Abstract
‘Wonhwang’ Asian pears could be intolerant to the incidence of physiological disorders during long-term storage. 1-Methylcyclopropene (1-MCP) technology is commonly used in the fruit industry to retain fruit quality during cold storage and the handling period. Thus, the objectives of this study were to evaluate the effectiveness of postharvest 1-MCP treatment on fruit physiological characteristics, physiological disorders, and the major metabolic responses of organic acids, soluble carbohydrates, and free amino acids in long-term cold-stored ‘Wonhwang’ pears. The 1-MCP treatment reduced flesh firmness but increased weight loss during storage. It also reduced the content of fructose, glucose, and malic acid but retained higher levels of sucrose and sorbitol during the second half of storage compared to the untreated control. The 1-MCP application enhanced the severity and incidence of cavities and water soaking during cold storage and increased methionine levels in cold-stored pears. The scores plot of principal component analysis indicated that 1-MCP treatment caused the response variables to diverge during the second half of storage. Additionally, the correlation coefficient network indicated that 1-MCP treatment differentially mediated the correlation coefficients of fruit quality parameters and targeted metabolites in long-term cold-stored pears. These results suggest that postharvest 1-MCP application affects the differential responses of fruit quality parameters in Asian pears stored at 0.5 °C for up to 6 months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
‘Wonhwang’ Asian pear is an early-season cultivar that is highly attractive to Asian pear growers and distributors because of the premium fruit quality at harvest (Kim et al., 1995; Seo et al., 2019). Nonetheless, the physiological quality of ‘Wonhwang’ pear fruit is highly influenced by the fruit harvest window (Choi et al., 2015b; Hong et al., 2004) and handling temperature during transport and distribution (Oh et al., 2010). Immediately after harvest, fruit quality was relatively stable during the short-term period of international trade (Seo et al., 2019). However, flesh firmness gradually declined with an increase in the shelf-life period starting immediately after harvest, regardless of fruit maturity (Lee et al., 2012b). The decrease in fruit firmness and titratable acidity (TA) and the increase in weight loss and soluble solids content (SSC) varied at room temperature during the shelf-life period starting immediately after harvest (Lwin et al., 2021a). Additionally, TA was not affected by handling temperature during transport and distribution, but fruit weight loss and firmness were significantly affected by the increase in the handling period (Oh et al., 2010). In contrast, the responses of SSC and TA in terms of fruit quality attributes were not highly affected during the handling period after 1 month of cold storage, but flesh firmness rapidly decreased (Choi et al., 2013). The fruit quality attributes of ethylene evolution and fruit CO2 production rate were greatly affected by handling temperature, depending on the period of short-term storage (Lee et al., 2002). However, fruit quality attributes were highly influenced by fruit size at 0.5°C for 6 months (Lwin and Lee, 2020). Storage temperature also affected fruit quality parameters along with physiological disorders during storage (Jia et al., 2018).
During cold chain and the distribution period, ‘Wonhwang’ Asian pears are vulnerable to the incidence of storage disorders, such as flesh browning, water soaking (Seo et al., 2019), mealiness, and pithiness (Oh et al., 2010). The severity and incidence of internal browning were mainly caused by the increases in storage temperature (Lee et al., 2016; Moon et al., 2008). Additionally, the incidence and severity of fruit shrivelling and decay were different depending on fruit size during storage at 0.5°C for 6 months (Lwin and Lee, 2020). The severity of core and flesh breakdown was increased with the augmentation in storage period and temperature (Lee et al., 2013; Lim et al., 2007). Even with a shelf-life period starting immediately after harvest, the severity of internal browning, core browning, water soaking, and cavity was increased with the prolonged fruit maturity and handling period (Lee et al., 2011; Lee et al., 2012b). In addition, the incidence of internal browning and mealiness was much more severe during shelf life with an increase in handling temperature (Lee et al., 2017b). The severity and incidence of internal breakdown, mealiness, and water soaking were worsened by an advance in shelf life after 1 month of storage (Choi et al., 2013). Pre-conditioning treatment at 10°C right after harvest significantly reduces the development of core breakdown during the handling period (Choi et al., 2015a).
‘Wonhwang’ Asian pears are primarily cold-stored during a short-term period immediately after harvest and then are distributed during the handling period (Choi et al., 2013; Choi et al., 2015a; Lee et al., 2017b; Seo et al., 2019). Nevertheless, their quality is highly dependent upon fruit size immediately after long-term cold storage (Lwin and Lee, 2020). In general, 1-methylcyclopropene (1-MCP) technology is commonly used in the pome fruit industry to maintain fruit quality for up to 1 year (Mattheis, 2008; Watkins, 2006; Watkins, 2008). Postharvest 1-MCP treatment could aid in retaining flesh firmness and fruit freshness during the handling period after a bilateral trade (Seo et al., 2019). Furthermore, the fruit quality of Asian pears was differently affected by preharvest 1-MCP treatment during the cold chain period (Lwin and Lee, 2021a). The effectiveness of preharvest 1-MCP spraying technology was dependent upon pear cultivars during cold storage (Lwin and Lee, 2021a, b). Although fruit maturity was different, the postharvest 1-MCP fumigation helped to maintain flesh firmness during shelf life in ‘Wonhwang’ pears (Lee et al., 2012b). The effectiveness of postharvest 1-MCP fumigation was dose dependent for ethylene production and SSC in ‘Yali’ pears stored at 0°C (Cheng et al., 2019). Furthermore, postharvest 1-MCP application decreased core breakdown severity, with positive effects on fruit quality attributes during shelf life in ‘Yali’ pears (Dong et al., 2015). The effectiveness of postharvest 1-MCP technology was more profound during the handling period after cold storage than during cold storage in ‘Jingbaili’ pears (Dong et al., 2014). However, there has been no such evaluation of postharvest 1-MCP application on fruit quality parameters and storage disorders in ‘Wonhwang’ Asian pears stored at 0.5°C for up to 6 months. Therefore, the objectives of this study were to evaluate the effectiveness of postharvest 1-MCP application on fruit quality characteristics, major metabolites, and the physiological disorders in ‘Wonhwang’ Asian pears stored at 0.5°C for 6 months.
2 Materials and methods
2.1 Plant material, 1-MCP treatment, and storage conditions
Asian pear (Pyrus pyrifolia Nakai cv. Wonhwang) fruit were picked at a conventional pear orchard in Naju, Jeollanam-do, Republic of Korea. Immediately after harvesting on August 22, 2018, the fruit were shipped to the Postharvest Physiology Laboratory at the Department of Plant Science and Technology, Chung-Ang University, Anseong, Republic of Korea. Fruit free from decay and damage were randomly screened and split into two batches: the untreated control and 1-MCP fumigation application. The average fresh weight of fruit used in this study were mostly from more than 470 g fruit-1 to less than 550 g fruit-1. For postharvest 1-MCP fumigation, pears were treated with 1 µL L-1 1-MCP (3.8% active ingredient (a.i.), SmartFresh™ powder, AgroFresh Inc., Seoul, Republic of Korea) at 22°C for 16 h. Then, pear fruit were held at 22°C with 60% relative humidity for 1 d after 6 months of cold storage at 0.5°C and 90% relative humidity.
2.2 Evaluation of fruit quality attributes
Fruit quality parameters were evaluated at harvest and 1 d after 3 and 6 months of storage, with five fruits per replicate and three replicates per treatment. Fifteen fruits were sampled for harvest time and storage period. The percentage of individual fruit weight loss was evaluated throughout the experimental period (Lwin et al., 2021b). The color variables were taken at the equator region of the peel, cortex, and core fruit tissues with three measurements per fruit using a chromameter (Minolta CR-200; Minolta Co., Osaka, Japan), and the color variables were expressed as lightness (L*), chroma (C*), and hue angle (h°) (McGuire, 1992). Flesh firmness (N) was measured with a texture analyser (TAHDi/500; TAHD, London, UK) using an 8-mm probe from opposite sides of individual fruits (Lwin et al., 2021b). The SSC in extracted juice was then measured using a hand-held reflectometer (Model PR-201α; Atago Co., Ltd., Tokyo, Japan) (Byeon and Lee, 2020a). TA was assessed by titration of 5 mL pear juice with 0.1 N sodium hydroxide to an endpoint of pH 8.2 (Seo et al., 2019).
During the experimental period, the incidence and severity of fruit storage disorder, including cortex browning, cavity, water soaking, and shrivelling, were evaluated for 15 pears from each treatment. The incidence was obtained from the percentage of symptomatic fruits among the total fruit (Lee et al., 2016; Seo et al., 2019). Then, the severity of the storage disorders was subjectively determined as follows: 0% = 0, 1–10% = 1, 11–25% = 2, 26–50% = 3, 51–75% = 4, and 76–100% = 5 based on the area affected for the peel, cortex, and core area (Lee et al., 2013; Lee et al., 2019b; Seo et al., 2019).
2.3 Measurement of soluble carbohydrates
Soluble carbohydrates were estimated using a previously provided protocol (Byeon and Lee, 2020a; Lwin et al., 2021b). Frozen ground samples (5 g) were homogenised with 15 mL of extraction solution containing 0.1 M perchloric acid, 0.1% meta-phosphate acid, and distilled water. The homogenate was centrifuged for 15 min at 15,000 ×g and 4°C after sonication with ice for 30 min. The aliquot was filtered through a 0.2-µm hydrophilic filter (Sartorius AG, Göttingen, Germany) and then used for further analysis. For individual soluble carbohydrate analysis, sample extract (10 µL) was injected into a Dionex Ultimate 3000 (Thermo Dionex Co., Sunnyvale, CA, USA), which consisted of a Sugar-Pak column (300 × 6.5 mm; Waters Co., Milford, MA, USA) and a Shodex RI-101 detector (Shodex, Tokyo, Japan). Distilled water was used as the mobile phase, and the flow rate of the mobile phase was set at 0.5 mL min−1. Chromatographs were acquired, and the data were recorded using Chromeleon Ver. 6 (Thermo Dionex Co., Sunnyvale, CA, USA).
2.4 Measurement of organic acids
Organic acids from pear cortex tissues were extracted following the same protocol as for the soluble carbohydrate extraction except using distilled water as the extraction solution. Organic acid analysis was performed with a Ultimate3000 HPLC system (Thermo Dionex Co., Sunnyvale, CA, USA), installed with an RI detector (RefractoMAX520, ERC Inc., Saitama, Japan) set at 210 nm using an Aminex 87 H column (300 × 10 mm, Bio-Rad Laboratories, Inc., Hercules, CA, USA), and column temperature was set at 40 °C. The elution solvent was 0.01 N H2SO4 at a flow rate of 0.5 mL min−1. The sample injection volume was 10 µL, and the run time was 30 min. The identification and quantification of individual organic acids were performed according to the protocol described by Byeon and Lee (2020b).
2.5 Measurement of free amino acids
The free amino acids were extracted according to a previously applied protocol for soluble carbohydrates (Byeon and Lee, 2020a; Lwin and Lee, 2021b). Approximately 5 g of frozen ground sample was homogenised with 15 mL of the same buffer used in soluble carbohydrate analysis. The mixture was then centrifuged for 15 min at 15,000 ×g and 4°C. The filtered sample was dissolved in borate buffer, and o-phthaldialdehyde (OPA)/meta-phosphoric acid and fluorenyl methoxycarbonyl (FMOC) were added to the sample for pre-column derivation. HPLC analysis was performed using a Dionex Ultimate 3000 with an Agilent 1260 Infinity Fluorescence Detector (Agilent Technologies, Santa Clara, CA, USA) at 450-nm emission and 340-nm excitation for OPA and 305-nm emission and 266-nm excitation for FMOC. Free amino acid separation was achieved on an Inno C18 column (4.6 × 150 mm, 5 µm; Youngjin Biochrom Co., Seoul, Korea). Detection was performed simultaneously using a UV detector at 338 nm. The column temperature was set at 40°C, and the derivatized extract (0.5 µL) was injected. Mobile phase A was sodium phosphate (40 mM, pH 7), and mobile phase B was distilled water, acetonitrile, and methanol at a ratio of 10:45:45 (v/v %) and a flow rate of 0.5 mL min−1. Gradient elution followed the previous protocol as described by Lwin et al. (2021).
2.6 Statistical analyses
Analysis of variance (ANOVA) was used to determine the effects of 1-MCP treatment, storage duration, and the interaction of the two using SAS software (version 9.3; SAS Institute Inc., Cary, NC, USA). All data were analysed using the least significance difference test to detect mean differences at P ≤ 0.05. MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/) was used to generate the normalised heatmap and to perform the principal component analysis (PCA) and the Pearson’s correlation coefficient (r) test (Chong et al. 2018). Normalised heatmap hierarchical clustering was produced for the comparison of the normalised responses of fruit quality attributes and targeted major metabolites using mean-centred, Euclidean distance measurements and the Ward clustering algorithm. PCA was performed as described previously by Lwin et al. (2021b), and the transformed data set was then adopted to the color system of the SigmaPlot 10.0 program (Systat Software, Inc., San Jose, CA, USA). Correlation coefficient analysis of fruit quality parameters and targeted major metabolites was performed with the MetScape (Version 3.1.3) plug-in for Cytoscape (Version 3.8.2; https://cytoscape.org/) (Basu et al. 2017). Pearson’s correlation coefficient analysis was applied to determine the correlation between fruit quality attributes and targeted major metabolites, and the resulting values, along with assigned major metabolites to any defined categories, were applied to detect the significance between variables (Lwin et al. 2021b). The range of edges for the r-values of correlation coefficients was set to a cut-off of r ≥ ± 0.90 to generate more relevant correlation coefficient-based networking for the final results.
3 Results
3.1 Fruit quality parameters and incidence of physiological disorder
Flesh firmness was slightly decreased during the first half of storage, irrespective of 1-MCP fumigation, but 1-MCP treatment sharply decreased flesh firmness, compared with the untreated control (Fig. 1). Weight loss gradually increased as the storage duration advanced. The 1-MCP application induced higher weight loss than the untreated control at the end of storage (Fig. 1). SSC also gradually increased during storage, but the effect of 1-MCP application was not detected (Fig. 1). TA content was greater in control fruit than in 1-MCP-treated fruit during storage. However, the amount of TA did not fluctuate in the 1-MCP treatment during storage (Fig. 1).
Physiological responses of flesh firmness, weight loss, soluble solids content (SSC), titratable acidity (TA), soluble carbohydrates (sucrose, fructose, glucose, and sorbitol), and organic acids (citric, malic, and shikimic acids) in ‘Wonhwang’ Asian pears treated with 0 or 1 µL L− 1 1-methylcyclopropene (1-MCP) at harvest and then stored at 0.5 °C for up to 6 months followed by 1 d at 22 °C. Each datum point indicates the mean of 15 fruit replicates (n = 15) ± standard error (SE) for firmness, weight loss, and SSC, and three replicates (n = 3) ± standard error for TA, soluble carbohydrates, and organic acids, where SE bars are larger than the symbols
Lightness (L*) was significantly greater in the 1-MCP treatment than in the control fruit of peel and cortex tissues but relatively lower in core tissues at 6 months (Fig. 2). Chroma (C*) was decreased in peel but increased in the cortex and core during storage (Fig. 2). C* values were lower in the 1-MCP application in the peel but higher in the cortex and core at 6 months. The hue angle (ho) values gradually decreased during storage (Fig. 2). Nonetheless, there were higher peel ho values at the end of storage and for the cortex ho in the middle of storage in the 1-MCP treatment than in the control.
Responses of lightness (L*), chroma (C*), and hue angle (ho) in peel, cortex, and core tissues of ‘Wonhwang’ Asian pears treated with 0 or 1 µL L− 1 1-methylcyclopropene (1-MCP) at harvest and then stored at 0.5 °C for up to 6 months followed by 1 d at 22 °C. Each datum point indicates the mean of 15 fruit replicates (n = 15) ± standard error (SE), which SE is larger than the symbol
The incidence of cortex browning was sharply increased in the 1-MCP treatment at 6 months, but the severity of cortex browning was not affected by 1-MCP fumigation application (Fig. 3). The severity and incidence of cavities were highly increased during cold storage, irrespective of the 1-MCP application (Fig. 3). Nonetheless, 1-MCP application enhanced the incidence and severity of cavities compared with the untreated control during storage. The incidence and severity of water soaking slowly increased during storage, irrespective of 1-MCP fumigation (Fig. 3). Although the incidence of water soaking tended to be higher in the 1-MCP application than in the control at 6 months, the severity of water soaking was not statistically different in the 1-MCP fumigation during storage. In contrast, the incidence and severity of shriveling occurred only at 6 months and were greater in the control fruit than in the 1-MCP treatment during storage (Fig. 3).
Incidence and severity of cortex browning, cavity, water soaking, and shriveling in ‘Wonhwang’ Asian pear fruit treated with 0 or 1 µL L− 1 1-methylcyclopropene (1-MCP) at harvest and then stored at 0.5 °C for up to 6 months followed by 1 d at 22 °C. Each datum point indicates the mean of 15 fruit replicates (n = 15) ± standard error, where larger than the symbols. The severity of physiological disorders was subjectively scored as 0 = 0%, 1 = 1–10%, 2 = 11–25%, 3 = 26–50%, 4 = 51–75%, and 5 = 76–100% for the coverage area of the peel, cortex, and core tissues based on the largest area with the corresponding symptoms
3.2 Responses of soluble carbohydrates, organic acids, and free amino acids
Sucrose level was not influenced by 1-MCP application through the whole storage period but sharply decreased in untreated fruit at 6 months (Fig. 1). The fructose and glucose contents highly increased during storage, irrespective of 1-MCP application (Fig. 1). Nevertheless, the glucose and fructose contents were greater in control fruit than in the 1-MCP application at 6 months. The sorbitol content was not affected during the first half of storage but substantially accumulated upon 1-MCP application compared to the untreated fruit at 6 months (Fig. 1).
The level of malic acid was not affected by 1-MCP application during the early stage of storage, but it highly increased during the later stage of storage (Fig. 1). Furthermore, the malic acid content was greater in the untreated fruit than in the 1-MCP application at 6 months. However, the level of citric acid was not statistically significantly influenced by 1-MCP (Fig. 1). Shikimic acid content was influenced not only by cold storage but also by 1-MCP application (Fig. 1).
The contents of aspartic acid, asparagine, arginine, threonine, GABA, methionine, valine, and phenylalanine were greater in the 1-MCP application than in the untreated group during the first half of storage (Fig. 4). However, the contents of aspartic acid, glutamic acid, glycine, proline, and serine were significantly lower in the 1-MCP application than in control fruit during the second half of storage (Fig. 4). The alanine content was greater in 1-MCP application than in untreated fruit at 6 months (Fig. 4). Furthermore, the levels of serine, histidine, valine, tryptophan, phenylalanine, and isoleucine gradually accumulated with storage period, irrespective of 1-MCP application (Fig. 4).
Metabolic responses of free amino acid contents in ‘Wonhwang’ Asian pear fruit treated with 0 or 1 µL L− 1 1-methylcyclopropene (1-MCP) at harvest and then stored at 0.5 °C for up to 6 months followed by 1 d at 22 °C. Each datum point is the mean of three replicates (n = 3) ± standard error, where larger than the symbols. Each datum unit is expressed as (mg kg−1) on a fresh weight basis
3.3 Normalised heatmap and PCA scores and loading plots
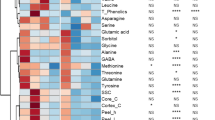
A normalised heatmap matrix system was applied to examine the overall responses of all variables to the 1-MCP application in pear fruit stored at 0.5 °C for 6 months (Fig. 5). Glutamine was highly responsive in untreated control fruit, but phenylalanine, GABA, methionine, leucine, and lysine were highest in the 1-MCP application in the middle of storage. At 6 months, the normalised responses of serine, valine, fructose, shriveling, and glucose were highest in the untreated fruit. Additionally, the relative levels of cortex browning and malic acid were also increased in untreated fruit. However, the relative responses of cavity, isoleucine, weight loss, and water soaking were highly detected in the 1-MCP application at 6 months. Interestingly, most variables in the 1-MCP treatment group were negatively expressed at 6 months, compared with the results of untreated fruit (Fig. 5).
Normalized heatmap responses and ANOVA results of fruit quality attributes, physiological disorders, and primary metabolites in ‘Wonhwang’ Asian pears treated with 0 or 1 µL L−1 1-methylcyclopropene (1-MCP) at harvest and then stored at 0.5 °C for up to 6 months followed by 1 d at 22 °C. NS, *, **, or ***, = non-significant or significant difference at P < 0.05, 0.01, or 0.001, respectively. GABA, γ-aminobutyric acid; TA, titratable acidity, and SSC, soluble solids content
ANOVA results indicated that 1-MCP application significantly affected only fructose, glucose, cavity, methionine, and peel ho during cold storage (Fig. 5). In contrast, storage duration significantly affected serine, valine, fructose, shriveling, glucose, phenylalanine, histidine, tryptophan, cavity, isoleucine, cortex browning, weight loss, water soaking, tyrosine, GABA, leucine, lysine, cortex ho, core ho, peel C*, asparagine, firmness, threonine, arginine, sorbitol, peel ho, core L*, cortex C*, glutamic acid, SSC, citric acid, and malic acid. However, there were significant differences detected among these variables. Additionally, significant interaction effects of 1-MCP fumigation application and storage period were detected for serine, fructose, glucose, phenylalanine, peel C*, sorbitol, peel ho, glutamic acid, and proline (Fig. 5).
A PCA was performed as a multivariate data analysis to evaluate the overall responsiveness, divergence, and association between the fruit quality attributes and targeted major metabolites in cold-stored 1-MCP-treated pear fruit (Fig. 6). The PCA scores plot was performed based on 44 predictor variables including fruit quality attributes and targeted major metabolites. Principal component one (PC-1) and two (PC-2) values explained 64.3% and 20.6% of the total X- and Y-variance, respectively. The results of the PCA indicated that the 1-MCP application caused a divergence of the overall responses during the second half of storage (Fig. 6 A). The data matrix with all response variables (1-MCP treatment and storage duration) with predicator variables (fruit quality attributes and targeted major metabolites) was applied to emphasize the association between response variables and predictor variables, and the total variances of X- and Y-axes from scores and loading plots were slightly different due to the addition of response variables. The PC-1 and PC-2 values explained 53.5% and 20.0% of the total X- and Y-variance, respectively (Fig. 6B). Although 1-MCP application was only closely linked with glutamine, storage duration was broadly clustered with physiological disorders, glucose, valine, serine, isoleucine, histidine, and tryptophan, along with weight loss, which was most closely linked with storage duration.
3.4 Pearson’s correlation coefficient analysis and correlation coefficient network
Correlation coefficient analysis was applied to evaluate the overall relationship between variables during cold storage. After combining all the results from the control and 1-MCP application, an overall correlation coefficient matrix was generated, as shown in Fig. 7. Irrespective of 1-MCP treatment, threonine, arginine, peel ho, core L*, peel C*, and core ho were strongly and positively related with each other and negatively correlated with core C*, sorbitol, phenylalanine, histidine, tryptophan, cortex C*, valine, isoleucine, shrivelling, malic acid, glucose, fructose, cortex browning, water soaking, weight loss, cavity, SSC, and serine. Additionally, phenylalanine, histidine, tryptophan, cortex C*, valine, and isoleucine, which were positively correlated with themselves, were positively correlated with weight loss, cavity, SSC, and serine. Malic acid was positively correlated with glucose and fructose. Furthermore, cortex browning, water soaking, weight loss, cavity, SSC, and serine were positively related with each other. Methionine and lysine were also positively related with glutamine and leucine (Fig. 7).
Heatmap matrix of Pearson’s correlation coefficients (r) among the variables from the results of fruit quality attributes, physiological disorders, and targeted primary metabolites in ‘Wonhwang’ Asian pears treated with 0 or 1 µL L− 1 1-methylcyclopropene (1-MCP) at harvest and then stored at 0.5 °C for up to 6 months followed by 1 d at 22 °C. Figure legend indicates the results of correlation coefficients (r). Red and blue indicate positive and negative correlation coefficients between variables, respectively
The networking system based on the Pearson’s correlation coefficient test was performed to evaluate the effectiveness of 1-MCP application on the relationship among the response variables, as shown in Fig. 8. In this case, we used r ≥ ± 0.90 for the correlation coefficient value to differentiate the effectiveness of 1-MCP application on fruit quality characteristics and targeted metabolites (n = 3) in cold-stored ‘Wonhwang’ pears for 6 months. Nineteen variables were significantly detected in the untreated fruit. Although core L* was negatively related with tryptophan and histidine, the other variables were positively correlated. Among them, core ho, cortex ho, cortex L*, peel C*, peel L*, and peel ho were positively related with each other. Threonine was positively related with all color variables in the untreated fruit. Serine was positively correlated with weight loss. Histidine was positively correlated with tryptophan and phenylalanine (Fig. 8). In contrast, 27 variables were significantly detected in the 1-MCP treatment. Core ho, core L*, cortex ho, cortex L*, peel ho, peel C*, and peel L* were strongly and positively associated with each other. In contrast, the cavity was negatively related with peel L*, peel C*, core ho, glutamic acid, asparagine, threonine, and shikimic acid. Weight loss was also negatively associated with firmness, glutamic acid, asparagine, threonine, and arginine. Citric and histidine were negatively correlated with citric acid, malic acid, and alanine but positively correlated with tryptophan. Asparagine was negatively correlated with cavity and weight loss but positively correlated with threonine (Fig. 8).
Pearson’s correlation network (r ≥│0.90│) among the response variables (n = 3) in ‘Wonhwang’ Asian pears treated with 0 or 1 µL L− 1 1-methylcyclopropene (1-MCP) at harvest and then stored at 0.5 °C for up to 6 months followed by 1 d at 22 °C. Figure legend indicates the results of correlation coefficient (r). Red and blue indicate positive and negative correlation coefficients between variables, respectively
4 Discussion
Although ‘Wonhwang’ Asian pears have excellent fruit quality at harvest, they are generally kept for short-term storage periods and then distributed during the fruit handling period (Lwin et al. 2021a; Park et al. 2016; Seo et al. 2019). Fruit maturity characteristics of ‘Wonhwang’ Asian pears are derived from that they are an early harvest season Asian pear cultivar (Hong et al. 2004). Nonetheless, a previous study showed that ‘Wonhwang’ Asian pears could be kept in long-term cold storage, depending on fruit size (Lwin and Lee 2020). During long-term cold storage, the increase in SSC and the decrease in TA were clear regardless of fruit size (Lwin and Lee 2020). However, a study reported that fruit fresh weight loss greatly increased, although the small ‘Wonhwang’ Asian pears had much higher weight loss than did the large pears during long-term cold storage. Additionally, we determined if fruit quality of ‘Wonhwang’ Asian pears is sufficient for a shelf-life period immediately after international trade, in which 1-MCP application could maintain fruit quality based on the results of fruit firmness during shelf life (Seo et al. 2019). Flesh firmness was much greater in the 1-MCP-treated pears than in control fruit during 1 month of shelf life (Lwin et al. 2021a). Nonetheless, no study has examined the effectiveness of 1-MCP application on the fruit quality characteristics and major metabolites for ‘Wonhwang’ Asian pears during long-term cold storage. Therefore, the objectives of this study were to test the hypothesis that 1-MCP treatment could retain fruit quality characteristics for ‘Wonhwang’ Asian pears in long-term cold storage compared with that of untreated fruit.
Flesh firmness tended to be greater in the 1-MCP application than in control fruit in the middle of storage but significantly lower at 6 months than untreated fruit. Weight loss was much higher in the 1-MCP application than in the untreated fruit at 6 months (Fig. 1). However, the incidence and severity of shriveling were less in the 1-MCP application than in the control fruit during storage (Fig. 3). Thus, although the reduction in flesh firmness and the enhancement in weight loss were influenced by the 1-MCP application, fruit shriveling was much lower in the 1-MCP treatment group. This controversial result could be attributed to the fact that the tested fruit were primarily placed under long-term cold storage. However, 1-MCP treatment induced greater fruit shriveling than the untreated control during the shelf-life period (Lwin et al. 2021a). Nevertheless, the absolute value of fruit shriveling severity was lower, approximately 0.5, given a possible overall rating of 5 (Fig. 3). It is also possible that the percentage of fruit weight loss was less than 10%, although there was a significant effect on weight loss in the 1-MCP treatment group. It is generally recognised that more than 10% weight loss could commercially affect the peel appearance, and consequently, fruit visual quality during the shelf-life period (Ben-Yehoshua and Rodov 2002; Lufu et al. 2020; Robinson et al. 1975). The results indicated that the incidence of fruit shriveling induced by 1-MCP treatment was negligible. The Pearson’s correlation coefficient network results provided supportive evidence that 1-MCP treatment did not induce any negative relationships between weight loss and fruit shriveling. However, there was a negative relationship between fruit firmness and weight loss in the 1-MCP application (Fig. 8). Additionally, the differential responses of flesh firmness to the 1-MCP application could result from a cultivar difference, the cold storage duration, or the shelf-life duration (Lafer 2005; Lee et al. 2012b). Flesh firmness and weight loss were affected by 1-MCP application in ‘Wonhwang’ pears, based on the designated temperature and period of shelf life (Lee et al. 2017b). Weight loss was highly influenced by storage temperature and duration rather than 1-MCP application in ‘Korla Xiang’ pears (Jia et al. 2018).
Although SSC gradually increased regardless of 1-MCP fumigation, the sorbitol and sucrose contents were higher with postharvest 1-MCP fumigation. However, the glucose and fructose contents were lower with postharvest 1-MCP application than in the untreated fruit at 6 months (Fig. 1). Although the individual soluble carbohydrates responded differently to 1-MCP application, 1-MCP fumigation did not affect SSC during cold storage. It was also reported that 1-MCP application did not affect SSCs during shelf life after bilateral trade (Seo et al. 2019). Furthermore, the SSC level was not influenced by 1-MCP fumigation in ‘Jingbaili’ pears during storage or shelf life after long-term cold storage (Dong et al. 2014). Irrespective of the storage regime, 1-MCP treatment did not influence the SSC level in ‘Conference’ and ‘Alexander Lucas’ European pears during long-term storage (Hendges et al. 2018). However, 1-MCP application reduced TA and the levels of malic and citric acids at the end of cold storage (Fig. 1). Typically, the 1-MCP application causes a decrease in fruit respiration during storage and shelf life, irrespective of storage regimes (Dong et al. 2014; Gapper et al. 2006; Hendges et al. 2018; Lee et al. 2017b) and then retains higher levels of TA, compared with that of control fruit (Chen et al. 2010; Jia et al. 2018; Lee et al. 2017a, b). A differential response of TA to 1-MCP treatment was also detected in ‘Chuhwangbae’ pears during cold storage and handling (Lwin et al. 2021b). TA level was decreased in ‘Wonhwang’ Asian pears stored at 1°C for 6 months, irrespective of fruit size (Lwin and Lee 2020). Therefore, the results indicated that the TA level inconsistently responds to storage regime, pear cultivar, and postharvest technology treatment. It is difficult to explain the responses of TA to 1-MCP treatment because of a lack of supportive evidence.
The incidence of cortex browning, cavity, and water soaking was greater in the 1-MCP application than in untreated fruit, but the severity of the cavity was only significantly greater in the 1-MCP application than in untreated fruit (Fig. 3). Nonetheless, the severity and incidence of storage disorders worsened with the prolonged storage. The severity and incidence of flesh browning were not affected by 1-MCP application in ‘Chuhwangbae’ Asian pears during short-term cold storage (Lwin et al. 2021b). During shelf life immediately after harvest, the incidence and severity of core and cortex browning were reduced by 1-MCP application in ‘Wonhwang’ Asian pears (Lwin et al. 2021a). However, the 1-MCP application caused a decrease in the incidence of senescent core breakdown in long-term, cold-stored ‘Bartlett’ pears, irrespective of orchard elevation or harvest maturity (Wang and Sugar 2015). That is, the physiological disorders in flesh tissues are highly affected by storage regimes rather than by 1-MCP application. Otherwise, long-term cold storage could have a negative effect on the development of storage disorders rather than shelf life or short-term cold storage. Nevertheless, 1-MCP treatment could be associated with developing physiological disorders in flesh tissues during storage (Lee et al. 2012a, 2016, 2019a). Although less is known about the physiological characteristics of fruit ripening and ethylene responses to 1-MCP application in Asian pear cultivars, 1-MCP application could be negatively involved in the severity and incidence of physiological disorders in long-term, cold-stored ‘Wonhwang’ pears.
The contents of free amino acids were varied in response to 1-MCP application during storage (Fig. 4). The levels of glycine, asparagine, aspartic acid, glutamic acid, arginine, and proline were lower with 1-MCP application than in untreated fruit at 6 months. Although the levels of glutamine, GABA, tyrosine, leucine, and lysine were not consistent with 1-MCP application, the contents of serine, histidine, valine, tryptophan, phenylalanine, and isoleucine gradually increased during cold storage, irrespective of 1-MCP application (Fig. 4), thereby contributing to the results of the PCA loading plot indicating that phenylalanine, histidine, tryptophan, isoleucine, serine, and valine were strongly linked with storage period variables, along with physiological disorders and weight loss (Fig. 6B). Aromatic and branch-chain amino acids are likely strongly linked with storage duration, provided that the amino acids are involved in producing volatile organic compounds (VOCs) as storage duration progresses (Byeon and Lee 2020a, 2021; Lwin and Lee 2020). It was also reported that 1-MCP application could influence the levels of certain aromatic and branch-chain amino acids in ‘Empire’ apples stored in a long-term controlled atmosphere (CA) (Lee et al. 2012a). Furthermore, the level of methionine was higher with 1-MCP application than in untreated fruit during cold storage (Fig. 4) and positively correlated with leucine, glutamine, lysine, isoleucine, valine, and cortex ho (Fig. 7). Asian pears are inconsistent in their climacteric type of fruit during ripening based on fruit respiration and ethylene production responses (Hwang et al. 2001; Jeong et al. 1998). Nevertheless, methionine highly accumulated in the 1-MCP treatment during cold storage. Although the levels of 1-aminocyclopropane-1-carboxylic acid (ACC) and ethylene, which are produced from methionine in the methionine cycle (Adams and Yang 1979), were not determined, 1-MCP could be involved in suppressing ethylene production by increasing methionine levels. Therefore, the results showed that although Asian pears did not belong to the climacteric type of fruit, 1-MCP could play a pivotal role in controlling the ethylene biosynthesis pathway based on the results for the accumulation of methionine.
The 1-MCP treatment is widely applied in the pome fruit industry to retain fruit freshness and firmness during long-term cold storage (Mattheis 2008; Park 2012; Watkins 2008). Although the effectiveness of 1-MCP application was much lower in Asian pears than in apple fruit (Lee et al. 2017b; Lwin et al. 2021a, b), the results of the PCA scores plot indicated that 1-MCP could be highly involved in delaying physiological and biochemical responses during storage, compared with the overall responses of the untreated control fruit (Fig. 6 A). Additionally, the PCA scores plot suggested that 1-MCP treatment could be much more effective during the second half of storage than the first half of cold storage because of the large divergence at the end of storage. Furthermore, the Pearson’s correlation coefficient network results indicated that the differential responses were more often significantly detected in the 1-MCP application than in control fruit; thus, 1-MCP treatment could enhance the highly interactive relationships among the variables during cold storage. As shown in Fig. 3, the incidence and severity of cavity were highly induced by 1-MCP treatment. In addition, cavity was negatively correlated with core ho, glutamic acid, asparagine, threonine, shikimic acid, and flesh firmness in 1-MCP treatment, compared with untreated fruit. Furthermore, weight loss was negatively related with flesh firmness, glutamic acid, asparagine, threonine, and arginine in the 1-MCP treatment. Interestingly, GABA, which plays a key role in controlling a wide range of physiological disturbances (Bouché and Fromm 2004), was detected only in the 1-MCP treatment. Therefore, the results indicated that 1-MCP treatment might cause another abiotic stress to cold-stored pear fruit during long-term cold storage, thus altering major metabolites including certain amino acids, thereby contributing to enhancing the incidence and severity of cavity and weight loss during long-term, cold-stored pear fruit.
5 Conclusions
Postharvest 1-MCP fumigation technology reduced flesh firmness and TA but enhanced weight loss, along with an increase in sucrose and sorbitol and a decrease in fructose, glucose, malic acid, and citric acid at 6 months. The severity and incidence of cavity and water soaking were much greater in the 1-MCP application than in the control fruit during cold storage. However, 1-MCP application reduced the severity and incidence of shriveling at 6 months. The contents of free amino acids differed in the 1-MCP application and storage period. Nonetheless, the methionine content was greater in the 1-MCP application than in the control fruit during cold storage. Additionally, the levels of certain aromatic and branch-chained amino acids were strongly linked with storage duration, according to the results of the PCA loading plot. Furthermore, the results of PCA scores plot indicated that overall physiological and metabolic responses could be suppressed by 1-MCP application, compared with the overall responses from untreated control fruit. Pearson’s correlation coefficient network results indicated that 1-MCP application could be positively related with more variables than the untreated control fruit. During long-term cold storage, the incidence and severity of the cavity could be negatively related to certain physiological and metabolic responses to 1-MCP treatment.
References
Adams DO, Yang SF (1979) Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Nat’l Acad Sci USA 76:170–174. https://doi.org/10.1073/pnas.76.1.170
Basu S, Duren W, Evans CR, Burant CF, Michailidis G, Karnovsky A (2017) Sparse network modeling and metscape-based visualization methods for the analysis of large-scale metabolomics data. Bioinformatics 33:1545–1553. https://doi.org/10.1093/bioinformatics/btx012
Ben-Yehoshua S, Rodov V (2002) Transpiration and water stress, In: Bartz, J.A., Brecht, J.K. (Eds.), Postharvest Physiology and Pathology of Vegetables. CRC Press, Boca Raton, pp. 111–159. https://doi.org/110.1201/9780203910092.ch9780203910095
Bouché N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9:110–115. https://doi.org/10.1016/j.tplants.2004.01.006
Byeon S-E, Lee J (2020a) Differential responses of fruit quality and major targeted metabolites in three different cultivars of cold-stored figs (Ficus carica L.). Sci Hortic 260:108877. https://doi.org/10.1016/j.scienta.2019.109160
Byeon S-E, Lee J (2020b) Fruit quality and major primary metabolites differ across production systems in cold-stored figs (Ficus carica L.). Sci Hortic 274:109669. https://doi.org/10.1016/j.scienta.2020.109669
Byeon S-E, Lee J (2021) Fruit maturity differentially affects fruit quality and responses of targeted metabolites in cold-stored figs (Ficus carica L.). J Sci Food Agric 101:673–683. https://doi.org/10.1002/jsfa.10680
Chen S, Zhang M, Wang S (2010) Physiological and quality responses of Chinese ‘Suli’ pear (Pyrus bretschneideri Rehd) to 1-MCP vacuum infiltration treatment. J Sci Food Agric 90:1317–1322. https://doi.org/10.1002/jsfa.3939
Cheng Y, Liu L, Feng Y, Dong Y, Guan J (2019) Effects of 1-MCP on fruit quality and core browning in ‘Yali’ pear during cold storage. Sci Hortic 243:350–356. https://doi.org/10.1016/j.scienta.2018.08.041
Choi J-H, Lee U-Y, Ahn Y-J, Hwang Y-S, Chun J-P (2013) Effects of ethephon and aminoethoxyvinylglycine on fruit quality and incidence of physiological disorders during simulated exportation environment in ‘Wonhwang’ pears. Kor J Agric Sci 40:79–86. https://doi.org/10.7744/cnujas.2013.40.2.079
Choi J-H, Yim S-H, Cho K-S, Kim M-S, Park Y-S, Jung S-K, Choi H-S (2015a) Fruit quality and core breakdown of ‘Wonhwang’ pears in relation to harvest date and pre-storage cooling. Sci Hortic 188:1–5. https://doi.org/10.1016/j.scienta.2015.03.011
Choi JH, Yim SH, Kim SJ, Lee HC, Kwon YH, Park YS, Jung SK, Choi HS (2015b) Effect of harvest date on fruit quality and core breakdown of ‘Wonhwang’ pears. Kor J Organic Agric 23:103–112. https://doi.org/10.11625/KJOA.2015.23.1.103
Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46:W486–W494. https://doi.org/10.1093/nar/gky310
Dong Y, Liu L, Zhang Y, Guan J (2014) Effects of 1-MCP on softening, yellowing and H2O2 content in post-harvest ‘Jingbaili’ pear fruit during and after cold storage. Hortic Environ Biotechnol 55:404–409. https://doi.org/10.1007/s13580-014-0184-5
Dong Y, Liu LQ, Zhao Z, Zhi HH, Guan JF (2015) Effects of 1-MCP on reactive oxygen species, polyphenol oxidase activity, and cellular ultra-structure of core tissue in ‘Yali’ pear (Pyrus bretschneideri Rehd.) during storage. Hortic Environ Biotechnol 56:207–215. https://doi.org/10.1007/s13580-015-0094-1
Gapper NE, Bai J, Whitaker BD (2006) Inhibition of ethylene-induced a-farnesene synthase gene PcAFS1 expression in ‘d’Anjou’ pears with 1-MCP reduces synthesis and oxidation of a-farnesene and delays development of superficial scald. Postharvest Biol Technol 41:225–233. https://doi.org/10.1016/j.postharvbio.2006.04.014
Hendges MV, Neuwald DA, Steffens CA, Vidrih R, Zlatić E, do Amarante CVT (2018) 1-MCP and storage conditions on the ripening and production of aromatic compounds in Conference and Alexander Lucas pears harvested at different maturity stages. Postharvest Biol Technol 146: 18–25. https://doi.org/10.1016/j.postharvbio.2018.08.006
Hong SS, Hong YP, Im BS, Jeong DS, Shin IS (2004) Influence of picking stage and storage type on the fruit respiration change and panel test in ‘Wonhwang’, ‘Hwasan’, and ‘Mansoo’ pear. Hortic Sci Technol 22:55–62. http://www.koreascience.or.kr/article/JAKO200410103459451.page
Hwang YS, Chun JP, Lee JC, Seo JH (2001) Storage response of ‘Kamchun’ and ‘Chuwhang’ pears by harvest dates. Hortic Sci Technol 19:48–53. http://www.dbpia.co.kr/journal/articleDetail?nodeId=NODE00891701
Jeong ST, Kim JG, Hong SS, Jang HS, Kim YB (1998) Influence of maturity and storage temperature on the respiration rate and ethylene production in ‘Kosui’, ‘Chojuro’ and ‘Niitaka’ pears. Hortic Environ Biotechnol 39:446–448
Jia X-h, Wang W-h, Du Y-m, Tong W, Wang Z-h, Gul H (2018) Optimal storage temperature and 1-MCP treatment combinations for different marketing times of Korla Xiang pears. J Integr Agric 17:693–703. https://doi.org/10.1016/S2095-3119(17)61872-0
Kim WC, Hwang HS, Shin YU, Lee DK, Kang SJ, Shin IS, Cheon BD, Moon JY, Kim JH, Kim SB (1995) ‘Wonwhang’, sweet and large sized pear cultivar with bright yellowish brown skin. Hortic Sci Technol 13:174–175
Lafer G (2005) Effects of 1-MCP treatments on fruit quality and storability of different apple varieties. Acta Hortic 682:1227–1232. https://doi.org/10.17660/ActaHortic.2003.599.6
Lee J, Cheng L, Rudell DR, Nock JF, Watkins CB (2019a) Antioxidant metabolism in stem and calyx end tissues in relation to flesh browning development during storage of 1-methylcyclopropene treated ‘Empire’ apples. Postharvest Biol Technol 149:66–73. https://doi.org/10.1016/j.postharvbio.2018.11.015
Lee J, Mattheis JP, Rudell DR (2013) Fruit size affects physiological attributes and storage disorders in cold-stored ‘Royal Gala’ apples. HortScience 48:1518–1524. https://doi.org/10.21273/HORTSCI.48.12.1518
Lee J, Mattheis JP, Rudell DR (2016) Storage temperature and 1-methylcyclopropene treatment affect storage disorders and physiological attributes of ‘Royal Gala’ apples. HortScience 51:84–93. https://doi.org/10.21273/HORTSCI.51.1.84
Lee J, Mattheis JP, Rudell DR (2019b) High storage humidity affects fruit quality attributes and incidence of fruit cracking in cold-stored ‘Royal Gala’ apples. HortScience 54:149–154. https://doi.org/10.21273/HORTSCI13406-18
Lee J, Rudell DR, Davies PJ, Watkins CB (2012a) Metabolic changes in 1-methylcyclopropene (1-MCP)-treated ‘Empire’ apple fruit during storage. Metabolomics 8:742–753. https://doi.org/10.1007/s11306-011-0373-5
Lee SJ, Park SM, Jeong CS, Ngo BX, Kim JH (2002) Changes of fruit quality by storage temperature for marketing during off-season in ‘Wonhwang’ pear. Hortic Environ Biotechnol 43:716–720
Lee U-Y, Choi J-H, Kim Y-K, Kim J-g, Chun J-P (2017a) Enhancement of shelf-life and decrease of physiological disorder incidence using 1-methylcyclopropene (1-MCP) in early season ‘Noksu’ pears. J Agric Life Sci 51:191–198. https://doi.org/10.14397/jals.2017.51.2.191
Lee U-Y, Choi J-H, Lee J-H, Oh K-S, Chun J-P (2017b) Effect of 1-MCP treatment on the early-season Asian pear cultivar ‘Wonhwang’ in response to different temperature conditions during simulated exportation. Hortic Sci Technol 35:568–576. https://doi.org/10.12972/kjhst.20170061
Lee U-Y, Oh K-Y, Choi J-H, Hwang Y-S, Choi J-M, Chun J-P (2011) Evaluation of fruit quality during shelf-life at high temperature environment in ‘Wonhwang’ and ‘Whasan’ pears. J Bio-Environment Contr 20:233–240
Lee U-Y, Oh K-Y, Moon S-J, Hwang Y-S, Chun J-P (2012b) Effects of 1-methylcyclopropene (1-MCP) on fruit quality and occurrence of physiological disorders of Asian pear (Pyrus pyrifolia), ‘Wonhwang’ and ‘Whasan’, during shelf-life. Hortic Sci Technol 30:534–542. https://doi.org/10.7235/hort.2012.12033
Lim B-S, Hwang Y-S, Chun J-P, Jung H-W (2007) Effect of storage temperature on the core breakdown of ‘Wonhwang’ and ‘Niitaka’ pear fruits. Hortic Sci Technol 25:212–216
Lufu R, Ambaw A, Opara UL (2020) Water loss of fresh fruit: Influencing pre-harvest, harvest and postharvest factors. Sci Hortic 272: 109519. https://doi.org/109510.101016/j.scienta.102020.109519
Lwin HP, Choi J-H, Chun J-P, Watkins CB, Lee J (2021a) 1-Methylcyclopropene treatment alters fruit quality attributes and targeted metabolites in ‘Wonhwang’ pears during shelf life. Sci Hortic 284:110125. https://doi.org/10.1016/j.scienta.2021.110125
Lwin HP, Lee J (2020) Fruit quality and major metabolites in cold-stored ‘Wonhwang’ Asian pears are differentially affected by fruit size. J Sci Food Agric 100:5117–5125. https://doi.org/10.1002/jsfa.10422
Lwin HP, Lee J (2021a) Differential effects of preharvest sprayable 1-methylcyclopropene application on fruit quality attributes and major targeted metabolites in cold-stored ‘Chuhwangbae’ pears. Hortic Environ Biotechnol 62:53–61. https://doi.org/10.1007/s13580-020-00289-9
Lwin HP, Lee J (2021b) Preharvest 1-methylcyclopropene treatment effects on fruit quality attributes and targeted metabolites in ‘Wonhwang’ pears stored at room temperature after cold storage. Sci Hortic 289:110480. https://doi.org/10.1016/j.scienta.2021.110480
Lwin HP, Rudell DR, Lee J (2021b) Metabolism and cold chain performance of ‘Chuhwangbae’ Asian pears as impacted by 1-MCP treatment. Sci Hortic 288:110357. https://doi.org/10.1016/j.scienta.2021.110357
Mattheis JP (2008) How 1-methylcyclopropene has altered the Washington State apple industry. HortScience 43:99–101. https://doi.org/10.21273/HORTSCI.43.1.99
McGuire RG (1992) Reporting of objective color measurements. HortScience 27:1254–1255. https://doi.org/10.21273/HORTSCI.27.12.1254
Moon SJ, Han CH, Lim BS, Lee CH, Kim MS, Hwang YS (2008) Effect of storage temperature and 1-MCP treatment on the incidence of flesh browning disorder in ‘Wonhwang’ pears. Hortic Sci Technol 26:144–148
Oh K-Y, Lee U-Y, Moon S-J, Kim Y-O, Yook H-S, Hwang Y-S, Chun J-P (2010) Transportation and distribution temperatures affect fruit quality and physiological disorders in ‘Wonhwang’ pears. Hortic Sci Technol 28:434–441
Park Y-M (2012) 1-MCP application for horticultural commodities in Korea: practical potential and future task. Hortic Environ Biotechnol 53:441–446. https://doi.org/10.1007/s13580-012-0174-4
Park Y, Lee B, Park H-S (2016) Observation of the anatomical causes of fruit softening during growth and storage periods in ‘Wonhwang’ oriental pear (Pyrus pyrifolia). Sci Hortic 210:250–257. https://doi.org/10.1016/j.scienta.2016.07.033
Robinson JE, Browne KM, Burton WG (1975) Storage characteristics of some vegetables and soft fruits. Ann Appl Biol 81:399–408. https://doi.org/10.1111/j.1744-7348.1975.tb01656.x
Seo H-J, Wang Y-S, Lwin HP, Choi J-H, Chun J-P, Roan S-F, Chen I-Z, Lee J (2019) Early season ‘Wonhwang’ pear fruit quality following international transport and storage is negatively impacted by fruitlet stage gibberellic acid4 + 7 (GA4 + 7) application but improved by postharvest 1-methylcyclopropene (1-MCP). Sci Hortic 256:108549. https://doi.org/10.1016/j.scienta.2019.108549
Wang Y, Sugar D (2015) 1-MCP efficacy in extending storage life of ‘Bartlett’ pears is affected by harvest maturity, production elevation, and holding temperature during treatment delay. Postharvest Biol Technol 103:1–8. https://doi.org/10.1016/j.postharvbio.2015.02.013
Watkins CB (2006) The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol Adv 24:389–409. https://doi.org/10.1016/j.biotechadv.2006.01.005
Watkins CB (2008) Overview of 1-methylcyclopropene trials and uses for edible horticultural crops. HortScience 43:86–94. https://doi.org/10.21273/HORTSCI.43.1.86
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant (2019R1F1A1062213) funded by the Korean Government (MSIT). We thank Ms. Boyeon Kim and Mr. Seungyeon Han at Chung-Ang University for their technical assistance and support.
Author information
Authors and Affiliations
Contributions
Hnin Phyu Lwin: Data curation, Formal analysis, Investigation, Methodology, Writing, Review & Editing. Jinwook Lee: Conceptualization, Data curation, Investigation, Writing, Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors have declared that no competing interests exist.
Additional information
Communicated by Eun Jin Lee.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lwin, H.P., Lee, J. Differential effects of postharvest 1-MCP treatment on fruit quality and targeted major metabolites in long-term cold-stored ‘Wonhwang’ pears. Hortic. Environ. Biotechnol. 63, 499–513 (2022). https://doi.org/10.1007/s13580-021-00412-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-021-00412-4