Abstract
Stingless bees were collected throughout the state of Rondônia in the southwestern Brazilian Amazon for 1 year. The impact of agricultural colonization and subsequent deforestation on species composition and richness is explored. Deforestation, around each of 187 sample sites, was characterized at the mesoscale, microscale, and local spatial scale. At the microscale, deforestation was measured using a data layer generated by satellite remote sensing and analyzed with the assistance of a geographic information system. We report perhaps the greatest richness of stingless bees ever recorded in the Tropics, collecting 9,555 individuals from 98 species of stingless bees. Ten of these are new species and 16 were first-ever records for Rondônia. Five new species were scientifically described from the study. We report statistical relationships between deforestation and species richness at all spatial scales of analysis, and we tentatively identify species that appear to be especially sensitive to deforestation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bees are considered to be some of the most important pollinators of both wild and cultivated plants (Michener 2000), responsible for pollinating at least 60 % of the nearly 1,500 species cultivated by humans (Garófalo 2009). Stingless bees have attracted the attention of researchers interested in the effects of deforestation and forest fragmentation on pollinators (Liow et al. 2001; Cairns et al. 2005; Villanueva-Gutiérrez et al. 2005; Ricketts et al. 2008; Brosi 2009; Brosi et al. 2007, 2008; Freitas et al. 2009; Fierro et al. 2012). The main conclusion from this work is that tropical deforestation affects greatly the abundance, diversity, and composition of stingless bees and that deforestation could have serious consequences for the pollination and reproduction of both native and cultivated plants. Few studies exist, however, from the Brazilian Amazon, in spite of its putative high diversity of bees (Oliveira et al. 1995; Oliveira 2001; Dick 2001; Brown and Albrecht 2001).
In Brazil, stingless bees comprise one of the country's most species-rich groups, with 192 recorded species (Silveira et al. 2002); the actual number of species is likely much higher, considering how poorly sampled bees are in the Brazilian Amazon (Overal 2001; Baccaro et al. 2008). There is a nearly 40-year gap in the generation of knowledge of these bees in the region. That gap runs from the last study of Adolpho Ducke (1925), who pioneered the study of these bees in the region, to expeditions that began in the region in 1963 (Camargo 1994). There are also major spatial gaps in our knowledge. Most of the work cited previously was carried out along the margins of major rivers (Camargo 1994) and near major urban areas (Oliveira et al. 1995; Oliveira 2001), with almost no work in the immense areas between rivers, near headwaters, or in mountainous areas (Oliveira et al. 2010). While our knowledge of these bees is relatively sparse in the region, deforestation due to the development of infrastructure, mining, and agricultural colonization continues throughout the Amazon, in spite of notable decreases in Brazil and several other Amazonian countries from 2005 to 2010 (Colombia, French Guiana, and Peru actually saw increases in deforestation rates) (PRODES 2011; RAISG—Amazonian Network of Georeferenced Socio-Environmental Information 2013).
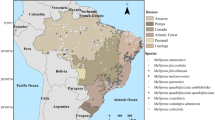
The landscape of the state of Rondônia, Brazil, the focus of the present study, is emblematic of the effects of development in the Brazilian Amazon on forest cover and biodiversity. Up until the 1960s, the forests of Rondônia were relatively intact, the main forest type being “tropical moist forest” in the northern half of the state and “subtropical moist forest” according the Holdridge Life-Zones map (International Institute for Applied Systems Analyses (IIASA) 1989). Agricultural colonization projects were established there beginning in the 1960s. Ouro Preto do Oeste (hereafter “Ouro Preto”) was the first colonization project established in Rondônia, it is 1 of 13 meso-regions of the state surveyed for the present study, and unlike the other regions, it was chosen for intensive monthly sampling during the study period for the following reasons. Ouro Preto's landscape is a microcosm of the range of deforestation landscapes found across the state, with environments representing some of the longest settlement history in the state and some much more recent, leading to areas ranging from very high to very low levels of deforestation, respectively (Figure 1). In general, then, farm lots within 16 km of the main BR-364 highway bisecting the state from southeast to northwest had very little forest remaining by 1996, when the present study was conducted; in contrast, there is much more forest remaining on farm lots in Mirante da Serra near the indigenous reserve and the national park. Other reasons for choosing Ouro Preto for year-round surveys include its convenient central location in the state and availability of laboratory space. Regular sampling in Ouro Preto during the entire study allowed us to test whether seasonality needs to be taken into account when conducting more rapid, one-time surveys of stingless bees in the state. The present study examines the relationship between deforestation, caused by modern settlement in the Amazon, and the composition and richness of stingless bees.
2 Materials and methods
2.1 Dependent and independent variables
Species composition and richness data (dependent variables) for each collection location were grouped in three main ways for analysis, each way representing the impact of colonization and subsequent deforestation at the mesoscale, microscale, and local scale. Deforestation levels at the mesoscale and microscale (independent variables) were determined by overlaying points recording the latitude and longitude of the sample locations over a data layer depicting forest and non-forest cover available from PRODES (Amazon Deforestation Calculation Program) from INPE (National Institute of Space Research) (Câmara et al. 2006). Circles with radii of numerous distances (0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 km) were drawn around each collection point, and the percent area deforested within each circle was calculated using ArcGIS (ESRI). (As shown in the succeeding paragraphs, the 0.5-km deforestation parameter was determined to be the most significantly related to the species variables, so it was used as the main deforestation variable at the meso-region level.)
Every meso-region of the state surveyed that has undergone modern agricultural settlement was characterized in terms of the year it received its first major influx of agricultural colonists, allowing two main types to be recognized: those settled 1980 and before are considered “older” and those settled 1981 and after are considered “newer.” These designations were made based on the history of each colonization area provided by Fearnside (1989). Meso-regions are labeled “areas of preservation” when they are under some form of permanent state or federal protection and have not undergone any modern agricultural settlement (indigenous reserves, extractive reserves, and state and national parks).
-
1.
Meso-regional scale: This scale of analysis allows comparisons of species composition and richness among colonization areas of different ages and to compare these with meso-regions that have experienced little or no impact of modern settlement because older areas have been disturbed for longer periods of time and have higher levels of deforestation and higher forest fragmentation.
-
2.
Micro-regional scale: This scale allows for analysis of impacts in the more immediate area of collection locations. Deforestation variables were generated for each sample location as described previously using the latitude and longitude of sample locations and a data layer from INPE depicting forest and non-forest cover in 1997.
-
3.
Local scale: This scale of analysis accounts for the immediate land cover of the collection location at the sub-location level. Each sub-location was characterized as closed canopy forest or open vegetation formations, which included savanna, secondary vegetation, cropland, and pasture.
Statistical analyses involved a number of different techniques to explore the relationships among deforestation, species richness, and composition at the previously described spatial scales. These included scatter plots, ordination (conducted using Canoco 5), ordinary least squares regression, and by comparing the summary statistics of all locations within particular ranges of deforestation level using box plots. Regarding ordination, detrended correspondence analysis of species composition across sample locations indicated the use of linear methods, so redundancy analysis (RDA) was used for all ordination. Before conducting the ordination, rare species were removed from the dataset by excluding species that appeared in <5 % of sample sites. For analyses involving just the Ouro Preto meso-region, this left 62 species, and for the statewide dataset, this left 63 species.
2.2 Choice of collection locations
Fieldwork was sponsored by the Second Approximation Project of the Socio-Economic–Ecological Zoning of Rondônia, funded by the World Bank, and executed by Tecnosolo/DHV Consultants. Selection of locations for sampling species composition and richness had to take into account the main access ways to the priority areas of the research for the zoning exercise: the federal highways BR-364 and BR-429, secondary roads, and the Ouro Preto and Cautário Rivers (Figure 1). Collections were most often done where access was easiest. Factors such as heavy rains, poor road conditions, and the need to obtain permission from landowners to enter properties often limited access. Examination of the number and spatial distribution of sample locations throughout the state, however, suggests that the data are unprecedented in spatial coverage in comparison to other stingless bee surveys, and they are representative of the state and its varied forest cover conditions. It bears repeating that the meso-region of Ouro Preto was surveyed every month of the study to allow for testing the hypothesis that seasonality must be taken into account when conducting stingless bee surveys. Other meso-regions of the state were surveyed only once.
2.3 Collection methods
From September 1996 to September 1997, six locations were sampled each month in the Ouro Preto meso-region. Each month, a separate meso-region of the state was selected for a survey expedition that lasted from 5 to 10 days. Independent of the location, collections were always made beginning after 7 h and ending before 18 h. Bees were sampled in a total of 187 locations during the study. In each meso-region sampled, care was taken to ensure that collections were done at least 1.5 km apart in an effort to decrease the chances of capturing bees from the same colony. The latitude and longitude of every location was recorded with the aid of a Garmin 45 GPS.
Collections were standardized in order to allow for comparisons across locations. Each location was divided into three sub-locations:
-
1.
Open area sub-location 1 (open canopy): Land cover in these areas was characterized by crops, savanna, pasture, or fallow, secondary vegetation. The nearest forest was approximately 250–500 m away, forming in most cases the very back of a farm lot. Each of three collectors then located a bush, with each bush separated by 50 m along a straight line parallel to the forest edge. Each collector took a plastic spray bottle filled with a 1:1 mixture of honey and water and sprayed an approximately 0.25 m2 surface area on each bush with 15 pumps of the spray bottle. Then, collectors waited at each bush for 60 min and captured bees as they arrived. This is a variation on a common technique first published by Wille (1962).
-
2.
Forest area sub-location (closed canopy): Collectors penetrated the nearest forest area by approximately 250 m, repeating the same honey and water spray procedure described previously. With an hour spent collecting in both sub-locations 1 and 2, 2 h was spent at spray locations as a whole in each sample location.
-
3.
Open area sub-location 2 (open canopy): Collectors returned to the initial open area sub-location and collected bees randomly found on flowers in the open.
The order of these collections was altered each time in order to generate heterogeneity in the relationship between land cover and the timing of the collections. At all times and in all land covers, bees were opportunistically collected when found on the following substrates: mud, human skin (collecting sweat), water, feces, and carcasses. When discovered, bees were collected at their nest entrances.
2.4 Mounting and identification of specimens
After capture, bees were killed with ethyl acetate and placed in labeled plastic film canisters lined with tissue paper. Samples were transported to Drs. João M. F. Camargo and Sílvia R. M. Pedro for species identification.
3 Results
3.1 Overall species composition in Rondônia
The study resulted in the collection of 9,555 individuals from 98 species of stingless bees, with 10 new species and 16 recorded for the first time ever in the state of Rondônia (Supplementary Material Table S1) (see Camargo and Pedro 2007). This diversity is very high in comparison to other surveys in the Tropics (Table I). Five new species were scientifically described from the study: Dolichotrigona mendersoni, Dolichotrigona browni, Dolichotrigona rondoni, Celetrigona hirsuticornis, and Leurotrigona gracilis. All indications are that D. rondoni is endemic to Rondônia (see Camargo and Pedro 2005). The other five new species are still waiting for description (Supplementary Material Table S1).
3.2 Statewide species composition by capture/substrate type
Most of the species in the current study were found on many different substrates (Supplementary Material Table S1). The exceptions are Duckeola ghilianii, Frieseomelitta flavicornis, Frieseomelitta portoi, Lestrimelitta limao, Melipona illustris, Schwarzula coccidophila, and some species of Paratrigona. Moreover, many Trigonisca, all relatively rare in the collection, were found on no more than two substrates.
3.3 Ouro Preto meso-region analysis
In 73 locations sampled during the year in the Ouro Preto meso-region, there were 82 species of stingless bees (Figure 2), which equals almost 74 % of the total species (98) that were found in the entire state of Rondônia. In an analysis of sampling effort and species accumulation, it was determined that sampling in five locations led to collection of 70 % of the species that would be found in the Ouro Preto meso-region.
In Figure 3a, the number of species found at each location is plotted with the distance in kilometers the location lies along the road that runs perpendicular to BR-364, from Ouro Preto (km 0) southwest through the urban centers of Nova União and Mirante da Serra all the way to the border with the area of Uru-Eu-Wau-Wau Indigenous Reserve and the Pacaás Novos National Park (km 84). There is great variation in the number of species per location, independent of the distance from BR-364. A Lowess smoother drawn through the scatter plot (Velleman 1980), however, shows a very slight trend toward more species with greater distance from BR-364. An ordinary least squares regression line fit to the data shows a statistically significant slope of positive correlation, but the slope is very slight. The effect of deforestation on species richness is most visible when plotting richness and the percentage of area deforested within 0.5 km of the sample location (Figure 3b), independent of distance from BR-364. RDA of the deforestation variables from all the distances indicated that deforested area within 0.5 km of the sample location had the greatest fit of all the deforestation variables in characterizing species composition (Figure 3c), though the results are marginally significant. The first axis eigenvalue in the RDA was 0.0435 (Monte Carlo permutation test (499 permutations), F ratio = 2.8, P = 0.066). In forward selection using just the 0.5-, 1-, and 2-km deforestation variables, the 0.5-km variable explained 3.3 % of species composition, and out of the three variables, it contributed 56 % to species variation (pseudo-F ratio = 2.4, P = 0.004) (Figure 3d).
a Scatter plot of stingless bee species richness in each sample location vs. the distance of the location from the BR-364 highway, Ouro Preto meso-region, Rondônia, Brazil. b Number of species found in each location vs. the percent area deforested within 0.5 km of the sample locations. Both linear regression lines are significant (P ≤ 0.0001). c Bi-plot of species and 0.5 km deforestation variable, all species. d Bi-plot showing only the 15 best-fitting species (DolcLong Dolichotrigona longitarsis, FrieSilv Frieseomelitta silvestrii, MelBr Melipona brachychaeta, MelpFulg M. fuliginosa, MelpSchw Melipona schwarzi, PartVici Partamona vicina, PlbAffMn Plebeia aff. minima, PlebKerr Plebeia kerri, PlebMarg Plebeia margaritae, PlebeSp1 Plebeia sp. 1, PlebeSp2 Plebeia sp. 2, SchwTimd Schwarzula timida, TetrgSpN Tetragona sp.n., TrigGrae Trigonisca graeffei, TrigHirt Trigonisca hirticornis)
Data were grouped into collections made during the dry season (May–September) and those made during the wet season (October–April), and the null hypothesis that there is no difference in species richness between them was tested. A visual examination of box plots showing the distribution of data in both wet and dry seasons shows there is no statistically significant difference between the groups because the shaded areas of the box plots (marking 95 % confidence intervals around the median) overlap one another (Figure 4).
3.4 Meso-regional analysis
Because of logistical reasons, the number of locations sampled in each meso-region was variable. The most extreme cases are Ouro Preto with 73 locations and some samples taken near BR-364 near Porto Velho with only 3 (Table II).
3.4.1 Species composition
The species found in each meso-region are listed in Supplementary Material Table S2). Thirty-eight species appeared in 10 (two thirds) of the 15 meso-regions sampled in the state, independent of the history of colonization or level of deforestation. Forty species were found in 5 (one third) or less regions. Six of the least common species were found exclusively in areas of preservation and four exclusively in areas of newer colonization and, hence, less deforested (Supplementary Material Table S2).
3.4.2 Species richness
The highest mean bee species richness was found in Extrema, Machadinho–Cujubim, São Miguel do Guaporé, Campo Novo, and Costa Marques, meso-regions of the state where we would expect to find a greater richness of species, given that they were colonized most recently and where deforestation levels are lower. In comparison, Linha D, Ouro Preto, Rolim de Moura, Chupinguaia, and Pimenteiras, all with a low mean number of species, were colonized much longer and, thus, have experienced much more deforestation. The difference between species richness found in older vs. newer settlements is statistically significant, as evidenced by the box plots in Figure 5.
Comparison of stingless bee species richness per sample location across type of meso-region in Rondônia state, Brazil. Shaded areas are 95 % confidence intervals around the median (AP, n = 54; newer, n = 31; older, n = 102). AP area of preservation, newer meso-regions receiving greatest influx of migrants 1981 and later, older meso-regions receiving greatest influx of migrants 1980 and earlier.
There was an unexpected low of mean species richness in official environmental preservation areas (AP in Figure 5): the Reserva Extrativista do Rio Cautário, the Reserva Extrativista do Rio Ouro Preto, the Estação Ecológica Cuniã, and the Parque Estadual Guajará-Mirim.
3.5 Micro-regional analysis
3.5.1 Species composition
Sixty-one species (62 %) were found at least once, no matter the level of deforestation, suggesting that these bees may have some level of tolerance to deforestation and fragmentation of the landscape (Supplementary Material Table S3). In contrast, 27 species (27 %) were the only species absent from highly deforested areas (80–100 % deforestation), suggesting a susceptibility to deforestation. RDA showed a statistically significant explanation of the variation in species composition using dummy variables for the meso-region of each sample site and the 0.5-km deforestation variable as environmental variable (adjusted explained variation, 3.5 %; Monte Carlo permutation test results (499 permutations): first axis (pseudo-F ratio = 4.4, P = 0.004), all axes (pseudo-F ratio = 1.5, P = 0.002) (Figure 6). The 0.5-km deforestation variable by itself was insignificant (results not shown).
a RDA species–environmental variables bi-plot of samples from all meso-regions (63 most common species, 0.5 km deforestation variable, and dummy variables for all meso-regions). b Plot from same analysis as a, but showing only the 20 species with the best fit; species in the two right hand quadrants are the most negatively correlated to the 0.5-km deforestation variable. DolcLong D. longitarsis, LeurMuel Leurotrigona muelleri, MelBr M. brachychaeta, MelpSchw M. schwarzi, NannMeln Nannotrigona melanocera, PartTest Partamona testacea, PlbAffMn Plebeia aff. minima, PlebeSp1 Plebeia sp. 1, PlebeSp2 Plebeia sp. 2, PtlLr Ptilotrigona lurida, ScaptSp2 Scaptotrigona sp. 2, TetrAngs T. angustula, TetrDors Tetragona dorsalis, TrigAmaz Trigona amazonensis, TrgCilCl Trigona cilipes, TrigChan Trigona chanchamayonensis, TrigCras Trigona crassipes, TrignSp2 Trigona sp. 2, TrignSp3 Trigona sp. 3, TrigTruc Trigona truculenta.
3.5.2 Species richness
A scatter plot of the percentage of area deforested within 0.5 km of the location of each sample site and species richness for all locations across Rondônia (Figure 7a) shows a similar situation to that found for the Ouro Preto meso-region (Figure 3). Results for the analyses performed using other radii (1 and 2 km) were insignificant, suggesting that the bees respond to more local, less regional deforestation patterns (results not shown). This test matched the results for species composition found using RDA (Figure 3). There is a wide diversity of bees that can be found at each level of deforestation. There is, however, a slight trend toward decreasing diversity when deforestation percentage rises. The scatter plot shows a line fit to the data using ordinary least squares regression, which is statistically significant (P ≤ 0.0001). The data were then grouped into sample sites of four different deforestation levels (0 to <10, 10 to <45, 45 to <80, and 80 to 100 %), with no significant differences among the groups (results not shown). A significant difference did appear, however, when a medium deforestation category was created from 10 to <80 %, compared to the 0 to <10 and 80 to 100 % categories (Figure 7b).
Relationship of stingless bee species richness and deforestation across the state of Rondônia, Brazil. a Scatter plot of the percentage of area deforested within 0.5 km of sample points and species richness. Slope of ordinary least squares regression line is significant at P ≤ 0.0001. b Summary statistics and box plots of distribution of data for groups of deforestation level (low 0 < 10 %, medium 10 < 80 %, high 80–100 %). Shaded areas of box plots are 95 % confidence intervals around the median.
3.6 Local-scale analysis
3.6.1 Species composition
A total of 79 (80 %) species were found in both open vegetation and closed canopy land covers, 7 only in closed canopy forest, and 8 only in open vegetation (Supplementary Material Table S4). RDA of a dataset that considered each open and closed canopy collection as a separate sample(n = 401) showed a significant difference in species composition between open and closed canopy sites (3 % adjusted explained variation, all axis permutation test (Monte Carlo, 499 permutations), pseudo-F ratio = 13.5, P = 0.004) (Figure 8).
RDA species–environmental variable bi-plot of 401 sample sites of open vs. closed canopy collections. a All species, b 20 best-fitting species on horizontal axis. DolcLong D. longitarsis, FrieTric Frieseomelitta trichocerata, MelpGran M. grandis, ParatSpN Paratrigona sp. n. aff. lineata, PartNham Partamona nhambiquara, PartVici P. vicina, PlbAffMn Plebeia aff. minima, PlebeSp1 Plebeia sp. 1, PlebeSp2 Plebeia sp. 2, PlebVari Plebeia variicolor, ScauLati Scaura latitarsis, ScauLong Scaura longula, TetrClav Tetragona clavipes, TetrGoet Tetragona goettei, TetrAngs T. angustula, TetrWeyr Tetragonisca weyrauchi, TrigAmaz T. amazonensis, TrigCras T. crassipes, TrigChan Trigona chanchamayoensis, TrignSpN Trigona sp. n.
3.6.2 Species richness
Species mean richness was slightly higher in open canopy vs. closed canopy environments (8.6 vs. 7.5 species), but as shown in the accompanying box plots, there is no significant difference between the two distributions (Figure 9).
We also analyzed a subset of our samples for only those bees found in nests at each location (Supplementary Material Table S5). Seven species were found only under open canopy, 15 only in closed canopy environments, and 9 in both closed and open canopy formations.
4 Discussion
There are at least 12 bees that have been recorded in the state, but they were not found in the present study, in spite of the immense spatial coverage and number of locations sampled: Lestrimelitta rufa, Lestrimelitta rufipes, Lestrimelitta maracaia, Melipona dubia, Melipona amazonica, Oxytrigona mulfordi, Plebeia alvarengai, Trigona lacteipennis, Trigona guianae, Trigonisca nataliae, Trigonisca pediculana, and Scaptotrigona sp. n. (Camargo and Pedro 2007). Rondônia can be said now to have 110 species, known as one of the most species-rich and sampled in the Tropics with respect to stingless bees. A discussion of potential taxonomic issues with our list of species can be found in the Supplementary Material. The large number of substrates examined for collecting in this study likely helped maximize the number of species found in each location, with nearly 80 % of all species captured from flowers, from honey baits, and on skin (collecting sweat) (Supplementary Material Table S1).
The evidence for the impact of colonization and subsequent deforestation on stingless bees was most visible from the more intensive yearlong collection in the Ouro Preto meso-region. More species were found there than in any other meso-region, likely due to the large number of samples taken throughout the year. On the left side of Figure 3a are data from collection sites that are closest to BR-364 and the urban center of Ouro Preto. Thus, they are within the oldest areas of colonization, ones that consequently are the most deforested as well. From km 4 to 32, the number of species tends to rise, indicating that, with distance from BR-364, the species richness rises. From km 32 to 60, however, richness decreases somewhat, which could be attributed to deforestation and urban impact, because km 40 is the center of the urban area of Nova União and km 60 is Mirante da Serra. Past km 60 to 84, the end of colonization and the border of the indigenous reserve and national park, richness tends to rise again, with three sites of very high richness at km 84. There was, however, a statistically significant relationship between species richness and deforestation within 0.5 km of the sample locations (Figure 3a, b). The RDA bi-plot in Figure 3d shows the 15 best-fitting species, with all but Melipona fuliginosa strongly negatively correlated with the deforestation variable, indicating sensitivity to higher levels of deforestation. The 0.5-km deforestation variable appears the most significantly related to species composition and richness (over other radii). We would expect a short-range variable like this to be significant given our understanding from the literature that stingless bee activity cannot be expected to extend >2–3 km (Kerr et al. 1962; Roubik and Aluja 1983; Souza et al. 1996; Nogueira-Neto 1997, p. 89; Carvalho-Zilse and Kerr 2004; Kuhn-Neto et al. 2009). Our results from Ouro Preto also indicate that species richness is not affected by seasonality, as evidenced by Figure 4. This matches our understanding that stingless bee nests are permanent and individuals are actively foraging throughout the year.
The meso-regional analysis showed that stingless bee richness is affected by deforestation in a statistically significant way, but not very substantively, with perhaps a few species less found on average between the most and least deforested sites across the state. Aggregating the numerical values to categorical levels of deforestation (high, medium, and low) did show significantly higher richness at medium levels when compared with high and low levels (Figure 7). High and low levels of deforestation with similar richness are difficult to explain. The low-level areas involve a significant number of samples from areas of preservation, where deforestation levels were near zero. Collections there did result in some sites with high richness, but many sites were very low, bringing down the mean. The low mean in the Estação Ecológica Cuniã, an official environmental preservation area, could be because of a strong cold front from Antarctica that penetrated the southern Amazon in June during the fieldwork. The temperature could have been outside the range of tolerance for the bees those days, explaining the low numbers found. The low mean species richness found in the other areas of preservation, however, was not related to any abnormal weather. One possible explanation for low richness is that, in very large preserved areas, the sampling methods used were simply inadequate to detect the existing diversity. In smaller forested patches, the chances may be higher to find greater diversity. M. L. Oliveira (personal observation) found a similar situation when sampling orchid bees (Euglossini) in the region of Manaus, and J. M. F. Camargo (unpublished data) remarked that stingless bees in the Amazon express very patchy distribution, with many species concentrated in few places, leaving some larger areas with low diversity within forested areas.
The RDA at the meso-region level revealed some important results. The 0.5-km deforestation variable alone was insignificant in explaining species composition, unlike the case when only the Ouro Preto meso-region alone was analyzed. An ordination that included dummy variables for the meso-region of each sample site, however, was statistically significant (Figure 6), and showing the 20 best-fitting species in the species–environmental variables bi-plot showed a group of species highly negatively corrected to deforestation, indicating possible susceptibility to deforestation. Finally, the local scale analysis showed no statistically significant difference in species richness, but RDA of species composition showed a statistically significant difference between closed and open canopy environments. An examination of the 20 best-fitting species along the horizontal axis allowed for identification of potentially susceptible species (Figure 8).
To sum up the effects of agricultural colonization and subsequent deforestation, we look to the redundancy analyses to identify particular species and groups that appear most strongly affected (Figures 3, 6, and 8). We start by selecting the best-fit species in each of the three ordinations and, from those, selecting the most negatively correlated with the deforestation variable. We then list those species as a first cut of the most affected by deforestation. Relative susceptibility within this list can be further determined by seeing which species appear most frequently in the list across the ordinations.
In Table III, the species that appear in the corresponding ordinations according to the previously discussed rules are marked with an “X.” It bears repeating that these ordinations are based on datasets that exclude rare species, so they are all species that are widespread in Rondônia and independent of the region in which they were found showed negative correlation with the 0.5-km deforestation variable and were mainly found in closed canopy environments.
Clearly, not all species known to prefer cavities in live trees are in our list of species most likely affected by deforestation. This may be because they were too rare in our survey to be included in the redundancy analyses or their presence has yet to be affected by deforestation. For any species to persist in an area undergoing deforestation, stingless bees must survive the physical destruction that occurs during tree felling, the initial burning of the trees, and subsequent burns, sometimes annual, especially in areas of cattle pasture. Bees that persist must have the ability immediately to rectify and rebuild nest architecture and then survive repeated burning and predation. As an example, Melipona seminigra abunensis and Melipona grandis appear able to do this better than other Melipona species in the Ouro Preto meso-region, or perhaps, these larger Melipona species respond to disturbance over a longer period of time (Brown and Albrecht 2001). We do not know whether the species and colonies found in open areas moved into those areas after disturbance or whether they survived the disturbance. In the long term, species must survive potential isolation and inbreeding.
There appears to be a consensus that stingless bees are essentially a forest group, but as generalists, they are able to forage away from their nests into disturbed environments in many cases, as supported by our analysis of species composition in open and closed canopy environments. There are likely to be several species that can survive quite well in disturbed environments, the classic species in this case being Tetragonisca angustula; it is very common in disturbed areas in the Americas (Oliveira 2001; Fierro et al. 2012), it is even well adapted to urban environments where it is commonly found in buildings, wooden posts, and walls, and it is widely managed for honey production. On the whole, it seems prudent to follow Brosi et al. (2008) and Brosi (2009) who recommend preservation of forest fragments wherever possible to maximize the possibility of colony survival.
Numerous questions remain for future research. It seems clear that stingless bee sampling is currently ineffective in very large, forested areas (Oliveira 2001). The bees may simply be easier to find and capture in deforested areas, so it would be helpful to understand better the spatial pattern of foraging by bees, perhaps by experimentation with managed colonies in forested areas and accompanying studies of pollen types found in honey throughout the year to determine the relative contributions of environments of various disturbance levels to colony survival. We also have little idea of how colonies survive the process of deforestation and subsequent burning of agricultural plots by settlers. It would seem plausible that maintenance of forest fragments is essential for stingless bee conservation, but future studies should attempt to determine what the minimum size and ideal spatial configurations are for species conservation.
It is our hope that this work brings greater attention to this group of bees as a resource that provides pollination services for both native and non-native plants and crops (Santos and Absy 2010; Rech and Absy 2011a, b). These bees are affected by agricultural settlement and deforestation and we have an opportunity to plan for their conservation as areas undergo development in years to come.
References
Baccaro, F.B., Schietti, J., Guariento, H.F., Oliveira, M.L., Magalhães, C. (2008) Avaliação de um patrimônio. Sci. Am. (Brasil) Especial Amazônia 2, 24–29
Brosi, B.J. (2009) The complex responses of social stingless bees (Apidae: Meliponini) to tropical deforestation. Forest Ecol. Manag. 258, 1830–1837
Brosi, B.J., Daily, G.C., Erlich, P.R. (2007) Bee community shifts with landscape context in a tropical countryside. Ecol. Appl. 17, 418–430
Brosi, B.J., Daily, G.C., Shih, T.M., Oviedo, F., Durán, G. (2008) The effects of forest fragmentation on bee communities in tropical countryside. J. Appl. Ecol. 45, 773–783
Brown, J.C., Albrecht, C. (2001) The effect of tropical deforestation on stingless bees of the genus Melipona (Insecta: Hymenoptera: Apidae: Meliponini) in central Rondonia. Brazil. J. Biogeogr. 28, 623–634
Cairns, C.E., Villanueva-Gutiérrez, R., Koptur, S., Bray, D.B. (2005) Bee populations, forest disturbance, and Africanization in Mexico. Biotropica 37, 686–692
Câmara, G., de Morisson Valeriano, D., Vianei Soares, J., Vianei, J. (2006) Metodologia para o Cálculo da Taxa Anual de Desmatamento na Amazônia Legal. INPE, São José dos Campos
Camargo, J.M.F. (1994) Biogeografia de Meliponini (Hymentopera, Apidae, Apinae): a fauna Amazônica. Anais do I Encontro sobre Abelhas de Ribeirão Preto, pp. 46–59. Editora Legis Summa Ltda., Ribeirão Preto
Camargo, J.M.F., Pedro, S.R.M. (1992) Systematics, phylogeny and biogeography of the Meliponinae (Hymenoptera, Apidae): a mini-review. Apidologie 23, 509–522
Camargo, J.M.F., Pedro, S.R.M. (2005) Meliponini neotropicais: o gênero Dolichotrigona Moure (Hymenoptera, Apidae, Apinae). Rev. Bras. Entomol. 49, 69–92
Camargo, J.M.F., Pedro, S.R.M. (2007) Meliponini Lepeletier. In: Moure, J.S., Urban, D., Melo, G.A.R. (eds.) Catalogue of bees (Hymenoptera, Apoidea) in the Neotropical region, pp. 272–578. Sociedade Brasileira de Entomologia, Curitiba
Carvalho-Zilse, G.C., Kerr, W.E. (2004) Substituição natural de rainhas fisogástricas e distância de vôo dos machos em Tiuba (Melipona compressipes fasciculata Smith, 1854) e Uruçu (Melipona scutellaris Latreille, 1811) (Apidae, Meliponini). Acta Amazonica 34, 649–652
Dick, C.W. (2001) Habitat change, African honeybees, and fecundity in the Amazonian tree Dinizia excelsa (Fabaceae). In: Bierregaard Jr., R.O., Gascon, C., Lovejoy, T.E., Mesquita, R. (eds.) Lessons from Amazonia: the ecology and conservation of a fragmented forest, pp. 146–157. Yale University Press, New Haven
Ducke, A. (1925) Die stachellosen Bienen (Melipona) Brasiliens, Nach morphologischen und ethologischen Merkmalen geordnet. Zool. Jahrb. Abt. Systm. Geogr. Biol. Tiere 49, 335–448
Fearnside, P.M. (1989) A ocupação humana de Rondônia: impactos, limites e planejamento. Assessoria Editorial e Divulgação Científica, Brasília
Fierro, M.M., Cruz-Lópes, L., Sánchez, D., Villanueva-Gutiérrez, R., Vandame, R. (2012) Effect of biotic factors on the spatial distribution of stingless bees (Hymenoptera: Apidae: Meliponini) in fragmented Neotropical habitats. Neotrop. Entomol. 41, 95–104
Freitas, B.M., Imperatriz-Fonseca, V.L., Medina, L.M., Kleinert, A.M.P., Galetto, L., Nates-Parra, G., Quezada-Euán, J.J.G. (2009) Diversity, threats and conservation of native bees in the Neotropics. Apidologie 40, 332–346
Garófalo, C.A. (2009) Patrimônio não avaliado. Scientific American (Brasil) 7, 50–51
International Institute for Applied Systems Analyses (IIASA). (1989) Holdredge Life-Zones Map. FAO, Rome. http://www.fao.org/geonetwork/srv/en/metadata.show?id=1006 (accessed June 6, 2013)
Kerr, W.E., Zucchi, R., Nakadaira, J.T., Butolo, J.E. (1962) Reproduction in the social bees (Hymenoptera, Apidae). J. New York Entomol. S. 70, 265–276
Kuhn-Neto, B., Contrera, F.A., Castro, M.S., Nieh, J.C. (2009) Long distance foraging and recruitment by a stingless bee, Melipona mandacaia. Apidologie 40, 472–480
Liow, H.L., Sodhi, N.S., Elmqvist, T. (2001) Bee diversity along a disturbance gradient in tropical lowland forest of south-east Asia. J. Appl. Ecol. 38, 180–192
Michener, C.D. (2000) The bees of the world. Johns Hopkins University Press, Baltimore
Nogueira-Neto, P. (1997) Vida e criação de abelhas indígenas sem ferrão. Editora Nogueirapis, São Paulo
Oliveira, M.L. (2001) Stingless bees (Meliponini) and orchid bees (Euglossini) in “terra firme” tropical forests and forest fragments. In: Bierregaard Jr., R.O., Gascon, C., Lovejoy, T.E., Mesquita, R. (eds.) Lessons from Amazonia: the ecology and conservation of a fragmented forest, pp. 208–218. Yale University Press, New Haven
Oliveira, M.L., Morato, E.F., Garcia, M.V.B. (1995) Diversidade de espécies e densidade de ninhos de abelhas sociais sem ferrão (Hymenoptera, Apidae, Meliponinae) em floresta de terra firme na amazonia central. Rev. Bras. Zool. 12, 13–24
Oliveira, M.L., Silva, S.J.R., Silva, M.C., Araújo, A.C.O., Albuquerque, M.I.C., Tavares, S.F. (2010) Abelhas de Roraima: Por que tantas espécies em tão pouco espaço? In: Barbosa, R.I., Melo, V.F. (eds.) Roraima: Homem, Ambiente e Ecologia, pp. 523–540. FEMACT, Boa Vista
Overal, W.L. (2001) O peso dos invertebrados na balança de conservação biológica da Amazônia. In: Capobianco J.P.R. (Org.) (ed.) Biodiversidade na Amazônia Brasileira: avaliações e ações prioritárias para a conservação, uso sustentável e repartição de benefícios, pp. 50–59. Instituto Socioambiental e Estação Liberdade, São Paulo
PRODES. (2011) Programa de Cálculo do Desflorestamento da Amazônia, Amazon Deforestation Calculation Program. http://www.obt.inpe.br/prodes/index.php (accessed on June 11, 2011)
RAISG—Amazonian Network of Georeferenced Socio-Environmental Information (2013) Amazonia under pressure. Instituto Socioambiental, São Paulo. http://raisg.socioambiental.org/system/files/Amazonia%20under%20pressure16_05_2013.pdf (accessed on June 6, 2013)
Rech, A.R., Absy, M.L. (2011a) Pollen sources used by species of Meliponini (Hymenoptera: Apidae) along the Rio Negro channel in Amazonas, Brazil. Grana (Stockholm) 50, 150–161
Rech, A.R., Absy, M.L. (2011b) Pollen storages in nests of bees of the genera Partamona, Scaura and Trigona (Hymenoptera, Apidae). Rev. Bras. Entomol. 55, 361–372
Ricketts, T.H., Regetz, J., Steffan-Dewenter, I., Cunningham, S.A., Kremen, C., et al. (2008) Landscape effects on crop pollination services: are there general patterns? Ecol. Lett. 11, 499–515
Roubik, D.W. (1989) Ecology and natural history of tropical bees. Cambridge University Press, Cambridge
Roubik, D.W., Aluja, M. (1983) Flight ranges of Melipona and Trigona in tropical forest. J. Kansas Entomol. Soc. 56, 217–222
Salmah, S., Inoue, T., Sakagami, S.F., Ohgushi R.I., Roubik, D.W. (1990) An analysis of apid bee richness (Apidae) in central Sumatra. In: Sakagami, S. F., Ohgushi, R. I., Roubik, D. W. (eds.) Natural history of social wasps and bees in equatorial Sumatra, pp. 139–174. Hokkaido University Press, Sapporo
Santos, C.F., Absy, M.L. (2010) Polinizadores de Bertholletia excelsa (Lecythidales: Lecythidaceae): Interações com abelhas sem ferrão (Apidae: Meliponini) e nicho trófico. Neotrop. Entomol. 39, 854–861
Silveira, F.A., Melo, G.A.R., Almeida, E.A.B. (2002) Abelhas Brasileiras: Sistemática e Identificação. Fundação Araucária, Belo Horizonte
Souza, I.C., Martins, M.A.S., Alves, R.M.O. (1996) Meliponicultura para iniciantes. In: Kerr, W.E., Carvalho, G.A., Nascimento, V.A. (eds.) Abelha Uruçu. Biologia, Manejo e Conservação, pp. 91–111. Fundação Acangaú, Belo Horizonte
Velleman, P.F. (1980) Definition and comparison of robust nonlinear data smoothing algorithms. J. Am. Stat. Assoc. 75, 609–615
Villanueva-Gutiérrez, R., Roubik, D.W., Colli-Ucán, W. (2005) Extintion of Melipona beecheii and traditional beekeeping in the Yucatán peninsula. Bee World 86, 35–41
Wille, A. (1962) A technique for collecting stingless bees under jungle conditions. Insectes Soc. 9, 291–293
Acknowledgments
The authors wish to thank Drs. João M. F. Camargo (in memoriam) and Sílvia R. M. Pedro for identification of the species, for their suggestions, and for their friendship; José Amilcar Tavares for his technical assistance with the collection; and Sandro Boina and José Aparecido Vieira for their field and laboratory assistance during the project. We also thank Denise Perpich who designed and managed the database of the project. We are grateful to Caio Márcio Vasconcellos Cordeiro de Almeida and Francisco Antônio Neto for providing laboratory space at our home base in Ouro Preto. The fieldwork for this study was funded by Tecnosolo and DHV Consultants, and it was conducted as part of the Second Approximation of the Socio-Economic–Ecological Zoning of the State of Rondônia. Additional funding was provided by the College of Liberal Arts and Sciences of the University of Kansas. Thanks as well to Chris Bishop, who helped construct the map and conduct the GIS-related analyses. We greatly appreciate the comments of the anonymous reviewers. All errors remain ours.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript editor: Klaus Hartfelder
Impact de l'extension des terres agricoles et de la déforestation sur la composition et la richesse des abeilles sans aiguillon (Apidae: Meliponini) dans l'état du Rondônia, Brésil
Abeilles socials / analyse de redondance / fragmentation de la forêt/utilisation des terres / Amazonie
Der Einfluss der landwirtschaftlichen Kolonisierung und der Entwaldung auf den Artenreichtum der stachellosen Bienen und die Zusammensetzung der Bienengemeinschaften in Rondonia, Brasilien
soziale Bienen / Redundanzanalyse / Waldzergliederung / Landnutzung / Amazonien
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 403 kb)
Rights and permissions
About this article
Cite this article
Brown, J.C., de Oliveira, M.L. The impact of agricultural colonization and deforestation on stingless bee (Apidae: Meliponini) composition and richness in Rondônia, Brazil. Apidologie 45, 172–188 (2014). https://doi.org/10.1007/s13592-013-0236-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-013-0236-3