Exp Bot 61(1):49–57
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sakr, M.T., El-Sarkassy, N.M. & Fuller, M.P. Osmoregulators proline and glycine betaine counteract salinity stress in canola. Agron. Sustain. Dev. 32, 747–754 (2012). https://doi.org/10.1007/s13593-011-0076-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13593-011-0076-3