Abstract
The effect of preharvest sprayable 1-methylcyclopropene (1-MCP) on fruit quality and certain targeted major metabolites was investigated in ‘Chuhwangbae’ pears during short-term cold storage and shelf life. Preharvest 1-MCP application affected methionine, sucrose, and total flavonoid content and fruit decay. During cold storage and shelf life, numerous fruit quality attributes and specific targeted metabolites were affected not by preharvest 1-MCP application but by the storage duration. Overall responses in the normalized heatmap matrix and principal component analysis (PCA) loading plot showed that preharvest sprayable 1-MCP treatment enhanced the incidence of physiological disorders and suppressed the responses of targeted metabolites compared with that in untreated fruit. The results obtained using the PCA score plot showed that physiological and biochemical parameters of the fruit after preharvest 1-MCP treatment were highly separated and diverged based on the storage duration. Nevertheless, preharvest 1-MCP treatment did not significantly affect the physiological and biochemical responses associated with fruit parameters or soluble carbohydrates and free amino acids contents in cold-stored pears. Therefore, the results suggest that preharvest sprayable 1-MCP application may not have strong positive effects on the postharvest quality attributes of ‘Chuhwangbae’ pears during cold storage and shelf life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The effect of 1-methylcyclopropene (1-MCP), an ethylene action inhibitor, has been actively studied in fruit and it has been commercialized for a wide range of horticultural applications, such as controlling fruit ripening in apples and pears (Huber 2008; Watkins 2008; Park 2012). The use of 1-MCP-based technology has had a huge impact on the handling of horticultural products, especially apples, and has provided insights into the biochemical reactions and mechanisms involved in fruit ripening and quality. The following two commercial formulas of 1-MCP exist: the first is the postharvest fumigation-type 1-MCP (SmartFresh™ Technology) and the second is the preharvest spray-type 1-MCP (Harvista™ Technology). The effectiveness of postharvest technologies of 1-MCP application on the inhibition of fruit ripening has been widely demonstrated and 1-MCP is excessively used at present as a commercial agent in storage and distribution systems.
Postharvest 1-MCP treatment is popularly employed to retain the quality of horticultural commodities and to extend the storability and shelf life (Watkins et al. 2000; Bai et al. 2005; Lee et al. 2012c). In addition, preharvest sprayable 1-MCP treatment has been reported to delay the ripening of many fruits (Elfving et al. 2007; Yuan and Carbaugh 2007; Yuan and Li 2008; McArtney et al. 2009), maintain fruit flesh firmness, and reduce the incidence of physiological disorders, for example, scald and internal breakdown in apples (McArtney et al. 2008; DeEll and Ehsani-Moghaddam 2010). Furthermore, it has been reported that preharvest sprayable 1-MCP treatment exhibits beneficial effects with respect to delaying the postharvest deterioration of fruits, such as ‘Scarletspur Delicious’ and ‘Cameo’ apples (Elfving et al. 2007), ‘Brown Turkey’ fig (Freiman et al. 2012), and yellow pitahaya (Cock et al. 2013). The application of sprayable 1-MCP to pome fruit trees has been reported to result in delayed premature fruit drop, reduced ethylene production rates, decreased rates of fruit softening, and loss of flesh firmness and acidity (DeEll and Ehsani-Moghaddam 2010; Villalobos-Acuña et al. 2010; Varanasi et al. 2013). The effects of preharvest sprayable 1-MCP treatment on fruit responses are dependent on the treatment amount (Elfving et al. 2007; McArtney et al. 2008) and timing before harvest (Elfving et al. 2007; Yuan and Li 2008; Varanasi et al. 2013).
The ‘Chuhwangbae’ Asian pear (Pyrus pyrifolia Nakai) cultivar, a cross between the ‘Geumchonjosaeng’ (Kim et al. 2003) and ‘20th century’ cultivars, was released by the Korean pear breeding program in 1985. As a late harvest cultivar, ‘Chuhwangbae’ is one of the most highly consumed varieties of pear in Korea. ‘Chuhwangbae’ pears weigh 400–500 g on average, are round in shape, have yellowish-brown skin, and are sweet and juicy, with firm and white flesh. Late-harvest pear cultivars are generally harvested from middle October to early November, depending on fruit maturity (Kitamura et al. 1981), harvest window or season (Lentheric et al. 1999), orchard location (Chiriboga et al. 2013) or variation, or year to year weather conditions. In general, late harvest-season Asian pears exhibit outstanding fruit quality attributes and storability, compared with the early harvest-season pear cultivars (Pasquariello et al. 2013). Several pre- and postharvest quality management technologies, such as preconditioning (Lee et al. 2016b), fruit bagging treatment (Li et al. 2020), delayed cooling (Lee et al. 2018), gradual cooling (Choi et al. 2020), and carbon dioxide treatment (Lee et al. 2018), have been employed to maintain fruit freshness and to prevent the incidence of peel blackening, a major physiological disorder in Asian pears during cold storage (Lee et al. 2018). Nevertheless, there is a lack of information regarding the effect of preharvest sprayable 1-MCP treatment on fruit quality and metabolic responses in ‘Chuhwangbae’ pears during short-term cold storage and shelf life. Therefore, the objective of this study was to determine the mechanism by which the preharvest application of sprayable 1-MCP affects the postharvest physiological and biochemical responses in cold-stored ‘Chuhwangbae’ pears.
2 Materials and methods
2.1 Plant material and storage conditions
1-MCP (Harvista™; AF10081; 1.8%; AgroFresh Inc., Spring House, PA, USA) treatment was performed on September 22, 2016, approximately 7 d before harvesting the ‘Chuhwangbae’ Asian pears (P. pyrifolia Nakai) at the experimental orchard of Pear Research Institute, NIHHS, RDA in Naju, Jeollanam-do, Republic of Korea. A sprayable formulation of 1-MCP was used on the pear trees, in accordance with the suggested concentration of 250.0 mg L−1 Harvista™, based on the preliminary results obtained upon the application of 0, 62.5, 125.0, and 250.0 mg L−1 Harvista™. Harvested pears without defects were stored at 1 °C with 90% relative humidity (RH) for up to 12 weeks (3 months) and then transferred at 22 °C and 60% RH for 2 weeks.
2.2 Measurement of fruit quality parameters
Fruit quality parameters were assessed at the time of harvest and after shelf life, with three replicates for each treatment and five pears per replicate. Fruit weight loss was measured in accordance with the methods described by Byeon and Lee (2020) and Lwin and Lee (2020), and described in terms of the percentage loss of initial weight. Fruit color parameters (L*, C*, and ho) were measured at the equator regions of the peel, cortex, and core tissues with three readings using a chromameter (Minolta CR-200; Minolta Co., Osaka, Japan) (Lwin and Lee 2020). Fruit firmness was assessed using a digital penetrometer at two opposite sides of each fruit and these results are expressed in newtons (N) (Seo et al. 2019). Then, soluble solids content (SSC) was estimated using a reflectometer (PR-201α; Atago Co., Ltd., Tokyo, Japan) and titratable acidity (TA) was measured using an automatic titrator. SSC and TA are expressed as a percentage (%) (Seo et al. 2019).
The incidence and severity of storage disorders, including peel staining, peel blackening, cortex browning, core browning, shriveling, and fruit decay, were assessed throughout the entire storage period as described in previous reports (Lee et al. 2013, 2016a; Seo et al. 2019).
2.3 Determination of soluble carbohydrate and free amino acid contents
The contents of soluble carbohydrates and free amino acids were investigated based on a previously described method (Byeon and Lee 2020; Lwin and Lee 2020). Ground frozen cortex (5 g) extracts were prepared using a buffer (15 mL) containing 0.1 M perchloric acid (Daejung Chemicals & Metals Co., Ltd., Siheung, Gyeonggi-do, Korea), 0.1% meta-phosphate acid (Junei, Tokyo, Japan), and distilled water. These extracts were filtered through a 0.2-μm hydrophilic filter (Sartorius Co., Göttingen, Germany) and the soluble carbohydrate and free amino acid contents were determined using high-performance liquid chromatography (Dionex ultimate 3000, Thermo Fisher Scientific, Korea Ltd., Seoul, Korea). The contents of soluble carbohydrates and free amino acids are presented as g kg−1 and mg kg−1 fresh weight, respectively.
2.4 Measurement of total phenolic compounds and flavonoid contents
The content of total phenolic compounds was determined using the Folin‒Ciocalteu method (Ainsworth and Gillespie 2007). Absorbance at 750 nm was measured using a spectrophotometer (Multiskan go, Thermo Scientific, Waltham, MA, USA). The content of total phenolic compounds was reported in terms of g gallic acid equivalent (GAE) kg−1 fresh weight.
The total flavonoid content was determined as previously described (Meyers et al. 2003) with some modifications. Absorbance was measured at 510 nm. The results are presented as g catechin equivalent (CE) kg−1 fresh weight. All assays were performed in triplicate.
2.5 Statistical analyses
Statistical analysis was conducted using SAS statistics software 9.3 (Version 9.3; SAS Institute Inc., Carry, NC, USA). Differences between the control and 1-MCP treatments were assessed using the least significance difference (LSD) test and significance was determined at P < 0.05. A normalized heatmap matrix system, principal component analysis (PCA) scores and loading plots, and the correlation coefficient (r) test were performed to determine the differential effects of 1-MCP on fruit quality parameters and targeted metabolites using the corresponding functions of MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/) (Chong et al. 2018).
3 Results
3.1 Fruit quality attributes
The weight of ‘Chuhwangbae’ pears decreased in the control and MCP-1 treatment groups following harvest, but preharvest treatment with sprayable 1-MCP delayed the weight loss at the end of the storage period. Flesh firmness was slightly lower in 1-MCP-treated fruits than that in untreated fruits at the time of harvest and then declined during cold storage and shelf life. Nevertheless, flesh firmness was slightly lower in 1-MCP-treated fruits than that in untreated fruits. No significant difference was detected in SSC and TA after preharvest treatment with sprayable 1-MCP. SSC gradually increased, but TA slowly decreased, regardless of 1-MCP treatment (Fig. S1).
The color responses of pear fruit tissues were not affected by preharvest sprayable 1-MCP application during cold storage and shelf life. Nonetheless, the L* value tended to be slightly lower in 1-MCP treated fruits than that in untreated fruits for core tissues. ho tended to be lower in 1-MCP-treated fruits than that in untreated fruits in cortex tissues. However, the L* and ho values responded inversely in core tissues, compared with that in the cortex tissues. L* and ho values were higher in the cortex and core tissues than those in the peel tissues, regardless of 1-MCP application and storage period. However, the C* value was lower in the core and cortex tissues than that in the peel tissues during cold storage and shelf life (Fig. S2).
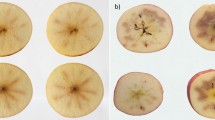
Peel blackening and internal browning were observed in control as well as 1-MCP-sprayed fruits after 6 weeks of cold storage and an additional 2 weeks of shelf life, and the incidence of peel blackening and internal browning was slightly higher in 1-MCP sprayed fruits than that in untreated fruits (Fig. S3). Shriveling was detected at the last stage of cold storage and shelf life, but the severity rate was not significantly different after sprayable 1-MCP treatment. Further, preharvest sprayable 1-MCP treatment increased the incidence and severity rate of fruit decay (compared with no treatment control).
3.2 Metabolic responses
The sucrose content was higher in preharvest 1-MCP-sprayed fruits than that in untreated fruits at the time of harvest. The level of sucrose gradually declined during cold storage and shelf life but tended to be higher in 1-MCP-sprayed fruits than that in untreated fruit. In contrast, the levels of glucose and fructose gradually increased during the entire storage period, regardless of sprayable 1-MCP treatment. The level of sorbitol was not changed upon 1-MCP application. Furthermore, the levels of total phenolic compounds and flavonoids were not influenced by preharvest 1-MCP spraying (Fig. S4).
The free amino acid contents responded differently to cold storage and shelf life but not to preharvest spraying with 1-MCP (Fig. S5). The contents of most amino acids were reduced during cold storage and shelf life, irrespective of preharvest sprayable 1-MCP treatment. However, the contents of aspartic acid, serine, and phenylalanine increased during the storage.
3.3 Normalized heatmap responses
A normalized heatmap matrix system was used to represent the overall responses of fruit quality parameters and targeted metabolites—to preharvest spraying with 1-MCP—during the entire storage period (Fig. 1). More positive responses were observed in the preharvest 1-MCP-sprayed fruits than those in the untreated fruits at the time of harvest. However, preharvest 1-MCP application reduced the responses, compared with those observed in untreated fruits, although the incidences of certain physiological responses were higher after sprayable 1-MCP treatment. In addition, the results of ANOVA based on two main effects and the interaction factor are presented. Fruit decay, cortex C* and core L* values, and methionine, sucrose, and total flavonoid contents were significantly affected by preharvest spraying of 1-MCP. On the contrary, the storage duration factor significantly affected internal browning, isoleucine, glucose, weight loss, shriveling, fructose, total phenolic compounds, glutamic acid, sorbitol, alanine, GABA, methionine, threonine, tyrosine, SSC, peel ho and L*, core L* and ho, arginine, sucrose, total flavonoids, firmness, and TA, with some differences detected at significant levels. The interaction effect was only detected for weight loss and total phenolic compounds.
Normalized heatmap responses of physiological fruit quality attributes, physiological disorders, and targeted major metabolites in ‘Chuhwangbae’ pears untreated (Control) or treated with 250.0 mg L−1 preharvest sprayable 1-methylcyclopropene (1-MCP; Harvista™) 1 week before harvest and after storage at 1 °C for 3 months and 22 °C for 14 d (2 weeks)
3.4 Association of fruit physiological and biochemical variables
PCA scores and loading plots were employed to estimate the overall association, divergence, and segregation with respect to the performances of fruit physiological parameters and major targeted metabolites in the preharvest sprayable 1-MCP-applied fruit during the entire storage period (Fig. 2). PC-1 and -2 values from PCA score plot accounted for 82.8% and 14% of the total X and Y variances, respectively. The results of PCA score plot showed that overall responses to preharvest sprayable 1-MCP treatment were more closely associated with each other than with those in the untreated fruit, regardless of storage duration (Fig. 2a).
Principal component analysis (PCA) scores plot a and loading plot b of response variables in ‘Chuhwangbae’ pears untreated (Control) or treated with 250.0 mg L−1 preharvest sprayable 1-methylcyclopropene (1-MCP; Harvista™) 1 week before harvest and after storage at 1 °C for 3 months (M) and 22 °C for 14 d (2 weeks; W). 0 M + 0 W = 0 W, 0 M + 2 W = 2 W, 1.5 M + 2 W = 8 W, and 3 M + 2 W = 14 W
Certain variables associated with storage duration and preharvest sprayable 1-MCP treatment were apparent in the PCA loading plot (Fig. 2b). The total variation in the PCA loading plot was accounted for by 27.4% in PC-1 and 24.5% in PC-2. The PCA loading plot showed the association of storage duration and 1-MCP treatment as a response variable with numerous predictor variables, including fruit physiological characteristics and targeted metabolites. Weight loss, glucose, and isoleucine were tightly associated with storage duration. Physiological disorders, such as internal browning, peel blackening, decay, and shriveling, were more closely associated with preharvest sprayable 1-MCP treatment and storage duration than no treatment. In addition, amino acids, such as glutamic acid, glycine, asparagine, serine, valine, leucine, aspartic acid, histidine, phenylalanine, and isoleucine, as well as sorbitol and glucose, were associated with storage duration and preharvest sprayable 1-MCP treatment. In contrast, untreated fruits were closely associated with color variables and total phenolic compounds. Firmness, TA, total flavonoids, sucrose, arginine, lysine, tyrosine, methionine, GABA, alanine, threonine, and glutamine were least associated with the storage duration, regardless of treatment.
3.5 Correlation between fruit quality parameters and targeted metabolites
The heatmap matrix of Pearson’s correlation coefficient test was used to understand the overall relationship between fruit quality parameters and major targeted metabolites, regardless of storage duration (Fig. 3). A positive correlation was observed with the preharvest sprayable 1-MCP treatment, whereas a negative correlation was detected with no treatment. In untreated fruits, SSC, color variables, and total phenolic compounds were highly positively correlated with each other. Additionally, firmness, TA, total flavonoids, and sucrose were positively correlated with each other. Another positive correlation observed was that of aspartic acid, histidine, leucine, phenylalanine, and isoleucine. The last positive correlation detected was among weight loss, shriveling, fructose, and glucose. On the contrary, in preharvest 1-MCP-sprayed fruit, SSC and cortex C* were highly positively correlated with firmness, TA, sucrose, and color variables. Weight loss was negatively correlated with glycine, sorbitol, tyrosine, arginine, alanine, glutamine, GABA, threonine, color variables, sucrose, TA, and firmness.
Pearson’s correlation coefficient heatmap matrices for the responses of physiological fruit quality attributes, severity of physiological disorders, and targeted metabolites in ‘Chuhwangbae’ pears untreated (Control) or treated with 250.0 mg L−1 preharvest sprayable 1-methylcyclopropene (1-MCP; Harvista™) 1 week before harvest and after storage at 1 °C for 3 months and 22 °C for 14 d
4 Discussion
Since its introduction in the fruit industry, postharvest 1-MCP fumigation technology has been exponentially used in the horticultural industry, including the pome fruit industry (Watkins 2006; Huber 2008; Park 2012). Based on the huge success of postharvest 1-MCP technology, preharvest sprayable 1-MCP technology has been developed to reduce the issues associated with labor intensiveness and technology, such as through the development of perfectly sealed facilities (Elfving et al. 2007; DeEll and Ehsani-Moghaddam 2010). As Asian pears are not highly responsive to ethylene during cold storage, postharvest 1-MCP technology has been adopted to retain fruit freshness after harvest (Lee et al. 2017b; Lee et al. 2017c). In addition, postharvest 1-MCP treatment could be used to retain fruit firmness and reduce the severity of internal browning during shelf life, followed by international transport (Seo et al. 2019). Thus, in this study, preharvest sprayable 1-MCP treatment was used to evaluate the physiological and biochemical characteristics of ‘Chuhwangbae’ Asian pears during cold storage and shelf life.
Preharvest spraying of 1-MCP did not have a huge impact on fruit freshness during storage. In addition, preharvest 1-MCP application does not have any significant effect on flesh firmness, ethylene production, peel color, and the incidence of internal browning and scald in cold-stored ‘Bartlett’ pears (Villalobos-Acuña et al. 2010). However, in mid-late harvest apple cultivars, such as the ‘McIntosh’ and ‘Delicious’ apple cultivars, preharvest 1-MCP treatment has demonstrated effectiveness with respect to retaining fruit firmness, compared with postharvest 1-MCP treatment (Watkins et al. 2010). In this study, fruit quality parameters were not affected by preharvest spraying of 1-MCP during short-term cold storage and shelf life. This difference could be attributed to the differences in the responsiveness of different horticultural crops to ethylene. Ethylene is more highly produced in apple fruit than in Asian pears, although there are variations in ethylene production among different Asian pear cultivars as well (Jeong et al. 1998).
Although the primary responses of fruit quality parameters and targeted metabolites to preharvest spraying with 1-MCP were not substantially outstanding during the entire storage period, the differences in the overall physiological and metabolic responses could be detected using the normalized heatmap system and the PCA scores plot. The PCA scores plot indicated that preharvest 1-MCP-sprayed fruit was less associated with the metabolic response than with the untreated fruit during the entire storage period. Although the fundamental results based on the responses of corresponding parameters were not significantly affected by preharvest spraying with 1-MCP, a multivariate data analysis system would be substantially useful to recognize any potential differences and associations depending on the treatment (Rudell et al. 2009; Lee et al. 2012a, b). The results obtained using the overall heatmap matrix system were highly affected either by preharvest spraying with 1-MCP or storage duration. In addition, the PCA score plot showed that the relationship and association of predictor variables were relatively suppressed by preharvest sprayable 1-MCP treatment. Furthermore, when predictor variables were bi-plotted with response variables, such as storage duration and preharvest sprayable 1-MCP treatment, the PCA loading plot provided further impact results in which preharvest sprayable 1-MCP treatment was much more closely associated with more free amino acids than the no treatment.
As a response variable, storage duration was tightly linked with weight loss and glucose but less so with sucrose and sorbitol. During the entire storage period, the content of soluble carbohydrates, in terms of SSC, was slightly increased with the increase in glucose and fructose levels, but sucrose and sorbitol levels decreased or remained unchanged. Thus, in the PCA loading plot, the biochemical responses of these individual soluble carbohydrates were similar in appearance to the storage duration variable. In addition, total phenolic compounds, total flavonoids, flesh firmness, and TA were least associated with storage duration. These predictor variables declined or remained unchanged after preharvest sprayable 1-MCP treatment. The incidence and severity of physiological disorders were higher in the preharvest 1-MCP-sprayed fruits than those in the untreated ones during the entire storage period. Otherwise, as the storage duration increased, fruit shriveling occurred, regardless of preharvest sprayable 1-MCP treatment. In the PCA loading plot, these physiological disorders were relatively closely associated with the storage duration and preharvest sprayable 1-MCP treatment.
Interestingly, two main clusters of free amino acids were detected in the PCA loading plot, and preharvest sprayable 1-MCP treatment was closely linked with these amino acids. Furthermore, higher positive correlations in the preharvest 1-MCP treatment group than in the untreated group were detected. This response could be strongly linked to a lower contrast response in the preharvest sprayable 1-MCP treatment group. This result would correspondingly be associated with overall normalized heatmap responses. That is, the contrast responses in the normalized heatmap matrix would be indirectly influenced by the overall correlation coefficients in untreated fruits. Although the physiological and biochemical responses of untreated fruits were not very comparable with those of the preharvest sprayable 1-MCP-treated fruits, these multivariate analyses provided more profound results regarding preharvest sprayable 1-MCP treatment.
Among the targeted metabolites, sucrose responded significantly to preharvest sprayable 1-MCP treatment. The higher level of sucrose at harvest in the preharvest sprayable 1-MCP-applied fruits could be involved in fruit ripening. Sucrose, galactose, and starch are highly linked with the maturity of tomato fruit (Tang et al. 2020; Zhang et al. 2020). During the entire storage period, the level of sucrose was higher in the preharvest sprayable 1-MCP treatment group than that in the untreated group. In addition, postharvest 1-MCP application results in a higher level of retained sucrose in cold-stored loquat fruit (Cao et al. 2011), kiwifruit (Hu et al. 2017), peaches (Yu et al. 2017), and Asian pears (Itai and Tanahashi 2008) but not in ‘Fuji’ apples (Lee et al. 2017a). Nonetheless, it can be assumed that the higher level of sucrose is associated with the lower conversion of glucose and fructose using invertase during cold storage, as shown previously in the lower progression of the degradation of sucrose and delayed accumulation of fructose and glucose in 1-MCP-treated pears (Itai and Tanahashi 2008).
In conclusion, preharvest sprayable 1-MCP treatment did not have a huge impact on cold-stored ‘Chuhwangbae’ pears in terms of the physiological and biochemical characteristics of the fruits. However, fruit quality parameters and targeted metabolites were substantially influenced by the storage period. Nonetheless, the results of multivariate data analyses, including a normalized heatmap matrix system, PCA scores and loading plots, and overall correlation coefficient test, provided certain profound and complex physiological and biochemical results regarding preharvest sprayable 1-MCP treatment, compared with no treatment. Unlike the primary responses of fruit physiological and biochemical characteristics, the secondary normalized results from the multivariate data analysis system could be used to elucidate any potential difference resulting from the treatment. Therefore, although preharvest spraying with 1-MCP did not directly impact affect fruit quality and metabolic responses, the secondary results suggest that further in-depth multivariate data analysis is required to elucidate the responses of cold-stored ‘Chuhwangbae’ pears to preharvest 1-MCP treatment.
References
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protocols 2:875–877. https://doi.org/10.1038/nprot.2007.102
Bai JH, Baldwin EA, Goodner KL, Mattheis JP, Brecht JK (2005) Response of four apple cultivars to 1-methylcyclopropene treatment and controlled atmosphere storage. HortScience 40:1534–1538. https://doi.org/10.21273/HORTSCI.40.5.1534
Byeon S-E, Lee J (2020) Differential responses of fruit quality and major targeted metabolites in three different cultivars of cold-stored figs (Ficus carica L). Sci Hortic 260:108877. https://doi.org/10.1016/j.scienta.2019.109160
Cao S, Zheng Y, Yang Z (2011) Effect of 1-MCP treatment on nutritive and functional properties of loquat fruit during cold storage. N Z J Crop Hortic Sci 39:61–70. https://doi.org/10.1080/01140671.2010.526621
Chiriboga M-A, Schotsmans WC, Larrigaudière C, Dupille E, Recasens I (2013) Responsiveness of ‘Conference’ pears to 1-methylcyclopropene: the role of harvest date, orchard location and year. J Sci Food Agric 93:619–625. https://doi.org/10.1002/jsfa.5853
Choi M-H, Choi HJ, Hong SS, Lim B-S (2020) Effects of gradual cooling treatment on the skin blackening and physicochemical characteristics of ‘Chuhwang’ pear fruit. Kor J Food Preserv 27:145–158. https://doi.org/10.11002/kjfp.2020.27.2.145
Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46:W486–W494. https://doi.org/10.1093/nar/gky310
Cock LS, Valenzuela LST, Alfredo AA (2013) Physical, chemical and sensory changes of refrigerated yellow pitahaya treated preharvest with 1-MCP. Dyna 80:11–20
DeEll JR, Ehsani-Moghaddam B (2010) Preharvest 1-methylcyclopropene treatment reduces soft scald in ‘Honeycrisp’ apples during storage. HortScience 45:414–417. https://doi.org/10.21273/HORTSCI.45.3.414
Elfving DC, Drake SR, Reed AN, Visser DB (2007) Preharvest applications of sprayable 1-methylcyclopropene in the orchard for management of apple harvest and postharvest condition. HortScience 42:1192–1199. https://doi.org/10.21273/HORTSCI.42.5.1192
Freiman ZE, Rodov V, Yablovitz Z, Horev B, Flaishman MA (2012) Preharvest application of 1-methylcyclopropene inhibits ripening and improves keeping quality of ‘Brown Turkey’ figs (Ficus carica L.). Sci Hortic 138:266–272. https://doi.org/10.1016/j.scienta.2012.01.007
Hu W, Sun D-W, Blasco J (2017) Rapid monitoring 1-MCP-induced modulation of sugars accumulation in ripening ‘Hayward’ kiwifruit by Vis/NIR hyperspectral imaging. Postharvest Biol Technol 125:168–180. https://doi.org/10.1016/j.postharvbio.2016.11.001
Huber DJ (2008) Suppression of ethylene responses through application of 1-methylcyclopropene: a powerful tool for elucidating ripening and senescence mechanisms in climacteric and nonclimacteric fruits and vegetables. HortScience 43:106–111. https://doi.org/10.21273/HORTSCI.43.1.106
Itai A, Tanahashi T (2008) Inhibition of sucrose loss during cold storage in Japanese pear (Pyrus pyrifolia Nakai) by 1-MCP. Postharvest Biol Technol 48:355–363. https://doi.org/10.1016/j.postharvbio.2007.10.015
Jeong ST, Kim JG, Hong SS, Jang HS, Kim YB (1998) Influence of maturity and storage temperature on the respiration rate and ethylene production in ‘Kosui’, ‘Chojuro’ and ‘Niitaka’ pears. J Kor Soc Hortic Sci 39:446–448
Kim M-S, Cho K-S, Hong S-J (2003) Determination of optimum harvest time of ‘Geumchonjosaeng’ pear (Pyrus pyrifolia) and its shelf life at ambient temperature. Hortic Sci Technol 21:120–123
Kitamura T, Iwata T, Fukushima T, Furukawa Y, Ishiguro T (1981) Studies on the maturation-physiology and storage of fruits and vegetables II. Respiration and ethylene production in reference to species and cultivars of pear fruit. J Jpn Soc Hortic Sci 49:608–616. https://doi.org/10.2503/jjshs.49.608
Lee J, Mattheis JP, Rudell DR (2012a) Antioxidant treatment alters metabolism associated with internal browning in ‘Braeburn’ apples during controlled atmosphere storage. Postharvest Biol Technol 68:32–42. https://doi.org/10.1016/j.postharvbio.2012.01.009
Lee J, Rudell DR, Davies PJ, Watkins CB (2012b) Metabolic changes in 1-methylcyclopropene (1-MCP)-treated ‘Empire’ apple fruit during storage. Metabolomics 8:742–753. https://doi.org/10.1007/s11306-011-0373-5
Lee U-Y, Oh K-Y, Moon S-J, Hwang Y-S, Chun J-P (2012c) Effects of 1-methylcyclopropene (1-MCP) on fruit quality and occurrence of physiological disorders of Asian pear (Pyrus pyrifolia), ‘Wonhwang’ and ‘Whasan’, during shelf-life. Hortic Sci Technol 30:534–542. https://doi.org/10.7235/hort.2012.12033
Lee J, Mattheis JP, Rudell DR (2013) Fruit size affects physiological attributes and storage disorders in cold-stored ‘Royal Gala’ apples. HortScience 48:1518–1524. https://doi.org/10.21273/HORTSCI.48.12.1518
Lee J, Mattheis JP, Rudell DR (2016a) Storage temperature and 1-methylcyclopropene treatment affect storage disorders and physiological attributes of ‘Royal Gala’ apples. HortScience 51:84–93. https://doi.org/10.21273/HORTSCI.51.1.84
Lee U-Y, Oh K-S, Hwang Y-S, Lim B-S, Ahn Y-J, Chun J-P (2016b) Effect of temperature pre-conditioning on fruit quality of early-season ‘Hanareum’ pears (Pyrus pyrifolia Nakai) during simulated marketing. Hortic Sci Technol 34:94–101. https://doi.org/10.12972/kjhst.20160003
Lee J, Jeong M-C, Ku K-H (2017a) Chemical, physical, and sensory properties of 1-MCP-treated Fuji apple (Malus domestica Borkh.) fruits after long-term cold storage. Appl Biol Chem 60:363–374. https://doi.org/10.1007/s13765-017-0288-6
Lee U-Y, Choi J-H, Kim Y-K, Oh K-S, Kim S-J, Chun J-P (2017b) Comparison of fruit maketability at various temperature environment in Asian pear ‘Jinhwang’ treated with 1-methylcyclopropene. Prot Hortic Plant Fact 26:19–26. https://doi.org/10.12791/KSBEC.2017.26.1.19
Lee U-Y, Choi J-H, Lee J-H, Oh K-S, Chun J-P (2017c) Effect of 1-MCP treatment on the early-season Asian pear cultivar ‘Wonhwang’ in response to different temperature conditions during simulated exportation. Hortic Sci Technol 35:568–576. https://doi.org/10.12972/kjhst.20170061
Lee U-Y, Wang M-H, Bae T-M, Kim S-J, Choi J-H, Ahn Y-J, Chun J-P (2018) Effects of pre-drying, delayed cooling, and carbon dioxide on skin blackening disorder in Asian pear (Pyrus pyrifolia Nakai) ‘Chuhwangbae’. Hortic Sci Technol 36:370–379. https://doi.org/10.12972/kjhst.20180036
Lentheric I, Pinto E, Vendrell M, Larrigaudiere C (1999) Harvest date affects the antioxidative systems in pear fruits. J Hortic Sci Biotechnol 74:791–795. https://doi.org/10.1080/14620316.1999.11511190
Li Q, Cheng C-x, Zhang X-f, Wang C-h, Yang S-l (2020) Preharvest bagging and postharvest calcium treatment affects superficial scald incidence and calcium nutrition during storage of ‘Chili’ pear (Pyrus bretschneideri) fruit. Postharvest Biol Technol 163:111149. https://doi.org/10.1016/j.postharvbio.2020.111149
Lwin HP, Lee J (2020) Fruit quality and major metabolites in cold-stored ‘Wonhwang’ Asian pears are differentially affected by fruit size. J Sci Food Agric. https://doi.org/10.1002/jsfa.10422
McArtney SJ, Obermiller JD, Schupp JR, Parker ML, Edgington TB (2008) Preharvest 1-methylcyclopropene delays fruit maturity and reduces softening and superficial scald of apples during long-term storage. HortScience 43:366–371. https://doi.org/10.21273/HORTSCI.43.2.366
McArtney SJ, Obermiller JD, Hoyt T, Parker ML (2009) ‘Law Rome’ and ‘Golden Delicious’ apples differ in their response to preharvest and postharvest 1-methylcyclopropene treatment combinations. HortScience 44:1632–1636. https://doi.org/10.21273/HORTSCI.44.6.1632
Meyers KJ, Watkins CB, Pritts MP, Liu RH (2003) Antioxidant and antiproliferative activities of strawberries. J Agric Food Chem 51:6887–6892. https://doi.org/10.1021/jf034506n
Park Y-M (2012) 1-MCP application for horticultural commodities in Korea: practical potential and future task. Hortic Environ Biotechnol 53:441–446. https://doi.org/10.1007/s13580-012-0174-4
Pasquariello MS, Rega P, Migliozzi T, Capuano LR, Scortichini M, Petriccione M (2013) Effect of cold storage and shelf life on physiological and quality traits of early ripening pear cultivars. Sci Hortic 162:341–350. https://doi.org/10.1016/j.scienta.2013.08.034
Rudell DR, Mattheis JP, Hertog MLATM (2009) Metabolomic change precedes apple superficial scald symptoms. J Agric Food Chem 57:8459–8466. https://doi.org/10.1021/jf901571g
Seo H-J, Wang Y-S, Lwin HP, Choi J-H, Chun J-P, Roan S-F, Chen I-Z, Lee J (2019) Early season ‘Wonhwang’ pear fruit quality following international transport and storage is negatively impacted by fruitlet stage gibberellic acid4+7 (GA4+7) application but improved by postharvest 1-methylcyclopropene (1-MCP). Sci Hortic 256:108549. https://doi.org/10.1016/j.scienta.2019.108549
Tang H, Zhang X, Gong B, Yan Y, Shi Q (2020) Proteomics and metabolomics analysis of tomato fruit at different maturity stages and under salt treatment. Food Chem 311:126009. https://doi.org/10.1016/j.foodchem.2019.126009
Varanasi V, Shin S, Johnson F, Mattheis JP, Zhu Y (2013) Differential suppression of ethylene biosynthesis and receptor genes in ‘Golden Delicious’ apple by preharvest and postharvest 1-MCP treatments. J Plant Growth Regul 32:585–595. https://doi.org/10.1007/s00344-013-9326-8
Villalobos-Acuña MG, Biasi WV, Flores S, Mitcham EJ, Elkins RB, Willits NH (2010) Preharvest application of 1-methylcyclopropene influences fruit drop and storage potential of ‘Bartlett’ pears. HortScience 45:610–616. https://doi.org/10.21273/HORTSCI.45.4.610
Watkins CB (2006) The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol Adv 24:389–409. https://doi.org/10.1016/j.biotechadv.2006.01.005
Watkins CB (2008) Overview of 1-methylcyclopropene trials and uses for edible horticultural crops. HortScience 43:86–94. https://doi.org/10.21273/HORTSCI.43.1.86
Watkins CB, Nock JF, Whitaker BD (2000) Responses of early, mid and late season apple cultivars to postharvest application of 1-methylcyclopropene (1-MCP) under air and controlled atmosphere storage conditions. Postharvest Biol Technol 19:17–32. https://doi.org/10.1016/S0925-5214(00)00070-3
Watkins CB, James H, Nock JF, Reed N, Oakes RL (2010) Preharvest application of 1-methylcyclopropene (1-MCP) to control fruit drop of apples, and its effects on postharvest quality. Acta Hortic 877:365–374. https://doi.org/10.17660/ActaHortic.2010.877.46
Yu L, Shao X, Wei Y, Xu F, Wang H (2017) Sucrose degradation is regulated by 1-methycyclopropene treatment and is related to chilling tolerance in two peach cultivars. Postharvest Biol Technol 124:25–34. https://doi.org/10.1016/j.postharvbio.2016.09.002
Yuan R, Carbaugh DH (2007) Effects of NAA, AVG, and 1-MCP on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of ‘Golden Supreme’ and ‘Golden Delicious’ apples. HortScience 42:101–105. https://doi.org/10.21273/HORTSCI.42.1.101
Yuan R, Li J (2008) Effect of sprayable 1-MCP, AVG, and NAA on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of ‘Delicious’ apples. HortScience 43:1454–1460. https://doi.org/10.21273/HORTSCI.43.5.1454
Zhang X, Tang H, Du H, Liu Z, Bao Z, Shi Q (2020) Comparative N-glycoproteome analysis provides novel insights into the regulation mechanism in tomato (Solanum lycopersicum L) during fruit ripening process. Plant Sci 293:110413. https://doi.org/10.1016/j.plantsci.2020.110413
Acknowledgements
This study was financially supported by a grant from the 2018 Research Fund (PJ01190804) of the Rural Development Administration, Republic of Korea. Ms. Hnin Phyu Lwin was supported by the Chung-Ang University Young Scientist Scholarship (CAYSS) in 2018 for her Ph.D. program. We thank Ms. Si-Eun Byeon at the Division of Special-purpose Trees, National Institute of Forest Science, and Ms. Boyeon Kim at Chung-Ang University for their technical assistance and support.
Author information
Authors and Affiliations
Contributions
HPL Data curation, Formal analysis, Investigation, Methodology, Writing, Revision. JL Conceptualization, Data curation, Investigation, Writing, Reviewing & Editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Communicated by Eun Jin Lee
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lwin, H.P., Lee, J. Differential effects of preharvest sprayable 1-methylcyclopropene application on fruit quality attributes and major targeted metabolites in cold-stored ‘Chuhwangbae’ pears. Hortic. Environ. Biotechnol. 62, 53–61 (2021). https://doi.org/10.1007/s13580-020-00289-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-020-00289-9