Abstract
NAM, ATAF1,2, and CUC2 (NAC) proteins constitute one of the largest families of plant-specific transcription factors. These proteins have diverse functions in biological processes. Particularly, NAC transcription factors have received considerable attention as regulators in stress signaling pathways. However, little is known about the NAC genes in tomato (Solanum lycopersicum). In this study, 104 NAC genes were identified in the tomato genome. The predicted NAC genes were distributed across all of 12 chromosomes at various densities and were phylogenetically clustered into six groups (I–VI), together with NAC genes from Arabidopsis and rice.The structure and motif compositions of the NAC genes in tomato were also analyzed. Analysis of available microarray data showed that most of the NAC genes in tomato had specific temporal and spatial expression patterns. Moreover, the expression profiles of eight selected NAC genes in tomato were analyzed in different tissues under different abiotic conditions by quantitative real-time RT-PCR. Except for one, all eight selected genes responded to one or more of the abiotic stress treatments. The results of this study provided insights into the classification and putative functions of this family.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant-specific NAM, ATAF1/2, and CUC2 (NAC) proteins constitute one of the largest transcription factor (TF) families. These proteins are characterized by a well-conserved N-terminal NAC domain (Olsen et al. 2005; Puranik et al. 2012). The NAC domain, which comprises nearly 160 amino acid residues, can be divided into five subdomains (A to E) based on its motif distribution (Aida et al. 1997; Ooka et al. 2003). The highly conserved subdomains C and D may be responsible for binding to DNA, subdomain A may be involved in homo- and heterodimerization, and the divergent subdomains B and E may be implicated in the functional diversity of NAC proteins (Ooka et al. 2003; Jensen et al. 2010; Chen et al. 2011). The transcriptional regulation region (TRR) of NAC TFs is generally located in the highly divergent C terminus; this region confers regulation diversity in transcriptional activation activity. The TRR contains group-specific motifs that are rich in serine and threonine, proline and glutamine, or acidic residues. At least 10 motifs have been identified in the TRR of rice (Oryza sativa) NAC proteins. These motifs are conserved for a given subgroup of NAC subfamilies but vary across different subfamilies (Fang et al. 2008).
NAC TFs regulate many biological processes, including shoot apical meristem formation and maintenance (Aida et al. 1997; Hibara et al. 2003), floral development (Sablowski and Meyerowitz 1998), embryo development (Duval et al. 2002), hormone signaling (Fujita et al. 2004), and regulation of secondary cell wall synthesis (Zhong et al. 2010). Particularly, NAC TFs have received considerable attention as regulators in both biotic and abiotic stress signaling pathways. Some of these TFs have been considered potential targets for the engineering of plant tolerance (Jensen et al. 2010; Puranik et al. 2012). Although considerable knowledge has been gained about the physiological and molecular functions of NAC proteins, this area of research is still in its infancy.
Tomato (Solanum lycopersicum) is an important vegetable crop worldwide. Numerous studies on fruit development have used tomato as a model. Intensive research has been conducted on NAC TFs in many plants, such as Arabidopsis, rice, soybean, apple and potato (Nuruzzaman et al. 2010; Le et al. 2011; Singh et al. 2013; Su et al. 2013). However, only a few studies have characterized the NAC TFs in tomato. The genome sequence of tomato has been decoded, and this has provided an excellent opportunity for genome-wide analysis of all the genes belonging to specific gene families (Mueller et al. 2005). Kou et al. (2013) have recently detected 74 putative NAC genes in tomato. In the present study, we performed a genome-wide analysis of the NAC gene family and identified 104 NAC genes in tomato. The tissue-specific expression profiles of the NAC genes in tomato were analyzed based on available microarray data. Given the potential of NAC genes as targets for the engineering of plant tolerance to different stresses, we searched Genevestigator to gain insights into the differential expression profiles of the SlNAC genes under various stress conditions. Furthermore, we selected eight tomato NAC genes and analyzed their expression profiles in different tissues under different abiotic stress conditions by quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR). The results of this study could serve as reference to select candidate NAC genes in tomato for further functional characterization.
Materials and methods
Identification and chromosomal location of the NAC genes in tomato
Two approaches were performed to identify the members of the NAC TFs in tomato. First, all known Arabidopsis and rice NAC gene sequences were used as queries in the multiple database searches that were performed against the proteome and genome files downloaded from the plantGDB database (http://www.plantgdb.org/). Stand-alone versions of Basic Local Alignment Search Tool (BLAST, http://blast.ncbi.nlm.nih.gov) from the NCBI were used with an e-value cutoff of 1e-003. All protein sequences derived from the collected candidate NAC genes were examined using the Protein family (Pfam) programs (http://pfam.sanger.ac.uk/) for domain analysis and Simple Modular Architecture Research Tool (SMART, http://smart.embl-heidelberg.de/) with the default cutoff parameters. Second, the domains of all tomato peptide sequences were analyzed using a Hidden Markov Model with Pfam searching. We obtained the sequences using the PF02365 Pfam number containing a typical NAC domain from the tomato genome sequences using a Perl-based script. Finally, all protein sequences were compared with known NAC sequences using ClustalX (http://www.clustal.org/) to verify that the sequences were candidate tomato NAC genes.
The isoelectric points (pIs) and molecular weights of the proteins were obtained using the proteomics and sequence analysis tools on the ExPASy Proteomics Server (http://expasy.org/).
The chromosome locations were retrieved from the tomato genome data downloaded from the plantGDB database. The remaining genes were mapped to the chromosomes using MapDraw.
Gene structure and motif analysis of the NAC genes in tomato
The gene structures of the NAC genes were generated using the Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/). Multiple EM for Motif Elicitation (MEME, http://meme.sdsc.edu) version 4.8.1 was used to elucidate the motifs in the 104 deduced tomato NAC protein sequences. MEME was run locally with the following parameters: number of repetitions, any; maximum number of motifs, 20; and optimum motif widths, between 6 and 200 residues (Bailey et al. 2006).
Sequence alignment and phylogenetic analysis of tomato NAC genes
The NAC TF sequences were aligned in ClustalX using BLOSUM 30 as protein weight matrix. The Multiple Sequence Comparison by Log-Expectation (MUSCLE) program (version 3.52) was also used to perform multiple sequence alignments to confirm the ClustalX results (http://www.clustal.org/). A phylogenetic tree for the NAC TF protein sequences was constructed using the neighbor-joining method of Molecular Evolutionary Genetics Analysis (MEGA5, http://www.megasoftware.net/) and the p-distance for the complete deletion option parameters. The reliability of the obtained trees was tested using a bootstrapping method with 1,000 replicates. The image of the phylogenetic tree was drawn using MEGA5.
Data analysis from the microarray database
Microarray expression data from various datasets were obtained using Genevestigator (https://www.genevestigator.com/gv/) with the tomato Gene Chip platform. The whole-genome sequences for tomato were used as query sequences to blast against all gene probe sequences in the Affymetrix Gene Chip (http://www.affymetrix.com/). The best homologous probe was used to annotate the corresponding gene. The identified NAC-containing probe IDs were selected by a Perl-based program and used as query sequences to perform searches in the tomato Gene Chip platform of Genevestigator.
Plant growth, treatments, and collection of tissues
Tomato (Solanum lycopersicum cv. Zhongshu 6) plants were grown under normal conditions. Four-week-old tomato seedlings were subjected to various stress treatments. For drought and salinity stress treatments, the seedlings were irrigated with 15 % PEG 6,000 and 250 mM NaCl solution for the given time periods, respectively. For low temperature treatment, whole plants in pots were placed in illuminated incubation chambers at 4 °C. For each treatment, plant samples were rapidly frozen in liquid nitrogen and then stored at −80 °C. Each treatment was performed at least thrice. For tissue expression analysis, tissues of the root, stem, leaf, flower, and fruits were harvested and stored at −80 °C until use.
Quantitative real-time RT-PCR
Total RNA was extracted using the PureLinkTM RNA Mini Kit (Invitrogen, USA) and treated with RNase-free DNase I. Approximately 2 μg of the extracted total RNA was used to synthesize first-strand cDNA using the PrimeScript First Strand cDNA Synthesis Kit (Takara, China).
Gene-specific primers for qRT-PCR were designed based on the nonconservative regions at the 3′ end (Supplementary Table S1). Primer specificity was analyzed by blasting each primer sequence against the tomato genome and then confirmed when the corresponding melting curves yielded a single sharp peak or a single amplified fragment with the correct predicted length. qRT-PCR was performed in 25 μL reaction volumes containing 10 μM of each primer, 50 ng of cDNA, and 12.5 μL of SYBR Premix Ex Taq II. The PCR amplification conditions included an initial heat-denaturing step at 95 °C for 3 min and then 40 cycles of 95 °C for 20 s, 56 °C for 20 s, and 72 °C for 20 s. Fluorescence was measured at the end of each cycle. A melting-curve analysis was performed by heating the PCR product from 55 °C to 95 °C. The expression data for the NAC genes were presented as relative units after their normalization to the tomato Ubiquitin, which served as the internal control, using the 2-∆∆CT method. The values for the mean expression and standard deviation were calculated from the results of three independent replicates.
Results and discussion
Identification and chromosomal localization of the tomato NAC gene family
We used bioinformatic methods to gather extensive information regarding the NAC gene family in tomato and identify its members. All putative NAC genes in the tomato genome were collected for sequence comparison. The presence of the NAM domain in these genes was confirmed by SMART and Pfam searches. Among the predicted NAC genes, 104 members contained full ORFs and named SlNAC1 to SlNAC104. The size of the NAC family in tomato is similar to that in other plant species, such as Arabidopsis (~105), rice (~140), and soybean (~138) (Hu et al. 2010). Thus, the presence of a large number of NAC genes in the tomato genome suggests that these genes are crucial in the complicated transcriptional regulations in this species.
The identified SlNAC genes encode proteins that range from 123 (Solyc03g062750) to 1,030 (Solyc02g081270) amino acids (aa) in length, with an average of 333 aa, and exhibit pIs that range from 4.58 (Solyc06g063380) to 9.9 (Solyc03g062750). The predicted SlNAC TFs were further analyzed using a local BLAST alignment to identify the relevant orthologs in Arabidopsis. Gene ontology analysis was also performed (Supplementary Table S2).
Among these 104 SlNAC genes, 103 (except for Solyc00g255510) were mapped onto the tomato chromosomes using MapDraw (Fig. 1). The SlNAC genes were distributed on every chromosome (Chr) in tomato. Chr 2 contained the highest number of SlNAC genes, with 18 out of 103 members (~17 %), whereas Chr 9 contained only one member (~1 %). Interestingly, many sister pairs of SlNAC genes, such as Solyc02g036430/Solyc02g081270, Solyc01g009860/Solyc03g097650, Solyc01g021730/Solyc04g072040, and Solyc01g094490/Solyc07g053680, were located on the same or different chromosomes. These results suggest that gene duplication participates in the expansion of the NAC gene family in tomato.
Gene structure and protein motif analyses of the NAC gene family in tomato
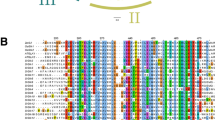
We analyzed the exon/intron organization in the coding sequences of individual SlNAC genes in tomato to determine the structural diversity of these genes. The exon/intron structures of these genes are shown in Fig. 2b. Among the 104 SlNAC genes, 18 had no intron, whereas the others had at least one intron. Solyc02g081270.1.1, which contained 16 introns, possessed the highest number of introns. In addition, a separate phylogenetic tree was generated from the complete protein sequences of all the SlNAC genes. The SlNACs were divided into 14 subfamilies (Fig. 2a). The most closely related members in the same subfamilies shared similar exon/intron structures in terms of intron number and exon length. For instance, most of the SlNAC genes in subgroups A, B, and C harbored no intron, whereas all members in subgroups L and M possessed two introns. By contrast, the members of subgroups H, K, and N displayed a large variability in the number and distribution of introns.
Phylogenetic tree, gene structure, and protein motif analysis of SlNAC transcription factors. (a) Phylogenetic tree. The amino acid sequences of the SlNAC proteins were aligned using Clustal X, and the phylogenetic tree was constructed using the neighbor-joining method in the MEGA5 software. Each node is represented by a number that indicates the bootstrap value for 1,000 replicates. The scale bar represents 0.1 substitutions per sequence position. (b) Gene structure. The center illustrates the exon/intron organization of the corresponding NAC genes. The exons and introns are represented by the boxes and lines, respectively. The scale bar represents 1.0 kb (middle). (c) Protein motif. Schematic representation of the conserved motifs in the NAC proteins in tomato, which were elucidated using MEME. Each motif is represented by a number in the colored box. The black lines represent the non-conserved sequences. The scale bar represents 200 aa
Putative motifs were predicted by MEME and 20 distinct motifs were identified to further reveal the diversity of the SlNAC genes. Most of the closely related members in the phylogenetic tree exhibited common motif compositions. This result suggests the presence of functional similarities among the NAC proteins within the same subgroup (Fig. 2c).
Phylogenetic analysis of the NAC TFs of Arabidopsis, rice, and tomato
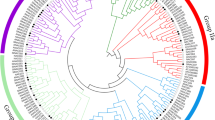
The full-length sequences of all SlNACs and NACs from dicot (Arabidopsis) and monocot (rice) model systems were subjected to multiple sequence alignment using MUSCLE to study the evolutionary relationship among the NAC TFs of different plant species. The multiple sequence alignment data were then used to construct an unrooted phylogenetic tree using MEGA5. As shown in Fig. 3, the phylogenetic tree divided the NACs into six groups (I to VI). Groups I, III, IV, and V were further divided into four, two, three, and two large subgroups, respectively. Group I constituted the largest clade with 35 SlNACs, followed by group IV (33 SlNACs), whereas group II had only one SlNAC and one NAC from Arabidopsis. Most subgroups were shared among the NACs from Arabidopsis, rice, and tomato. Subgroup Id did not include any NACs from Arabidopsis and rice but included NACs from tomato. These results indicate that these genes are either lost in Arabidopsis and rice or acquired in tomato after their divergence from the last common ancestor. Therefore, these genes may have specialized functions in tomato.
Phylogenetic analysis of NAC transcription factors of Arabidopsis, rice and tomato. The full-length sequences of the NAC proteins were aligned with Clustal X, and the phylogenetic tree was constructed using the neighbor-joining method in MEGA5 software. Each node is represented by a number that indicates the bootstrap value for 1,000 replicates
The NAC genes with the same functions clustered into one subgroup, providing an excellent reference to explore the functions of the SlNAC genes. For example, a subset of SlNAC genes, including Solyc10g047060, Solyc07g063410, Solyc04g009440, and Solyc12g013620, were clustered with the well-known stress-related marker genes (e.g., ATAF1/2, ANAC019, RD26, SNAC1/2, and OsNAC10) in group V (Fujita et al. 2004; Bu et al. 2008; Hu et al. 2008; Jensen et al. 2010; Jeong et al. 2010). This result implies that these SlNAC genes are involved in stress responses. This hypothesis was supported by the expression profile analysis discussed below. The results of the present study provided insights into functions of the SlNAC genes.
Most SlNACs were widely scattered across the phylogenetic tree. This finding indicates that these SlNACS belong to the NAC subgroups that are also present in Arabidopsis and rice. Meanwhile, no NACs in Arabidopsis and rice but 26 SlNACs clearly formed a clade in subgroup Id. This result suggests that the clade of these NACs is absent in the other two plant genomes but is present and may have special functions in tomato. A novel subgroup of NACs termed TNACs (tobacco NAC) was found in tobacco. This subgroup contained the NAC domain at the N-terminal distinguished from other NACs and appeared unique to Solanaceaea. Therefore, we hypothesized that some members of subgroup Id are the TNACs in tomato (Rushton et al. 2008). To test this hypothesis, we constructed an unrooted phylogenetic tree of the NAC domains of SlNACs (Supplementary Figure S1). As illustrated in Figure S1, 10 members of group Id and tobacco TNACs clustered into one group marked with red shadow. Furthermore, the partial NAC domain sequence (subdomains A to C) of the 10 candidate TNACs identified in tomato shared typical characteristics with the TNACs in tobacco. These results suggest that the 10 SlNACs are the TNACs in tomato.
Differential expression profiles of the SlNAC genes
We searched the literature for relevant tomato probes and available microarray data in the database to investigate the potential functions of the SlNAC TFs in tomato development. We then analyzed the temporal and spatial expression patterns of the SlNAC genes under normal growth conditions using Genevestigator (Fig. 4). The expression profiles of 58 genes (55.8 %) were obtained, whereas those of the other 46 genes were not detected. Possibly, the 46 genes showed transcript levels that were too low to be detected, their special temporal and spatial expression patterns were not examined, or some of them are pseudogenes.
As illustrated in Fig. 4, the 25 SlNAC genes denoted by stars showed distinct stage-specific expression profiles, whereas the others were expressed at all six stages tested from main shoot growth to fruit ripening, complete with different expression levels. The expression profiles of the 58 SlNAC genes in 14 tissues were also analyzed. Most of the SlNAC genes showed diverse tissue-specific expression profiles. This result indicates that SlNAC TFs have multiple functions in the regulation of tomato growth and development.
We also utilized Genevestigator to analyze the different expression profiles of the SlNAC genes under various stress conditions. As predicted by Genevestigator analysis, most of the SlNAC genes were downregulated or upregulated in response to biotic or abiotic stress treatments (Supplementary Figure S2). In addition, the different genotypes of the tomato strain had different expression profiles of the SlNAC genes. The diverse expression profiles of the SlNAC genes under stress conditions may reflect their multiple functions in regulating the stress signaling pathway.
Expression analysis of the selected SlNAC genes by qRT-PCR
Basing on the results of Genevestigator analysis, we selected eight SlNAC genes, namely, Solyc10g005010, Solyc11g005920, Solyc08g079120, Solyc10g047060, Solyc07g063410, Solyc04g009440, Solyc12g013620, and Solyc01g094490, for expression analysis. Consistent with the above data analysis from the microarray database, the eight SlNAC genes showed distinct tissue-specific expression patterns, which represented the distinct functions of the individual SlNAC genes in tomato development (Fig. 5a). For instance, Solyc01g094490 was highly expressed in the roots, whereas Solyc04g009440 and Solyc12g013620 were highly expressed in all tested tissues. Solyc11g005920, Solyc08g079120, Solyc10g047060, and Solyc07g063410 were preferentially expressed in the flowers and fruits. This result indicates that these genes have important functions in regulating the development of flowers and fruits. Substantial evidence has indicated that NAC TFs have important functions in plant growth, development, and stress responses. However, only a few studies have explored the functions of NAC TFs in fruit ripening (Olsen et al. 2005; Puranik et al. 2012). Six NAC genes, designated as MaNAC1 to MaNAC6, have been recently characterized from banana fruit. These genes were suspected to participate in banana fruit ripening by interacting with ethylene signaling components (Shan et al. 2012). The results of this study provided insights into the possible functions of SlNAC genes in the fruit development of tomato.
NAC TFs are involved in stress response and are potential targets in the development of improved stress-tolerant transgenic plants. Therefore, we utilized qRT-PCR to analyze the different expression patterns of the eight SlNAC genes in the seedlings subjected to three abiotic stress treatments: low temperature (4 °C), drought (15 % PEG 6,000), and high salinity (250 mM NaCl). Except for one (i.e., Solyc08g079120), all the selected SlNAC genes responded to one or more stress treatments (Fig. 5b). Solyc10g047060, Solyc07g063410, Solyc04g009440, and Solyc01g094490 were significantly upregulated by all the three treatments, whereas Solyc12g013620 was induced by high salinity and slightly repressed by the other two treatments. These results indicate that SlNACs have multiple functions in the regulation of abiotic stress signaling pathways and thus may be excellent candidates for the engineering of tomato plants with improved stress resistance.
Kou et al. (2013) have recently identified 74 putative SlNAC genes in tomato using 10 reported NAC genes as queries for BLASTP search. They have also analyzed the expression profiles of six selected SlNACs in different tissues during fruit development and senescence, focusing on the relationship of these genes to hormone responsiveness. In this study, we performed a comprehensive genome-wide analysis of the NAC gene family in tomato using all known Arabidopsis and rice NAC gene sequences as queries for database search and identified 104 NAC genes. Furthermore, we identified candidate tissue-specific and/or stress-responsive SlNAC genes. The results of this study could be used as reference to further characterize the functions of the NAC gene family in tomato. Moreover, these studies may facilitate the understanding of the molecular basis of many agronomically important traits of tomato, such as fruit development and defense against biotic and abiotic stresses.
Abbreviations
- NAC NAM, ATAF, and CUC:
-
transcription factor
- TF:
-
transcription factor
- TRR:
-
transcriptional regulation region
- BLAST:
-
the Basic Local Alignment Search Tool
- SMART:
-
a Simple Modular Architecture Research Tool
- HMM:
-
Hidden Markov Model
- MEGA:
-
Molecular Evolutionary Genetics Analysis
- MEME:
-
Multiple Em for Motif Elicitation
- NJ:
-
the neighbor-joining
- NCBI:
-
National Center for Biotechnology Information
References
Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M (1997) Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell 9:841–857
Bailey TL, Williams N, Misleh C, Li WW (2006) MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res 34:W369–W373
Bu Q, Jiang H, Li CB, Zhai Q, Zhang J, Wu X, Sun J, Xie Q, Li C (2008) Role of the Arabidopsis thaliana NAC transcription factors ANAC019 and ANAC055 in regulating jasmonic acid-signaled defense responses. Cell Res 18:756–767
Chen Q, Wang Q, Xiong L, Lou Z (2011) A structural view of the conserved domain of rice stress-responsive NAC1. Protein Cell 2:55–63
Duval M, Hsieh TF, Kim SY, Thomas TL (2002) Molecular characterization of AtNAM: a member of the Arabidopsis NAC domain superfamily. Plant Mol Biol 50:237–248
Fang Y, You J, Xie K, Xie W, Xiong L (2008) Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice. Mol Genet Genomics 280:535–546
Fujita M, Fujita Y, Maruyama K, Seki M, Hiratsu K, Ohme-Takagi M, Tran LS, Yamaguchi-Shinozaki K, Shinozaki K (2004) A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J 39:863–876
Hibara K, Takada S, Tasaka M (2003) CUC1 gene activates the expression of SAM-related genes to induce adventitious shoot formation. Plant J 36:687–696
Hu H, You J, Fang Y, Zhu X, Qi Z, Xiong L (2008) Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol Biol 67:169–181
Hu R, Qi G, Kong Y, Kong D, Gao Q, Zhou G (2010) Comprehensive analysis of NAC domain transcription factor gene family in Populus trichocarpa. BMC Plant Biol 10:145
Jensen MK, Kjaersgaard T, Nielsen MM, Galberg P, Petersen K, O’Shea C, Skriver K (2010) The Arabidopsis thaliana NAC transcription factor family: structure-function relationships and determinants of ANAC019 stress signaling. Biochem J 426:183–196
Jeong JS, Kim YS, Baek KH, Jung H, Ha SH, Do Choi Y, Kim M, Reuzeau C, Kim JK (2010) Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol 153:185–197
Kou X, Wang S, Wu M, Guo R, Xue Z, Meng N, Tao X, Chen M, Zhang Y (2013) Molecular characterization and expression analysis of NAC family transcription factors in Tomato. Plant Mol Biol Rep. doi:10.1007/s11105-013-0655-3
Le DT, Nishiyama R, Watanabe Y, Mochida K, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS (2011) Genome-wide survey and expression analysis of the plant-specific NAC transcription factor family in soybean during development and dehydration stress. DNA Res 18:263–276
Mueller LA, Tanksley SD, Giovannoni JJ et al (2005) The Tomato Sequencing Project, the first cornerstone of the International Solanaceae Project (SOL). Comp Funct Genom 6:153–158
Nuruzzaman M, Manimekalai R, Sharoni AM, Satoh K, Kondoh H, Ooka H, Kikuchi S (2010) Genome-wide analysis of NAC transcription factor family in rice. Gene 465:30–44
Olsen AN, Ernst HA, Leggio LL, Skriver K (2005) NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci 10:79–87
Ooka H, Satoh K, Doi K, Nagata T, Otomo Y, Murakami K, Matsubara K, Osato N, Kawai J, Carninci P, Hayashizaki Y, Suzuki K, Kojima K, Takahara Y, Yamamoto K, Kikuchi S (2003) Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res 10:239–247
Puranik S, Sahu PP, Srivastava PS, Prasad M (2012) NAC proteins: regulation and role in stress tolerance. Trends Plant Sci 17:369–381
Rushton PJ, Bokowiec MT, Han S, Zhang H, Brannock JF, Chen X, Laudeman TW, Timko MP (2008) Tobacco transcription factors: novel insights into transcriptional regulation in the Solanaceae. Plant Physiol 147:280–295
Sablowski RW, Meyerowitz EM (1998) A homolog of NO APICAL MERISTEM is an immediate target of the floral homeotic genes APETALA3/PISTILLATA. Cell 2:93–103
Shan W, Kuang JF, Chen L, Xie H, Peng HH, Xiao YY, Li XP, Chen WX, Lu WJ (2012) Molecular characterization of banana NAC transcription factors and their interactions with ethylene signalling component EIL during fruit ripening. J Exp Bot 63:5171–5187
Singh AK, Sharma V, Pal AK, Acharya V, Ahuja PS (2013) Genome-wide organization and expression profiling of the NAC transcription factor family in potato (Solanum tuberosum L.). DNA Res 20:403–423
Su H, Zhang S, Yuan X, Chen C, Wang XF, Hao YJ (2013) Genome-wide analysis and identification of stress-responsive genes of the NAM-ATAF1,2-CUC2 transcription factor family in apple. Plant Physiol Biochem 71:11–21
Zhong R, Lee C, Ye ZH (2010) Functional Characterization of Poplar Wood-Associated NAC Domain Transcription Factors. Plant Physiol 152:1044–1055
Acknowledgments
This work was supported by the National Natural Science Foundation (Grant No. 31000307; 31100218), and the Natural Science Foundation of Shandong Province (No. ZR2013CM018) in China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hongyan Su and Shizhong Zhang the two authors contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Primers used for the qRT-PCR analysis (DOC 33 kb)
Table S2
The NAC transcription factors in tomato. (DOC 229 kb)
Figure S1
Phylogenetic analysis of tomato NACs and amino acid sequence comparisons of the N-terminal half of TNAC domains. (A) Phylogenetic analysis of tomato NACs. 25 tobacco TNACs were included as reference. The NAC domains are aligned with Clustal X and the phylogenetic tree of is constructed using the neighbor-joining method in MEGA5. The color shadow marks the TNACs group, which is further divided into three subgroups (TNAC A-C). Each node is represented by a number that indicates the bootstrap value for 1,000 replicates. (B) Amino acid sequence comparisons of the N-terminal half of TNAC domains. The domain sequences are aligned with Clustal X. Areas of significant difference are indicated by red lines at the bottom of the alignment. (PDF 134 kb)
Figure S2
The expression profiles of the SlNAC genes under various stress conditions. The red or green shading represents an up-regulated or down-regulated expression level, respectively. The scale represents the relative expression level. (JPEG 2268 kb)
Rights and permissions
About this article
Cite this article
Su, H., Zhang, S., Yin, Y. et al. Genome-wide analysis of NAM-ATAF1,2-CUC2 transcription factor family in Solanum lycopersicum . J. Plant Biochem. Biotechnol. 24, 176–183 (2015). https://doi.org/10.1007/s13562-014-0255-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-014-0255-9