Abstract
Human-dominated land uses constitute a large and growing proportion of global land cover, so understanding their potential to support biodiversity is critical for effective conservation. Here, we asked how bee diversity and community composition differ among common human dominated land uses (teak plantations, coffee agroforestry, and pastures) during wet and dry seasons in the Nicoya Peninsula of Costa Rica. We sampled over two years using blue vane and pan traps, collecting 47 genera and 119 species and morphospecies of bees. We observed similar numbers of bees in coffee farms and high elevation pastures, but collected substantially more bees in low elevation pastures relative to teak plantations during both seasons. Shannon diversity was greatest in pastures, with an estimated 20 more common species in low elevation pastures relative to teak plantations and estimated 13 more common species in high elevation pastures relative to coffee. Teak plantations were dominated by distinct taxa during the dry season compared to the other land uses, hosting stem-nesting genera such as Ceratina but lacking oil collecting groups like Centris and Epicharis. Our findings reflect the seasonal availability of habitat: teak is managed as a monoculture and blooms during the wet season, when fewer bees are active. In contrast, shade trees in pastures provide nectar, pollen, and nesting substrates throughout the year.
Implications for insect conservation:
Our study provides baseline information on regional bee biodiversity in a tropical agroecosystem, demonstrating the influence of both season and land use on an important group of pollinators in this system. To avoid biodiversity trade-offs, policies that incentivize the establishment of teak plantations as a form of reforestation should incorporate land management that enhances habitat of important insects such as native bees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biodiversity loss is occurring rapidly in the 21st century, with extinctions taking place at 100–1000 times the background rate (Dirzo et al. 2014; Pimm et al. 2014). This is in part due to land use change and intensification, which reduces and fragments natural habitats (Foley et al. 2005; Flynn et al. 2009; Allan et al. 2015). The establishment and maintenance of protected areas is one essential strategy for preventing the loss of remaining natural habitats, but with the rapid expansion of human-dominated land uses, especially in the tropics, there is an increased focus on the conservation potential of privately managed lands (Bawa et al. 2004; Harvey et al. 2008; Chazdon et al. 2009; Watson and Venter 2017). Supporting both biodiversity and resource production for a growing human population will require a better understanding of the extent to which strategically managed agroecosystems can provide habitats that conserve the maximum number of taxa (DeFries et al. 2007).
Payments for Environmental Services (PES) address this need for conservation outside of conventional protected areas. Defined as voluntary transactions designed to generate offsite services from natural resource management on private land (Wunder 2015), PES typically provide payments to landowners for maintaining certain land uses, land cover types, or management practices that support biodiversity and ecosystem services in working landscapes (e.g., Bennett 2008; Pagiola 2008; Muñoz-Piña et al. 2008). In Costa Rica, one of the first countries to adopt PES, incentives are available in exchange for the establishment or maintenance of native forests, certain agroforestry systems, and tree plantations (Pagiola 2008; FONAFIFO 2020a). From 2010–2019, the PES program in Costa Rica maintained > 9500 contracts supporting environmental services on privately owned forests and agroforestry systems (FONAFIFO 2020b).
One of the objectives of Costa Rica’s PES program is to reforest or slow deforestation on privately owned land, including pastures (Pagiola 2008). Though some pastures are left to naturally develop into secondary forest, some have been replaced with tree plantations, including those of non-native teak (Tectona grandis) (Healey and Gara 2003; Vallejo et al. 2006). Biodiversity of mammals, invertebrates, birds, and plants is lower in monoculture tree plantations compared to secondary forests (Barlow et al. 2007; Stephens and Wagner 2007; Yue et al. 2015), but little is known about the relative biodiversity of animals in teak versus the human-dominated land uses such as pastures that plantations often replace (Brockerhoff et al. 2008; Hallet et al. 2011). For PES and similar conservation strategies to successfully support biodiversity and ecosystem services in tropical agroecosystems, decisionmakers need baseline data on ecologically important species within common human-dominated land uses, including PES-incentivized plantations.
Pollinators are important components of natural and human-dominated systems, as they support both biodiversity maintenance (Ollerton et al. 2011) and crop production (Klein et al. 2007; Garibaldi et al. 2013). Bees (Hymenoptera: Apiformes) are the primary animal pollinators in many regions of the world (Klein et al. 2007), and they are sensitive to various anthropogenic activities such as land use change and intensification (Winfree et al. 2009; Potts et al. 2010). However, most studies on bee populations in agricultural settings have been done in North America and Europe, where landscapes typically have a low proportion of remaining natural habitat and farms are managed intensively (Winfree et al. 2009, Winfree et al. 2011; Archer et al. 2014). In less intensively managed agroecosystems, human-dominated land uses may provide a variety of nesting substrates and foraging resources for bees (Tylianakis et al. 2005; Tscharntke et al. 2005, Winfree et al. 2011, Kennedy et al. 2013). Though deforestation and agricultural intensification may pose threats for bee populations, we lack reliable data on which to base pollinator conservation efforts in the Neotropics (Freitas et al. 2009; Archer et al. 2014; De Palma et al. 2016; Bartomeus et al. 2019).
In this study, we surveyed wild bee communities among common human-dominated land uses in a tropical agroecosystem, asking: 1) How do bee abundance and diversity differ among common human-dominated land uses (i.e., coffee agroforestry, teak plantations, and pasture)? 2) How does community composition (i.e., the relative abundance of bee genera) differ among these land uses? 3) How are these patterns influenced by seasonality? We hypothesized that bee abundance and diversity would be lowest in teak plantations, which are managed as monocultures, and highest in coffee agroforestry, which is typically managed with diverse flowering shade trees in the region, supporting high biodiversity (Perfecto et al. 1996; Bhagwat et al. 2008). Furthermore, tropical bee communities are highly variable over space and time in human-dominated land uses (Tylianakis et al. 2005). Many bee species in the seasonally dry tropics are more active during the dry season, but the majority can be found in both the dry and wet season (Heithaus 1979). Thus, we expected that the abundance of bee communities would change between the dry and wet seasons, peaking in teak plantations and coffee agroforestry during their respective blooming seasons, and that diversity would be greatest during the dry season among all land uses.
Bee community composition is often different among distinct habitats even when diversity measures are the same (e.g., Tylianakis et al. 2005; Brosi et al. 2008; Harrison et al. 2018), and tropical bees are diverse in their floral resource and nesting preferences (Roubik 1989). We therefore also hypothesized that bee communities in teak plantations, which flower in the wet season only, would be taxonomically distinct relative to coffee agroforestry and pastures, which harbor native shade trees that generally bloom in the dry season (Frankie et al. 2004). Furthermore, teak plantations lack habitat diversity (e.g., nesting locations for cavity-nesting bees in older trees), so we expected that a wider range of bee functional groups would be supported in coffee agroforestry and pastures (Jha et al. 2014; Caudill et al. 2017).
Methods
Study region, land use descriptions, and site selection
We tested our hypotheses in the Nicoya Peninsula of Northwestern Costa Rica (Fig. 1). The peninsula, bordered by the Pacific Ocean to the west and the Gulf of Nicoya to the east, is a mix of seasonally dry and moist tropical ecological life zones (Calvo-Alvarado et al. 2009). About 95% of the average 1800 mm of rainfall occurs from May to November (Mata and Echeverria 2004), with dry weather extending from approximately December through April. Tropical dry forests are among the most endangered ecosystems in the world with about 97% currently exposed to a high level of threats, including conversion to agriculture (Miles et al. 2006). The Nicoya Peninsula has undergone substantial land use change from the mid-1900s to the present because of deforestation for livestock production, then subsequent land abandonment and reforestation (Vallejo et al. 2006). Currently, the peninsula is comprised of a heterogeneous landscape including secondary forest regrowth, pastures, tree plantations, and agricultural crops (Calvo-Alvarado et al. 2009).
Location of study region within the context of Central America (upper inset) and location of study sites within a land use map of the central Nicoya Peninsula (main map). Study sites are shaded in black, with surrounding land uses including forest and mangrove (darkest grey), pasture (mid-grey), non-forest (lightest grey; representing agricultural, urban, or fallow land), and tree plantations (white). Photographs show a teak plantation (lower left), pasture (lower center) and coffee farm (lower right) during the dry season.
We selected three of these land uses for this study—coffee agroforestry, pasture, and teak plantations—based on their relation to PES policy (FONAFIFO 2013), prevalence in the Nicoya Peninsula, and importance in contributing to local livelihoods (Vallejo et al. 2006; Calvo-Alvarado et al. 2009). Coffee is the third largest crop in Costa Rica in terms of export value (SEPSA 2019) and the land use covers nearly 85,000 ha of the country (INEC 2015). The “Agroforestry Systems” PES modality (FONAFIFO 2020a) provides benefits to landowners who manage their coffee farms to support biodiversity, and there is evidence that coffee agroforestry supports diversity of bees and other wildlife (e.g., Jha and Vandermeer 2010; Caudill et al. 2017). Local coffee management in the Nicoya Peninsula (which was reflected in our study sites) is typically non-organic and includes maintaining shade tree species such as avocado (Persea americana), orange (Citrus cinensis) and Poro (Erythrina fusca) (see Supplementary materials S3). None of the farms selected for this study were under certification schemes.
Pastures are one of the most dominant land uses in Costa Rica, covering approximately 1,100,000 hectares and representing 21.5% of the national territory (Serna et al. 2017). Regional pastures, which can also be supported through the Agricultural Systems PES modality, typically contain live fences and shade trees (Gutierrez et al. 2014; see Supplementary materials S3). Regional pastures (as typified in our study sites) use grasses known locally as ‘pasto mejorado’ or ‘improved pasture’ (Hyparrhenia rufa), which were introduced to support grazing during the long dry season (Pohl 1983). Our sites were used for grazing cattle and occasionally horses. High-elevation pastures selected for this study ranged from 587-748 MASL, and low elevation pastures ranged from 35-347 MASL.
Plantations of teak, a tropical hardwood deciduous species native to Southeast Asia, are incentivized by the ‘Reforestation’ PES modality in Costa Rica (FONAFIFO 2020a). Though teak plantations are less common than coffee or pastures, they currently cover approximately 47,000 ha in Costa Rica (INEC 2015) and teak is one of the most economically important timber species in the tropics (FAO 2015). Plantations used in this study were between 15 and 20 years old. The understory was heavily managed via manual weeding and herbicide (Galbraith, personal observation) and no other tree species were inter-planted with the teak, though a few trees remained in the land uses from before the plantations were established.
Though we were interested in comparing bee communities among these three land uses, the elevational difference between coffee agroforestry and teak plantations was a potential barrier to making direct comparisons. To address this issue, we paired both teak plantations and coffee agroforestry with nearby pastures and made comparisons within elevational groups. We refer to the pastures that were paired with coffee agroforestry as ‘high elevation pasture’ and those paired with teak as ‘low elevation pasture’, and we focus on comparisons within these elevational categories throughout.
We selected 20 farms (referred to as “sites”) for this study. This included five sites each of coffee farms, high elevation pasture, low elevation pasture, and teak plantations. To select farms for sampling, we first established a list of potential locations for the study based on farmer willingness to participate and site location, seeking farms located within Hojancha and Nicoya counties. We then selected farms from this list that represented the elevational and moisture gradient of the Nicoya Peninsula (Fig. 1) and that fit the typical size range and management regime for the region, as described above. To ensure independence within land use replicates, sites within the same land use category were > 2 km apart. A detailed description of each site, including area and location, is included in the Supplementary Materials (S1).
Sampling design
We sampled bee populations on the 20 sites over a 2-year period: March 2013–November 2014. Bees were collected with blue vane traps (BVTs, SpringStar Inc.™; Stephen and Rao 2005) during both years and pan traps (Prado et al. 2017) were added during the second year of sampling only. We initially used only BVTs because they were better suited to the extreme differences between the wet and dry season sampling conditions, as we could prevent them from overflowing during periods of rain. However, although BVTs have been shown to be effective in temperate agricultural systems (Stephen and Rao 2007), they have rarely been used in tropical settings (but see Samnegård et al. 2015). In addition, studies increasingly recommend using a combination of methods when sampling bees to reduce issues stemming from trap bias (Prendergast et al. 2020). To address this shortcoming, we added pan traps during the second year because they have been used extensively for bee research (Westphal et al. 2008) and are recommended for estimating bee richness in tropical systems (Prado et al. 2017). We made pan traps by painting the inside of 12-oz plastic Solo cups with fluorescent paint (for blue and yellow traps) and leaving some cups unpainted (for white traps) (Droege 2008). We added small slits at the top of each cup to allow rainwater to drain from the traps without losing specimens.
We placed one BVT and three pan traps (one of each white, yellow, and blue) at 0, 100, and 200 meters from a secondary forest location at the edge of each site. BVTs were set at floral height, so that traps had maximum visibility and sun exposure in all farm types. They did not contain any killing agent, and we fixed small drains covered in mosquito netting at the bottom of the traps to ensure that they remained dry throughout the sampling period to prevent damage to the specimens. Pan traps were filled with a solution of water, soap, and salt (as a preservative) and placed at a uniform 1.5 m from the ground on raised platforms. All traps remained in place for 72 h before collection.
We completed ten rounds of sampling over 2 years (see Supplementary Materials S2 for sampling dates), including five sampling rounds during the wet season (June–November) and five during the dry season (December–May). The late dry season sampling dates coincided with the coffee blooming period, which occurs after the first few rain showers of the year. The mid-wet season sampling dates coincided with the blooming of teak, which occurs in June and July.
After collection, the specimens were washed, pinned and labeled, then frozen inside collection boxes to prevent molding or insect damage until they could be transferred to the U.S. All specimens were identified to genus using “The Bee Genera of North and Central America” (Michener et al. 1994), then to the lowest possible taxon at the National Biodiversity Institute in Costa Rica and the USDA Bee Lab in Logan, Utah. Several genera of neotropical bees do not have available species-level keys, but we were able to use synoptic collections at the USDA Bee Lab to assign these genera to morphospecies. Select specimens were deposited at the USDA Bee Lab for reference, and remaining voucher specimens will be deposited at the University of Idaho William Barr Entomological Museum upon publication.
Data analysis
We used Generalized Linear Mixed Models (‘GLIMMIX’ in SAS 9.4) to compare bee abundance among land uses, assuming a completely random design (Stroup 2014). The response variable (bee abundance) was calculated by pooling results from all traps and sampling dates for each site × season combination to avoid pseudoreplication. Models thus included sampling season (2 levels: dry season and wet season), land use (4 levels), and the interaction between season and land use as fixed effects. We also included the individual site as a random effect.
The GLMMs assumed a negative binomial error distribution with a log link to account for overdispersion due to count data with many zero observations. We evaluated model fit by assessing residuals plots and checking that the Pearson Chi-square/DF fit statistic for conditional distribution was < 1. To account for the occasional damaged trap, we log-transformed the number of traps successfully collected per observation and included this as an offset in the model. We included sites from all land uses in a single model, but present differences in least squares means among land uses from the same elevation category only. We present estimates from least squared means with 95% confidence intervals. All results have been back-transformed to the response scale. We generated separate abundance models for BVTs and pan traps due to their potential for distinct trapping biases, then ran the models with all traps combined. Results for the two trap types are only presented separately when results differed between the two methods.
Simple counts of species often underestimate true species richness and they are biased by sampling effort and completeness, so we compared bee species richness and diversity among sites using species accumulation curves. We generated interpolated and extrapolated Hill number curves from individual-based abundance data using package ‘iNEXT’ in R version 1.2.1335 (Hseih et al. 2016). Hill numbers are a diversity measure that represents the effective number of species in a sample (Hill 1973) and can be modelled using different orders of ‘q’. When q = 0, Hill numbers represent species richness, counting species equally without regard to their relative abundances. When q = 1, Hill numbers represent Shannon diversity, giving less weight to rare species in the sample (Hseih et al. 2016). We compared species richness and Shannon diversity by interpolating separate sampling curves and 95% confidence intervals from bootstrapping for collections from each land use. We then interpolated curves to 3× the minimum observed sample size (Hsieh et al. 2016).
To describe differences in bee composition among land use types, we explored the spatial clustering of sampling units using nonmetric multidimensional scaling (NMDS; R version 3.4.2 vegan package; Oksanen 2013). NMDS is a method for graphically demonstrating the dissimilarity between variables in a reduced number of dimensions based on the pairwise distances between sites given the metrics of interest. It then positions the sites graphically in an assigned number of dimensions to maximize the rank correlation between the pairwise inter-site distances of the population metric and those of the graphical ordination (Quinn and Keough 2002). We assessed differences in communities by genus and species, and report results by genus because stress plots were < 0.2 for genus only (Oksanan 2013).
We performed separate NMDS for wet and dry seasons because of the temporal change in habitat availability among the focal land uses. For each of the figures, we calculated the goodness of fit for the environmental variable (i.e., land use category) using the ‘envfit’ command in the vegan package. We plotted the results for each site as a point, then drew a polygon ‘hull’ to demonstrate the space each land use occupied in the two dimensions. Finally, we superimposed scores from each genus onto the same graph to identify genera that made samples from the different land uses more or less similar.
Results
We collected a total of 1751 bees representing five families, 47 genera, and 119 species and morphospecies (Table 1). Overall, more bees were collected during the dry season (69%) than the wet season (31%) despite equal collecting effort. Among all bees collected, the most common genera were Ceratina, Xylocopa, Euglossa, Apis, Epicharis, Trigona, and Eulaema. These genera, which all belong to the family Apidae, composed 71% of the bees collected in the study. Eleven genera were represented by only a single individual, including cleptoparasitic bee genera Coelioxys and Osiris. The managed European honeybee (Apis mellifera), was one of the most common species collected (176 individuals, 10.3% of collected specimens). As such, we removed honeybees from datasets before conducting GLIMMIX abundance tests but kept them in diversity curves and descriptive comparisons of community composition. The number of bee species differed between trap types, so details of results by collection method can be accessed in the Supplementary Materials (S4).
Differences in bee abundance and diversity
Bee abundance differed between land uses (F3,16 = 6.13, p = 0.006), and seasons (F1,16 = 26.83, p < 0.001), but we did not detect a substantial interaction between these two effects (F3,16 = 3.07, p = 0.058). On average, we collected > 2.5x more bees per site in the dry season relative to the wet season (Est = 2.74, SE = 0.19, t = 5.18, 95% C.I. [1.81, 4.10]). Bee abundance was greater in coffee farms during the dry season compared to the wet season (Est = 5.97, SE = 0.37, t = 4.78, 95% C.I. [2.70, 13.20]). Bee abundance was also greater in nearby high elevation pastures during the dry season compared to the wet season (Est = 3.48, SE = 0.38, t = 2.31, 95% C.I. [1.53, 7.94]). However, we did not find evidence for seasonal differences in bee abundance in low elevation pasture (Est = 1.28, SE = 0.37, t = 0.69, 95% C.I. [0.58, 2.87]) or teak plantations (Est = 2.03, SE = 0.39, t = 1.79, 95% C.I. [0.88, 4.70).
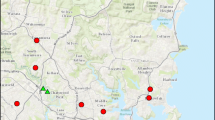
During the dry season, we did not find evidence for differences in bee abundance between coffee farms and high elevation pastures (Fig. 2a; Est = 0.89, SE = 0.39, t = − 0.30, 95% C.I. [0.38, 2.06]). We did not find evidence for differences in bee abundance in these land uses during the wet season, either (Fig. 2b; Est = 0.52, SE = 0.42, t = − 1.52, 95% C.I. [0.21, 1.26]). We did detect differences in bee abundance between land uses at lower elevations: bee abundance was > 2.5x greater on average in low elevation pastures compared to teak plantations during the dry season (Fig. 2a; Est = 2.68, SE = 0.36, t = 2.54, 95% C.I. [1.10, 6.51] and > 4x greater in the wet season (Fig. 2b; Est = 4.21, SE = 0.36, t = 3.40, 95% C.I. [1.78, 9.99]). Abundance comparisons were consistent between trapping methods, except among high elevation sites during the wet season, when there was evidence for lower abundance in coffee versus high elevation pastures in the BVT only model (Est = 0.37, SE = 0.44, t = − 2.24, 95% C.I. [0.15, 0.95]), but not the pan trap only model (Est = 0.89, SE = 0.37, t = − 0.31, 95% C.I. [0.40, 1.97]).
Estimated mean bee abundance among land uses during the dry season (white background, a) and wet season (background shaded grey, b). Points represent least squared means per site with both trapping methods combined, and a correction in effort for the different number of traps used in the 2 years. Error bars represent 95% confidence intervals
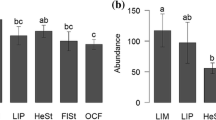
Interpolated species richness curves show lower observed species richness in teak plantations compared to low elevation pastures (Fig. 3a) and similar observed species richness in coffee agroforestry and high elevation pastures (Fig. 3b). However, species richness curves did not reach asymptote, even when extrapolated to 3× the number of individuals collected in teak plantations, where we had the smallest sample. Thus, we do not have enough evidence to draw conclusions about differences in species richness between land uses despite frequent sampling. However, Shannon diversity curves did reach asymptote (Fig. 3c and d). Extrapolated models estimated 20 fewer species in teak plantations relative to low elevation pastures (Fig. 3c). Likewise, high elevation pastures had an estimated 13 more common species than coffee agroforestry based on the Shannon diversity Hill numbers, which give rare species less weight relative to richness estimates (Fig. 3d).
Accumulation curves of Hill numbers representing species richness (a-b) and Shannon diversity (c-d). Lines represent interpolated (solid line) and extrapolated (dotted line) estimates and points show the observed species richness for low elevation pastures (square), teak plantations (cross), high elevation pastures (triangle), and coffee farms (circle)
Bee community composition
Bee community composition was distinct among the studied land uses. The most common bee collected was different in each land use, with the non-native honeybee composing almost 25% of the trapped specimens in coffee agroforestry, and a single morphospecies in subgenus Ceratina (Calloceratina) composing nearly 50% of the collection in teak plantations (Table 2). High elevation pastures were dominated by Epicharis angulosa, though they composed only ~12% of specimens collected. One morphospecies of Euglossa made up ~14% of bees collected in low elevation pastures, and the same morphospecies of Ceratina (Calloceratina) as in teak plantations was almost equally abundant in this land use. No single species appeared as one of the five most common species in all four land uses (Table 2).
NMDS plots demonstrated patterns of differences in bee community composition during the dry season (Fig. 4a; goodness of fit R2 = 0.46, p = 0.001). Specifically, bee communities in the dry season were distinct in teak compared to the other land uses. The observations are grouped in the portion of the graph associated with genera Caenaugochlora, Augochlorella, Ceratina, and Euglossa. We did not find evidence for substantial differences in community composition during the wet season (Fig. 4b; goodness of fit R2 = 0.10, p = 0.745).
Discussion
Understanding the potential of human-dominated land uses for supporting biodiversity is crucial for conservation outside of protected areas. Though numerous studies have compared biodiversity in human-dominated land uses to forest habitats in the tropics, few studies have looked at how biodiversity compares among common human-dominated land uses in such regions. It is particularly important that we include novel land uses such as tree plantations in these comparisons, as they are sometimes incentivized as a method of reforestation despite varied impacts on biodiversity (Stephens and Wagner 2007; Bremer and Farley, 2010; Hallet et al. 2011). Here, we collected 2.5 – 4× more individuals on average in low elevation pastures relative to teak plantations, depending on the sampling season. By contrast, we did not find sufficient evidence for differences in bee abundance between high elevation pastures and coffee agroforestry in either season. Despite conducting sampling over a 2-year period during the wet and dry seasons, we found that sample sizes were insufficient to compare species richness between sites even when we pooled samples between the seasons. However, both interpolated and extrapolated estimates of Shannon diversity were greater in pastures relative to teak and- to a lesser degree- coffee sites.
Although conservation strategies often target pastures as biodiversity-poor regions, traditionally managed pastures in the tropics have the potential to support a high diversity of pollinators and other organisms that thrive in open habitats (Harvey and Haber 1998; Tscharntke et al. 2005; Milder et al. 2010). Despite a relatively small sample size, we collected 84 species of bees in low elevation pastures and 76 species of bees in high elevation pastures during this study, representing a substantial proportion of the estimated 785 species in the country (Griswold 2000). Traditionally managed pastures in the tropics typically contain live fences and shade trees that support animal diversity, but these resources come under threat in more intensively managed pasture systems (Harvey et al. 2011). The Costa Rican PES program has an “Agroforestry Systems” modality that serves to support the conservation of pollinator biodiversity in agroecosystems by establishing agricultural systems that include trees interspersed with crops and/or pastures (FONAFIFO 2020a). Our study indicates that this modality would provide better habitat for pollinators than the modalities that encourage the conversion of pastures into less habitat-rich land uses (e.g., teak plantations), as it supports the availability of flowering plants and nesting substrates within existing pastures.
In contrast to pastures, observed bee abundance and species diversity were relatively low in teak plantations. Bee abundance in coffee farms roughly coincided with coffee bloom in the late dry season-early wet season. In contrast, bee abundance did not increase in teak farms during the blooming period (June–July). Bees are the main pollinators of teak in its native range (Tangmitcharoen et al. 2006, 2009), but teak blooms during the wet season in Costa Rica, when fewer bee species are active (Heithaus 1979; Frankie et al. 1983). The lack of bees in this land use throughout the year is particularly notable given the deciduous nature of teak, which has almost no canopy cover during the dry season. Because trapping results in teak plantations were unaffected by season, results do not appear to be driven by trap visibility or the availability of UV light. Instead, we expect that lower abundance and richness of bee populations in teak relative to low altitude pastures is driven by the lower floral diversity in teak plantations.
In coffee and high elevation pastures, collection results varied with season. As expected, we observed greater bee abundance and richness overall in the dry season relative to the wet season. This is consistent with previous studies in the seasonally dry tropics, though many bee taxa are also active year-round (Janzen 1967; Heithaus 1979; Frankie et al. 1983; Roubik 1989). The abundance and richness of bees collected in coffee agroforestry was markedly higher in the dry season compared to the wet season. Coffee agroforestry is associated with a diversity of tree species that are maintained to provide shade and fix nitrogen within the farms (Perfecto et al. 1996; Bhagwat et al. 2008; S3), and this is known to benefit tropical bee communities (Jha and Vandermeer 2010). Both coffee and most native tree species used as shade trees in the Nicoya Peninsula flower in the dry or shoulder seasons and thus bees are likely responding to the temporal availability of flowering plants. Furthermore, the seasonality of common bees in coffee agroforestry may also drive this trend: though the most common species in coffee agroforestry (A. mellifera) was not included in GLMMs, the second and third most common species (Xylocopa gualanensis and X. fimbriata) influenced model results. Previous studies in similar regions of Costa Rica have observed that Xylocopa spp. are most active during the dry season preceding the coffee bloom (Heithaus 1979). This genus of large bees prefers flowering trees such as Cassia grandis, which were common in the coffee agroforestry sites we sampled (S3), and bloom in the dry season (Janzen 1967).
Bee seasonality, floral resource availability, and bee nesting ecology likely drove differences in the relative abundance of different bee genera and their functional characteristics (see S5 for a table of bee functional groups) among the four land uses based on Table 2 and Figure 4. Genera Caenaugochlora, Augochlorella, Ceratina, and Euglossa, while also present in pastures and coffee farms, made up a relatively larger portion of bees collected in teak plantations. One likely reason for the abundance of Caenaugochlora and Augochlorella in teak plantations is because these and other genera of halictids are often active during the wet season (Heithaus 1979), when teak blooms. Ceratina species have been observed visiting teak in its native range (Tangmitcharoen et al. 2006), so they may be more physiologically matched to collect resources from the small flowers on teak. In contrast, it may not be energetically efficient for buzz pollinating Centris, Epicharis, and Xylocopa to forage within teak plantations. Furthermore, though we found few nests while conducting this study, it is likely that wild bees nest within some of the examined land uses. Ground-nesting Caenaugochlora and Augochlorella and stem-nesting Ceratina (Roubik 1989) may be able to find nesting locations between rows of teak and in the sparse understory brush. Large cavity nesters like Xylocopa and Centris were almost completely absent from teak plantations, which are harvested after 15–25 years (Vallejo et al. 2006), and therefore likely lack locations for cavity-nesting bees relative to older shade trees in pasture or coffee farms.
We chose to survey bee communities using passive sampling so we could sample across more sites and more frequently than logistically possible with active capture methods, such as hand-netting. However, despite frequent sampling, we collected a relatively small sample of bees in this study. Sampling in tropical regions presents unique challenges, such as high canopy cover and frequent rain (Prado et al. 2017), so it is common to collect fewer individuals compared to an equal sampling effort in temperate regions. Like other studies, we observed some distinct seasonal responses in BVT and pan trapped bee communities in the tropics (Samnegård et al. 2015). More data are needed to inform robust pollinator monitoring schemes and improve our understanding of how these communities shift in space and time, especially outside of the U.S. and Europe, where fewer studies overall have been conducted (Freitas et al. 2009; Archer et al. 2014; De Palma 2016; Bartomeus et al. 2019).
Our study shows that land use and season interact to influence bee abundance and diversity in a tropical agroecosystem. Importantly, we found no evidence that teak plantations can support bee abundance or diversity to the same extent as other human-dominated land uses in the Nicoya Peninsula region. This mirrors local perceptions that relatively few resources are available for bees in teak plantations (Galbraith et al. 2017). These findings bring into question the effectiveness of incentivizing teak plantations via PES in Costa Rica with respect to biodiversity protection, which is one of the stated ecosystem services expected from PES programs. Tree plantations are often considered to be a potential means of reforestation, and forest plantations increased by > 100 M ha from 1990 to 2015 (Keenan et al. 2015), so understanding the impact of converting pastures and other land uses to tree plantations is critical for protecting biodiversity worldwide (Barlow et al. 2007; Bremer and Farley 2010; Hallet et al. 2011). Future research should focus on ways in which management activities within human-dominated land uses in the tropics, including novel land uses like teak plantations, could increase habitat for pollinators to better support biodiversity outside of protected areas. Without considering management actions that increase native habitats, reforestation via monoculture plantations is not likely to support the biodiversity of important pollinators relative to the traditional human-dominated land uses that it is replacing.
Data availability
Reference collection data will be made available upon publication.
References
Archer CR, Pirk CWW, Carvalheiro LG, Nicolson SW (2014) Economic and ecological implications of geographic bias in pollinator ecology in the light of pollinator declines. Oikos 123:401–407
Allan E, Manning P, Alt F, Binkenstein J, Blaser S, Blüthgen N, Böhm S, Grassein F, Hölzel N, Klaus VH, Kleinebecker T (2015) Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol Lett 18:834–843
Bartomeus I, Stavert JR, Ward D, Aguado O (2019) Historical collections as a tool for assessing the global pollination crisis. Phil Trans Royal Soc Lond B, Biol Sci 374:20170389
Brockerhoff EG, Jactel H, Parotta JA, Quine CP, Sayer J (2008) Plantation forests and biodiversity: oxymoron or opportunity? Biodivers Conserv 17:925–951
Barlow J, Gardner TA, Araujo IS, Ávila-Pires TC, Bonaldo AB, Costa JE, Esposito MC, Ferreira LV, Hawes J, Hernandez MI, Hoogmoed MS (2007) Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc Natl Acad Sci 104:18555–18560
Bawa KS, Kress WJ, Nadkarni NM, Lele S, Raven PH, Janzen DH, Lugo AE, Ashton PS, Lovejoy TE (2004) Tropical ecosystems into the 21st century. Science 306:227–228
Bennett MT (2008) China's sloping land conversion program: institutional innovation or business as usual? Ecol Econ 65:699–711
Bhagwat SA, Willis KJ, Birks HJB, Whittaker RJ (2008) Agroforestry: a refuge for tropical biodiversity? Trends Ecol Evol 23:261–267
Brosi BJ, Daily GC, Shih TM, Oviedo F, Durán G (2008) The effects of forest fragmentation on bee communities in tropical countryside. J Appl Ecol 45:773–783
Bremer LL, Farley KA (2010) Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers Conserv 19:3893–3915
Calvo-Alvarado J, McLennan B, Sánchez-Azofeifa A, Garvin T (2009) Deforestation and forest restoration in Guanacaste, Costa Rica: putting conservation policies in context. For Ecol Manage 258:931–940
Caudill SA, Brokaw JN, Doublet D, Rice RA (2017) Forest and trees: shade management, forest proximity and pollinator communities in southern Costa Rica coffee agriculture. Renew Agric Food Sys 32:417–427
Chazdon RL, Harvey CA, Komar O, Griffith DM, Ferguson BG, Martínez-Ramos M, Morales H, Nigh R, Soto-Pinto L, Van Breugel M, Philpott SM (2009) Beyond reserves: a research agenda for conserving biodiversity in human-modified tropical landscapes. Biotropica 41:142–153
DeFries R, Hansen A, Turner BL, Reid R, Liu J (2007) Land use change around protected areas: management to balance human needs and ecological function. Ecol Appl 17:1031–1038
De Marco P, Coelho FM (2004) Services performed by the ecosystem: forest remnants influence agricultural cultures' pollination and production. Biodivers Conserv 13:1245–1255
De Palma A, Abrahamczyk S, Aizen MA, Albrecht M, Basset Y, Bates A, Cruz-López L (2016) Predicting bee community responses to land-use changes: effects of geographic and taxonomic biases. Sci Rep- UK 6:1–14
Droege S (2008) The very handy manual: how to catch and identify bees and manage a collection. USGS Native Bee Inventory and Monitoring Lab: 1-65.
Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJ, Collen B (2014) Defaunation in the anthropocene. Science 345:401–406
FAO (Food and Agriculture Organization) 2015 Global teak trade in the aftermath of Myanmar’s log export ban. Rome, Italy. https://www.fao.org/3/a-i5023e.pdf
Flynn DF, Gogol-Prokurat M, Nogeire T, Molinari N, Richers BT, Lin BB, Simpson N, Mayfield MM, DeClerck F (2009) Loss of functional diversity under land use intensification across multiple taxa. Ecol Lett 12:22–33
Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH (2005) Global consequences of land use. Science 309:570–574
FONAFIFO (Fondo Nacional de Financiamiento Forestal) (2013) Distribución de hectáreas contratadas en pago por servicios ambientales, por año y por modalidad: Período 1997–2012. https://www.fonafifo.go.cr/es/servicios/estadisticas-de-psa/
FONAFIFO (Fondo Nacional de Financiamiento Forestal) (2020a) Pagos de Servicios Ambientales. https://www.fonafifo.go.cr/es/servicios/pago-de-servicios-ambientales
FONAFIFO (Fondo Nacional de Financiamiento Forestal) (2020b) Programa de Pago por Servicios Ambientales: Distribución de las hectáreas contratadas, por año y por actividad. Período 2010-2019. https://www.fonafifo.go.cr/es/servicios/estadisticas-de-psa/
Frankie GW, Haber WA, Opler PA, Bawa KS, (1983) Characterstics and organization of the large bee pollination system in the Costa Rican dry forest. Handb Exp Pollinat Biol 411–447
Frankie GW, Haber WA, Opler PA, Bawa, KS (2004) Flowering phenology and pollination systems diversity in the seasonal dry forest. In: Frankie GW, Mata A, Vinson S Bradleigh (ed) Biodiversity conservation in costa rica: learning the lessons in a seasonally dry forest. University of California Press, Berkeley and Los Angeles, CA, pp 17-29
Freitas BM, Imperatriz-Fonseca VL, Medina LM, Kleinert ADMP, Galetto L, Nates-Parra G, Quezada-Euán JJG (2009) Diversity, threats and conservation of native bees in the Neotropics. Apidologie 403:332–346
Galbraith SM, Hall TE, Tavárez HS, Kooistra CM, Ordoñez JC, Bosque-Pérez NA (2017) Local ecological knowledge reveals effects of policy-driven land use and cover change on beekeepers in Costa Rica. Land Use Policy 69:112–122
Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, Cunningham SA, Kremen C, Carvalheiro LG, Harder LD, Afik O and Bartomeus I (2013) Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339:1608–1611
Griswold T, Parker FD, Hanson PE, (2000) An inventory of the bees of Costa Rica: the myth of the depauperate tropics. In: Proceedings of the Sixth International Conference on Apiculture in Tropical Climates, 152-156
Gutiérrez C, MarioSepúlveda J, Ibrahim C, López MT, Villanueva D, Rodríguez C (2014) Caracterización de fincas ganaderas con y sin pago por servicios ambientales bajo el marco de los capitales de la comunidad en la península de Nicoya, Costa Rica (No. Thesis C266b). Masters Thesis: CATIE
Harvey CA, Villanueva C, Esquivel H, Gómez R, Ibrahim M, Lopez M, Martinez J, Muñoz D, Restrepo C, Saénz JC, Villacís J (2011) Conservation value of dispersed tree cover threatened by pasture management. For Ecol Manage 261:1664–1674
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54(2):427–432
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456
Hallett JT, Díaz-Calvo J, Villa-Castillo J, Wagner MR (2011) Teak plantations: economic bonanza or environmental disaster? J For 109:288–292
Harrison T, Gibbs J, Winfree R (2018) Forest bees are replaced in agricultural and urban landscapes by native species with different phenologies and life-history traits. Glob Chang Biol 24:287–296
Harvey CA, Haber WA (1998) Remnant trees and the conservation of biodiversity in Costa Rican pastures. Agroforestry Systems 44:37–68
Harvey CA, Komar O, Chazdon R, Ferguson BG, Finegan B, Griffith DM, Martínez-Ramos M, Morales H, Nigh R, Soto-Pinto L, Van Breugel M (2008) Integrating agricultural landscapes with biodiversity conservation in the Mesoamerican hotspot. Conserv Biol 22:8–15
Healey SP, Gara RI (2003) The effect of a teak (Tectona grandis) plantation on the establishment of native species in an abandoned pasture in Costa Rica. For Ecol Manage 176:497–507
Heithaus ER (1979) Community structure of neotropical flower visiting bees and wasps: diversity and phenology. Ecology 60:190–202
INEC (Instituto Nacional de Estadística y Censos (2015) VI Censo Nacional Agropecuario: Resultados Generales. San José, Costa Rica
Janzen DH (1967) Synchronization of sexual reproduction of trees within the dry season in Central America. Evolution 21:620–637
Jha S, Vandermeer JH (2010) Impacts of coffee agroforestry management on tropical bee communities. Biol Conserv 143:1423–1431
Jha S, Bacon CM, Philpott SM, Ernesto Mendez V, Läderach P, Rice RA (2014) Shade coffee: update on a disappearing refuge for biodiversity. BioScience 64:416–428
Keenan RJ, Reams GA, Achard F, de Freitas JV, Grainger A, Lindquist E (2015) Dynamics of global forest area: results from the FAO global forest resources assessment 2015. For Ecol Manage 352:9–20
Kennedy CM, Lonsdorf E, Neel MC, Williams NM, Ricketts TH, Winfree R, Bommarco R, Brittain C, Burley AL, Cariveau D, Carvalheiro LG (2013) A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol Lett 16:584–599
Klein AM, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc Biol Sci 274:303–313
Mata A, Echeverria J (2004) Introduction In: Frankie GW, Mata A, Vinson S Bradleigh (ed) Biodiversity Conservation in Costa Rica: Learning the lessons in a seasonally dry forest. University of California Press, Berkeley and Los Angeles, pp 1-17
Michener CD, McGinley RJ, Danforth BN (1994) The bee genera of North and Central America (Hymenoptera: Apoidea) Smithsonian Institution Press, Washington
Milder JC, DeClerck FA, Sanfiorenzo A, Sánchez DM, Tobar DE, Zuckerberg B (2010) Effects of farm and landscape management on bird and butterfly conservation in western Honduras. Ecosphere 1:1–22
Miles L, Newton AC, DeFries RS, Ravilious C, May I, Blyth S, Kapos V, Gordon JE (2006) A global overview of the conservation status of tropical dry forests. J Biogeogr 33:491–505
Muñoz-Piña C, Guevara A, Torres JM, Braña J (2008) Paying for the hydrological services of Mexico's forests: analysis, negotiations and results. Ecol Econ 65:725–736
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H, Oksanen MJ (2013) Package ‘vegan’: Community ecology package. https://cran.ism.ac.jp/web/packages/vegan/vegan.pdf
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326
Pagiola S (2008) Payments for environmental services in Costa Rica. Ecol Econ 65:712–724
Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, Sexton JO (2014) The biodiversity of species and their rates of extinction, distribution, and protection. Science 344
Perfecto I, Rice RA, Greenberg R, Van der Voort ME (1996) Shade coffee: a disappearing refuge for biodiversity: shade coffee plantations can contain as much biodiversity as forest habitats. BioScience 46:598–608
Pohl RW (1983) Hyparrhenia rufa (Jaragua). In: Janzen DH (ed) Costa Rican Natural History. University of Chicago Press, Chicago, p 256
Prendergast KS, Menz MH, Dixon KW, Bateman PW (2020) The relative performance of sampling methods for native bees: an empirical test and review of the literature. Ecosphere 11:e03076
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353
Prado SG, Ngo HT, Florez JA, Collazo JA (2017) Sampling bees in tropical forests and agroecosystems: A review. J Insect Conserv 21:1–18
Quinn GP, Keough MJ, Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, New York, USA
Roubik D (1989) Ecology and natural history of tropical bees. Cambridge University Press, Cambridge, UK
Samnegård U, Hambäck PA, Eardley C, Nemomissa S, Hylander K (2015) Turnover in bee species composition and functional trait distributions between seasons in a tropical agricultural landscape. Agric Ecosyst Environ 211:185–194
SEPSA (Secretaría Ejecutiva de Planificación Sectorial Agropecuaria) (2019) Informe: Comercio Exterior del Sector Agropecuario 2017-2018. Sector de Desarollo Agropecuario y Rural, Costa Rica. https://www.sepsa.go.cr/docs/2019-004-Comercio_Exterior_Sector_Agropecuario_2017-2018.pdf
Serna L, Escobar D, Tapasco J, Arango J, Chirinda N, Chacon M, Segura J, Villanueva C (2017) Challenges and opportunities for the development of the livestock NAMA in Colombia and Costa Rica. CCAFS Info Note. Wageningen, Netherlands: CGIAR Research Program on Climate Change, Agriculture and Food Security (CCAFS).
Stephen WP, Rao S (2005) Unscented color traps for non-Apis bees (Hymenoptera: Apiformes). J Kansas Entomol Soc 78:373–380
Stephen WP, Rao S (2007) Sampling native bees in proximity to a highly competitive food resource (Hymenoptera: Apiformes). J Kans Entomol Soc 80:369–376
Stephens SS, Wagner MR (2007) Forest plantations and biodiversity: a fresh perspective. J For 105:307–313
Stroup WW (2014) Rethinking the analysis of non-normal data in plant and soil science. Agron J 106:1–17
Tangmitcharoen S, Takaso T, Siripatanadilox S, Tasen W, Owens JN (2006) Behavior of major insect pollinators of teak (Tectona grandis Lf): a comparison of clonal seed orchard versus wild trees. For Ecol Manage 222:67–74
Tangmitcharoen S, Tasen W, Owens JN, Bhodthipuks J (2009) Fruit set as affected by pollinators of teak (Tectona grandis Lf) at two tree spacings in a seed orchard. For Res 31:255–259
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity–ecosystem service management. Ecol Lett 8:857–874
Tylianakis JM, Klein AM, Tscharntke T (2005) Spatiotemporal variation in the diversity of Hymenoptera across a tropical habitat gradient. Ecology 86:3296–3302
Vallejo Á, Gutiérrez I, Chacón M, van Rooij W, Dávila MS, Arce JJC, Villalobos R (2006) Biodiversity-human well-being linkages for teak farms in Hojancha, Nicoya peninsula. Costa Rica, CATIE, Turrialba, Costa Rica
Vinson SB, O’Keefe, ST, Frankie GW (2004) The conservation values of bees and ants in the Costa Rican dry forest. In: Frankie, GW, Mata, A, Vinson, S Bradleigh (ed) Biodiversity conservation in Costa Rica: learning the lessons in a seasonally dry forest. University of California Press, Berkeley and Los Angeles, pp 67–79
Watson J, Venter O (2017) A global plan for nature conservation. Nature 550:48–49
Westphal C, Bommarco R, Carré G, Lamborn E, Morison N, Petanidou T, Potts SG, Roberts SP, Szentgyörgyi H, Tscheulin T, Vaissière BE (2008) Measuring bee diversity in different European habitats and biogeographical regions. Ecol Monogr 78:653–671
Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA (2009) A meta-analysis of bees' responses to anthropogenic disturbance. Ecology 90:2068–2076
Winfree R, Bartomeus I, Cariveau DP (2011) Native pollinators in anthropogenic habitats. Annu Rev Ecol Evol Syst 42:1–22
Wunder S (2015) Revisiting the concept of payments for environmental services. Ecol Econ 117:234–243
Yue S, Brodie JF, Zipkin EF, Bernard H (2015) Oil palm plantations fail to support mammal diversity. Ecol Appl 25:2285–2292
Acknowledgements
This research was funded by grants from the National Science Foundation Integrative Graduate Education and Research Traineeship and the United States Agency for International Development Borlaug Fellowship in Food Security. We thank the many landowners who granted us to access their farms for this study, the National System of Conservation Areas office in Hojancha and Emel Rodríguez for logistical support, and Facundo Mendoza Castrillo for field assistance.
Funding
This research was funded by grants from the National Science Foundation Integrative Graduate Education and Research Traineeship (# 0903479) and the United States Agency for International Development Borlaug Fellowship in Food Security (# 12724). Author Galbraith also received support from the United States Department of Agriculture National Institute of Food and Agriculture (# 2018-67013-27535) while writing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose related to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Galbraith, S.M., Griswold, T., Price, W.J. et al. Biodiversity and community composition of native bee populations vary among human-dominated land uses within the seasonally dry tropics. J Insect Conserv 24, 1045–1059 (2020). https://doi.org/10.1007/s10841-020-00274-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-020-00274-8