Abstract
Growth, osmotic adjustment, antioxidant enzyme defense and principle medicinal component bacoside A was studied in in vitro raised shoots of Bacopa monnieri under different concentrations of KCl and CaCl2 (0, 50, 100, 150 or 200 mM). Significant reduction was observed in shoot number per culture; shoot length, fresh weight, dry weight and tissue water content (TWC) when shoots were exposed to increasing KCl and CaCl2 concentrations (50–200 mM) as compared to control. Minimum damage to the membrane as assessed by malondialdehyde (MDA) content was noticed in control in contrast to sharp increase in KCl and CaCl2 stressed shoots. Higher amounts of free proline, glycine betaine and total soluble sugars (TSS) accumulated in KCl and CaCl2 exposed shoots compared to the controls. Among different concentrations of KCl and CaCl2, increasing concentration of CaCl2 showed more increase in osmolyte accumulation. Na+ content decreased with increasing concentrations of KCl and CaCl2. Accumulation of K+ increased significantly in KCl (50–100 mM) stressed shoots as compared to control, while it decreased in CaCl2 treated shoots indicating that it prevents the uptake of K+ ions. Ca2+ accumulation significantly increased with increasing concentrations of CaCl2 up to 150 mM but decreased at higher concentrations. Shoots treated with KCl and CaCl2 (0–100 mM) showed higher antioxidant enzyme (SOD, CAT, APX and GPX) activities but KCl suppressed the activities at higher concentrations. Accumulation of bacoside A was enhanced with an increase in KCl and CaCl2 concentration up to 100 mM. It appears from the data that accumulation of osmolytes, and elevated activities of antioxidant enzymes play an important role in osmotic adjustment in shoot cultures of Bacopa and the two salts tested have a positive effect on bacoside accumulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The stress factors such as salt, drought, low temperature, flooding, heat, oxidative stress, heavy metal toxicity and pathogenic stress adversely affect the plant growth and productivity. These stresses affect almost every aspects of plants physiology which leads to a series of morphological, physiological, biochemical and molecular changes. It is estimated that every year about 6 million arable lands are lost from agriculture use. On the other hand existing agricultural land fails to fulfill the food, feed, fodder and industrial raw material requirement of ever growing population. The new land is becoming unavailable for the cultivation of crops. Rapid increase in population pressure are leading to greater utilization of remaining meager fresh water supplies for drinking, living, and even less for agriculture. In this complex scenario, it has become crucial to consider development of non-conventional technologies which might more effectively utilize degraded, marginal and saline lands for agriculture by using the ‘so-called’ poor water. Despite advances in increasing plant productivity and resistance to a number of pests and diseases, improving salt tolerance in crop plants remains elusive. Furthermore, limited success in increasing the yield stability of crop plants grown in saline soil might be due to lack of understanding about salinity and other abiotic stresses that affect the cell division, differentiation and expansion which have a sustainable impact on plant growth and development. Salinity is one of the major abiotic stresses affecting more than 800 million hectares of arable land throughout the world (Munns and Tester 2008). Depletion of cellular water content due to abiotic stresses such as drought, high soil salinity and temperature extremes is responsible for the greatest agricultural losses (Jaleel et al. 2007; Lokhande et al. 2011). Upon exposure to stresses many plants show changes in chla/chlb ratio (Lin and Kao 1998; Lutts et al. 1996); membrane permeability and/or increase in lipid peroxidation (Dhindsa and Matowe 1981). There are many cellular mechanisms by which plants ameliorate the effects of environmental stresses such as accumulation of compatible osmolytes, most commonly amino acids and quaternary ammonium compounds (Bohnert and Jensen 1996; Kavi Kishor et al. 2005). The increase in cellular osmolarity resulted due to accumulation of osmolytes is accompanied by the influx of water into, or reduced efflux from cells which helps to provide the necessary turgor for cell expansion (Cuin and Shabala 2005; Meloni and Martínez 2009). Both K+ and Ca2+ are involved in photosynthesis and in protein synthesis regulation and their depletion may lead to serious inhibition of these processes (Santos et al. 2001). Similarly, if they are accumulated in higher concentrations, they may inhibit photosynthesis and other vital activities. Maintenance of membrane integrity and the selective uptake of essential ions like K+ and Ca2+ are related with the acquisition of salt tolerance (Salama et al. 1994).
Plants prevent the damaging effects of free radicals by evolving several non-enzymatic and enzymatic mechanisms that efficiently scavenge ROS (Irigoyen et al. 1992; Shalata and Tal 1998). Enzymatic protection is partly performed by SOD that eliminates superoxide radicals and by catalases and peroxidases that degrade H2O2 which influence the levels of lipid peroxidation (Santos et al. 2001). Recent studies have demonstrated that activities of these antioxidant enzymes and levels of antioxidant molecules increase and are correlatable to various environmental stresses (Hernández et al. 2000; Sekmen et al. 2007). Such a correlation was observed between NaCl induced salt stress tolerance and antioxidative responses in different plant systems (Vaidyanathan et al. 2003; Benavente et al. 2004; Jogeswar et al. 2006; Lokhande et al. 2011). In recent years, medicinal and aromatic plants or products are on an increasing demand in agro alimentary, perfumes, pharmaceutical and natural cosmetic industries (Baatour et al. 2010; Tarchoune et al. 2012). As in cultivated species, growth and yield of medicinal and aromatic plants are affected by environmental constraints such as salinity and drought (Baatour et al. 2010). However, little information is available about the physiological basis and effects on secondary metabolite accumulation under different abiotic stress conditions in medicinal plants.
In Indian medicine, certain herbs have been used traditionally as brain or nerve tonics. Bacopa monnieri (L.) Pennell (Scrophulariaceae) is commonly known as ‘Brahmi’ or ‘Nir Brahmi’. It is one of the sources of the medhya rasayan drugs (that counteracts stress and improves intelligence and memory) of Ayurveda. Recently, B. monnieri was placed second in a priority list of the most important Indian medicinal plants evaluated on the basis of their medicinal importance, commercial value and potential for further research and development. According to the National Medicinal Plant Board (NMPB), Government of India, annual demand of Bacopa is increasing day by day due to the popularity of the Bacopa based drugs.
Efforts were made earlier to induce the stress in vitro using NaCl (Ali et al. 1999; Debnath 2008), mannitol (Debnath 2008), heavy metals like copper (Ali et al. 1998a), cadmium, zinc (Ali et al. 2000) and aluminium (Ali et al. 1998b). But these authors did not identify the stress tolerance mechanisms, antioxidative enzyme responses and accumulation of bacoside in Bacopa monnieri. While some plants accumulate only proline and sugars under stress, others accumulate glycine betaine in addition to proline. Our results indicate that Bacopa accumulates glycine betaine also. Potassium (K+) plays an important role in the regulation of osmotic potential of plant cells and calcium (Ca2+) is the structural element essential in the synthesis of cell wall. The calcium is said to antagonize the uptake of excess K+. Many soils have an excess of certain elements, particularly sodium (Na+), K+ or Ca2+ (Taiz and Zeiger 2010) which results in severe stress and subsequently decline in productivity. Detailed understanding of the basic mechanisms involved in plant salt tolerance is an important prerequisite to improve the performance of plants in saline soils (Binzel and Reuveni 1994). Since plants are affected not only by Na+ salts but also by K+ salts, in the present study, the effects of KCl and CaCl2 stress on growth of shoot cultures and nutritional imbalance were studied in Bacopa monnieri. The effect of KCl and CaCl2 stress on lipid peroxidation, osmolyte accumulation, antioxidant enzyme activities (SOD, CAT, APX, GPX) and accumulation of medicinally active component bacoside A in in vitro shoot cultures were carried out.
Materials and methods
Plant material, establishment of in vitro cultures and salt stress treatment
The shoot cultures of B. monnieri were established using nodal explants and were maintained in liquid Murashige and Skoog’s (MS 1962) medium containing 1 mg/l 6-benzyladenine (BA) (Ahire et al. 2012a). For analysis of in vitro stress studies, shoots of Bacopa were subjected to the treatment of different concentrations of KCl and CaCl2 separately in liquid MS medium supplemented with 1 mg/l BA. About 4 shoots (5 nodes each/bottle) were inoculated in glass bottles containing 50 ml of MS liquid medium fortified with 1 mg/l BA and respective KCl and CaCl2 (0–200 mM) concentrations separately. Cultures were incubated under controlled conditions at 25 ± 2 °C temperature, 60 ± 10 % relative humidity and 8 h photoperiod (PFD 40 μmol m−2 s−1) provided by white fluorescent tubes (Philips, India).
Determination of mineral nutrients
Shoots were washed with distilled water and excess water was soaked on blotting paper followed by drying at 60 °C for 48 h in an oven. Dried shoots were ground to powder and 200 mg powder from each treatment was soaked in 10 ml of 35 % (v/v) HNO3 (Qualigens, Mumbai, India) overnight at room temperature followed by acid digestion at 100 °C till the acid was evaporated and finally the residue was dissolved in 30 ml of distilled water. Samples were filtered using Whatman filter paper No. 1 (Whatman International Ltd., Maidstone, England). Cations such as Na+, K+ and Ca2+ were measured by atomic absorption spectrophotometer (AA-7000, Labindia Analytical Instruments Pvt. Ltd., Mumbai, India). Standard solutions of Na+, K+ and Ca2+ were purchased from Qualigens, Mumbai, India and were used for quantification of ion content.
Growth analysis and tissue water status
Growth analysis was carried out after 28 days of incubation. Morphological observations like number of shoots per culture and shoot length (cm) were recorded. Tissue water status was determined by measuring fresh (FW) and dry weights (DW). Shoots were harvested and excess medium was soaked on blotting paper and FW of shoots was measured. Shoots were kept in an oven at 60 °C separately till constant weight was obtained and noted as DW. FW and DW of the shoots obtained from each treatment were used to determine the water status, which is expressed as percentage tissue water content (TWC %) calculated using the equation as described by Lokhande et al. (2011).
Determination of chlorophyll, lipid peroxidation and osmolyte content
Chla, Chlb and total chlorophyll contents in the control and treated shoots were estimated as per the standard method described by Arnon (1949). The level of lipid peroxidation was measured in terms of malondialdehyde (MDA) content as described by Heath and Packer (1968), with some modifications as described by Lokhande et al. (2011). Glycine betaine was estimated by the periodide colorimetric method according to Grieve and Grattan (1983) as described by Lokhande et al. (2010) and proline content was estimated as per Bates et al. (1973).
Determination of antioxidant enzyme activities
Treated and control fresh samples (500 mg) were homogenized in 5 ml of ice cold 50 mM sodium phosphate buffer (pH 7.0) containing 0.1 mM EDTA and 1 % (w/v) polyvinylpyrrolidone with chilled mortar and pestle. The homogenate was filtered with single layered cheese cloth and centrifuged at 10,000 rpm for 20 min at 4 °C. Appropriate aliquot/dilution of the supernatant was used as a crude enzyme(s) for determination of antioxidant enzyme activities. Soluble protein content in the enzyme extract was determined according to Lowry et al. (1951) using bovine serum albumin as standard. Total superoxide dismutase (SOD) enzyme (EC 1.15.1.1) activity was assayed according to Becana et al. (1986) by inhibition of the photochemical reduction of nitroblue tetrazolium (NBT). The reaction mixture (1 ml) containing 50 mM phosphate buffer (pH 7.0) and 0.1 mM EDTA to which an oxygen-generating system containing 14.3 mM methionine, 82.5 μM NBT, and 2.2 μM riboflavin, prepared freshly in situ, was added. Reaction was initiated by adding 25 μl of crude enzyme. The entire system was kept 30 cm below the light source (six 15 W fluorescent tube light) for 30 min. Reaction was stopped by switching off the tube light. For light blank, all the reactants without enzyme extract was incubated in light as for the samples, whereas all the reactants along with 25 μl enzyme extract were incubated in dark for dark blank. Reduction in NBT was measured by monitoring the change in absorbance at 560 nm. SOD activity is expressed as μKat of SOD mg−1 protein. Catalase (CAT) enzyme (EC 1.11.1.6) activity was measured by following the decomposition of hydrogen peroxide (H2O2) as described by Cakmak and Marschner (1992) with minor modifications. Activity was measured in a reaction mixture (1 ml) containing 50 mM phosphate buffer (pH 7.0) and 300 mM H2O2. The reaction was initiated by adding 50 μl enzyme extract and the activity was determined as a result of H2O2 decomposition by monitoring the decrease in absorbance at 240 nm (ε = 36 mM−1 cm−1) for 2 min at an interval of 15 s. The slope of readings between the time interval considered as ∆A and enzyme activity is expressed as μ Kat of CAT mg−1 protein. Ascorbate peroxidase (APX) enzyme (EC 1.11.1.11) activity was determined according to Nakano and Asada (1981). The reaction mixture (1 ml) contained 50 mM phosphate buffer (pH 7.0), 0.5 mM ascorbate and 0.1 mM H2O2. Reaction was started by adding 50 μl of crude enzyme. Ascorbate oxidation was monitored for 1 min by measuring the decrease in absorbance at 290 nm at every 15 s (ε = 2.8 mM−1 cm−1). Enzyme activity is expressed as μKat of APX mg−1 protein. Guaiacol peroxidase (GPX) enzyme (EC 1.11.1.7) activity was assayed according to Hemeda and Klein (1990). The reaction mixture (1 ml) contained 50 mM phosphate buffer (pH 7.0), guaiacol, 200 mM H2O2 and 10 μl enzyme extract. The reaction was started by adding 200 mM H2O2. The increase in absorbance due to oxidation of guaiacol (ε = 26.6 mM−1 cm−1) was monitored at 470 nm. Enzyme activity is expressed as μKat mg−1 protein.
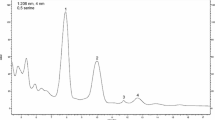
Estimation of bacoside A
The dried samples (control and treated) were ground to fine powder and used for estimation. Bacoside-A was extracted as per Watoo et al. (2007) with some modifications as described by Parale et al. (2010). One g of powder was soaked in 10 ml of distilled water for 2 h and the mixture was squeezed. Plant residue was extracted thrice in 20 ml of 95 % (v/v) ethanol for 24 h on a rotary shaker at 80 rpm at 25 °C. The pooled filtrate was evaporated to dry under vacuum. Dry residue was dissolved in methanol at a concentration of 1 mg/ml and used for HPTLC analysis. The standard bacoside-A was procured from Natural Remedies, Bangalore, India and dissolved in HPLC grade methanol at the concentration of 1 mg/ml and used to plot the standard curve. HPTLC was performed on 20 × 10 cm aluminum foil plates coated with 200 μm layer of silica gel 60 F254 (E. Merck, Germany). Plates were prewashed with methanol and dried in an oven for 5 min at 105 °C temperature. The standard and samples were loaded with Linomat 5 semiautomatic applicator (CAMAG, Switzerland) fitted with 100 μl syringe as bands 8 mm in width. The delivery speed of the syringe was 90 nl/s. HPTLC plates were developed in a twin-trough chamber (20 × 10 × 4 cm; CAMAG, Switzerland). Chamber saturation was carried out using 20 × 10 cm Whatman filter paper for 20 min. The plates were developed in toluene : ethanol : methanol : glacial acetic acid (4:3:3:1 v/v), until 80 mm from the lower edge of the plate. After development, the plates were dried using hair dryer. The dried developed plates were immersed for 2 s in 5 % methanolic sulphuric acid. After immersion, the plates were dried in an oven for 5 min at 100 °C. Documentation of the plates was carried out using CAMAG TLC Scanner III system at 500 nm with winCATS software 1.4.3.
Data analysis
A completely randomized design (CRD) was used in all experiments. The experiments were repeated at least thrice. The data were subjected to analysis of variance (ANOVA) followed by Duncan’s multiple range tests (DMRT) at P ≤ 5 %.
Results
Effect of KCl and CaCl2 stress on growth
In the present investigation, shoots of Brahmi were exposed to different salt (KCl and CaCl2: 0, 50, 100, 150 and 200 mM) concentrations. Significant reduction in number of shoots per culture was observed with increasing salt concentrations. Maximum number of shoots (138.0 ± 5.7) was observed in control after 28 days of incubation and least number of shoots per culture (15.9 ± 1.8) was recorded at 200 mM KCl stress. Similarly, shoot length, shoot fresh and dry weights decreased significantly with increasing concentrations of KCl and CaCl2 (Table 1). Tissue water content (TWC %) decreased with increasing KCl and CaCl2 concentrations followed by fresh and dry weights of tissues (Fig. 1; Table 1). Among KCl and CaCl2, higher concentration of KCl showed more pronounced effect on the number of shoots per culture.
Influence of KCl and CaCl2 stress on chlorophyll content and lipid peroxidation
Decrease in chla, chlb and total chl was observed with increasing KCl and CaCl2 concentrations (Table 2). Maximum chla (9.16 ± 0.68 mg g−1 FW), chlb (3.96 ± 0.31 mg g−1 FW) and total chl (13.61 ± 0.97 mg g−1 FW) were recorded in control shoots. A sharp decrease was noticed with increasing KCl and CaCl2 concentrations. Considerable reduction in chla (3.63 ± 0.5 mg g−1 FW), chlb (1.36 ± 0.2 mg g−1 FW) and total chl (4.99 ± 0.7 mg g−1 FW) was noticed at 200 mM CaCl2 concentration. Progressive decrease in chlorophyll content was observed with increasing concentrations of KCl and CaCl2 more so with CaCl2. Lipid peroxidation in terms of MDA content was enhanced significantly with increasing KCl and CaCl2 concentration from 0 to 200 mM (Fig. 2). In shoots treated with KCl, MDA content increased up to 150 mM level but decreased thereafter (Fig. 2). About 1.51 times more MDA content was observed in the shoots treated with 150 mM KCl (Fig. 2). At lower CaCl2 concentrations (50 and 100 mM), MDA content was not high. Concentration of MDA increased at higher CaCl2 concentrations (150 and 200 mM). Higher lipid peroxidation (2.16 times more MDA content over control) was observed at 200 mM CaCl2 (Fig. 2) indicating more damage of membrane at higher KCl and CaCl2 concentrations due to lipid peroxidation.
Osmolyte accumulation in response to salt stress
A progressive increase in free proline content was recorded in KCl treated shoots as compared to control (Fig. 3). Elevation in proline content was 3.77 times higher at 200 mM KCl concentration (Fig. 3). In CaCl2 treated shoots, proline content increased up to 150 mM (6.21 times more over control) but decreased at 200 mM concentration. Accumulation of glycine betaine in the shoots of Brahmi was low in control, whereas a steep increase was recorded with an increase in 50–200 mM KCl and CaCl2 stresses (Fig. 4). About 3.46 and 4.04 times more glycine betaine content was observed over control in shoots treated with 200 mM KCl and CaCl2 respectively. Treatment of KCl and CaCl2 resulted in higher accumulation of total soluble sugar (TSS) in shoots of Bacopa as compared to the controls (Fig. 5). Increased TSS content up to 150 mM levels of KCl was noticed though it declined slightly thereafter. Similarly, CaCl2 increased the TSS and 3.64 times more over control was observed at 200 mM (Fig. 5). Among the different concentrations of KCl and CaCl2, increasing concentration of CaCl2 showed more increase in osmolyte accumulation.
Effect of KCl and CaCl2 on the accumulation of ions
Salt stress (0–200 mM KCl and CaCl2) promoted the accumulation of cations in the shoots of Bacopa in contrast to the control (Table 3). Decrease in Na+ concentration was observed with increasing KCl and CaCl2 levels (Table 3). Contrary to this, K+ concentration was enhanced significantly in KCl-stressed shoots as compared to the control (Table 3), while the opposite effect was seen in CaCl2 treated shoots. Accumulation of K+ increased by 1.72 times at 100 mM KCl level and decreased thereby. Ca2+ accumulation slightly increased in the shoots when they were exposed to 0–100 mM KCl but declined at higher concentrations. However, Ca2+ accumulated significantly when exposed to different concentrations (50–150 mM) of CaCl2 (Table 3), maximum being 7.85 times at 150 mM CaCl2 (Table 3).
Effect of KCl and CaCl2 stress on antioxidant enzyme activities
KCl and CaCl2 stresses (0–100 mM) stimulated the antioxidant enzyme (SOD, CAT, APX and GPX) activities in shoots of Bacopa. Increasing concentration of KCl and CaCl2 suppressed the activities (Fig. 6). Both KCl and CaCl2 (50–100 mM), increased the SOD activity (Fig. 6a). SOD specific activity was 1.35-folds higher at 150 mM KCl while it displayed almost the same activity at 100 mM CaCl2. Activity of CAT was also enhanced by 1.2-folds at 100 mM KCl and 1.4-folds at 100 mM CaCl2 concentration (Fig. 6b). The trend declined at higher concentrations of KCl and CaCl2 (150 and 200 mM). Control shoots (without KCl and CaCl2 in the medium) displayed lowest APX activity in contrast to treated tissues. While shoots cultured on medium containing 100 mM KCl showed 2.63-folds higher APX activity than controls (Fig. 6c), activity was 1.94-folds higher in 100 mM CaCl2 treated tissues. A steep increase in GPX activity was also recorded as the KCl and CaCl2 concentrations increased up to 100 mM (Fig. 6d). About 1.65-folds and 2.8-folds higher GPX activity was recorded in shoots treated with 100 mM KCl and CaCl2 respectively (Fig. 6d).
Effect of different concentrations of KCl and CaCl2 on activity of antioxidant enzymes in shoots of Bacopa monnieri. Shoots were transferred to media containing 0–200 mM KCl and CaCl2 and the SOD (a), CAT (b), APX (c) and GPX (d) activities were measured after 28 days of culture. Each value represents mean of three replications and vertical bars indicate SE. The bars with different letters on the same color columns are significantly different at p ≤ 0.05
Bacoside A accumulation in response to KCl and CaCl2 stress
In the present investigation, effect of KCl and CaCl2 stress on the accumulation of the principle medicinal compound bacoside A was studied. Content of bacoside A was enhanced with an increase in KCl and CaCl2 concentrations in the medium up to 100 mM. Highest bacoside A (2.37 ± 0.29 mg g−1 DW) was noticed in the shoots cultured on 100 mM CaCl2 (Fig. 7). Higher concentration of KCl and CaCl2 (150 and 200 mM) resulted in a drastic decline in the bacoside A content in shoots of Bacopa.
Discussion
Salinity is one of the major environmental constraints inducing a wide range of effects at the cellular and whole-plant levels. It causes significant yield reductions in different plant species (Belkheiri and Mulas 2013). The ability to maintain turgor induced by salt stress may preserve the metabolic processes, and thus the growth of a plant (Martinez et al. 2004). K+ is the only important monovalent cation, that represents the major inorganic constituent essential for all higher plants (Tester and Davenport 2003; Lv et al. 2012). It contributes to many physiological processes like osmotic pressure and ionic strength, cell elongation, growth of shoot and root, stomatal movement (Maathuis and Amtmann 1999; Tester and Davenport 2003); due to its relatively low charge:mass ratio, resulting in a small hydration shell and it has a low tendency to modify protein conformation (Maathuis and Sanders 1996; Rascio et al. 2001). K+ is also essential for enzyme activation, protein synthesis and photosynthesis (Belkheiri and Mulas 2013). Calcium on the other hand is an essential plant nutrient required for structural roles in the cell wall and membranes as a counter-cation for inorganic and organic anions in the vacuole. Besides, it is necessary for cell elongation and known to activate a number of enzymes such as phospholipase D, lecithinase, ATPase and amylase (Hepler 2005). It plays an important role in various physiological processes by acting as intracellular messenger in the cytosol and second messenger in the transduction of exogenous signals (Marschner 1995). The continuous supply, uptake, transportation, and metabolism of different ions seem to vary from the initiation of organs and meristematic tissue towards plant growth as has been pointed out by Kanashiro et al. (2009). The increase in Cl− concentration in the medium leads to a reduction of nitrate levels in tissues of several plant species (Gratten and Grieve 1994). Similar observations were made by Santos et al. (2001) in KCl-stressed sunflower plants and calli.
Like many other abiotic stresses, salt stress inhibits plant growth. Salt stress may promote the synthesis of several osmolytes necessary to combat stress thus relocating the valuable resources (Sabir et al. 2012). When shoots of Brahmi were exposed to different concentrations (0, 50, 100, 150 and 200 mM) of KCl and CaCl2, significant reduction in number of shoots, fresh and dry weight of shoots per culture and TWC were noticed (Table 1; Fig. 1). At low concentrations of KCl and CaCl2 (50–100 mM), shoot growth was less affected compared to 150 and 200 mM. Similarly, KCl stress decreased sunflower plant and calli growth (Santos et al. 2001). CaCl2 dihydrate (CaCl2 2H2O) also decreased the growth of the plants. CaCl2 in excess (3.35 mM) decreased both the fresh and dry mass of Aechmea blanchetiana plantlets (Kanashiro et al. 2009). Sabir et al. (2012) made identical observations in in vitro differentiating shoots of Withania somnifera Dunal. Among the KCl and CaCl2 stresses, CaCl2 showed more deleterious effect than KCl. Increasing concentration of KCl displayed more pronounced effect on the number of shoots per culture. Both growth and relative water content of salt-treated shoots of Withania somnifera were decreased with increasing salt concentration (Sabir et al. 2012). The growth reduction may be due to the decrease in the turgor. The saline solution establishes a water potential imbalance between the apoplast and symplast that leads to decrease in turgor (Sangwan et al. 1994; Bohnert et al. 1995). When the turgor is reduced below the threshold level of the cell wall, it results in the reduced growth responses (Sabir et al. 2012). If the water potential difference is greater than it can be compensated for by turgor loss, then cellular dehydration starts. This growth cessation finally leads to the low dry weight accumulation (Molassiotis et al. 2006; Sabir et al. 2012).
Decrease in chla, chlb and total chl was observed with increasing KCl and CaCl2 concentrations (Table 2). Salinity induces various biochemical and physiological responses in plants and affects almost all plant functions including chlorophyll content, photosynthesis, growth and development (Aghaleh et al. 2009). Chlorophyll content is directly related to the growth and productivity of the plant. High salt concentration results in decreased chlorophyll pigments; and this might be due to the interference of salt ions in chlorophyll biosynthesis. High salt concentration creates an imbalance in ion homeostasis which restrains the iron to the protoprophyrin molecule. This results in decreased synthesis of chlorophyll pigments (Agastian et al. 2000; Sabir et al. 2012). Lipid peroxidation as measured by MDA content increased with increasing KCl and CaCl2 concentration from 0 to 200 mM (Fig. 2). At higher KCl and CaCl2 concentrations (150 and 200 mM), membrane damage was severe due to lipid peroxidation. These observations are in agreement with the hypothesis that the amount of MDA content is a direct sign of oxidative stress caused by damage to the lipid molecules of the cell membrane (Erturk et al. 2007). Similar results were recorded by Santos et al. (2001) in the KCl treated sunflower plants and calli. An increase in lipid peroxidation was recorded in suspension cultures of C. roseus under salt stress (Elkahoui et al. 2005). Increase in MDA content was also noticed in KCl and CaCl2 treated in vitro differentiating shoots and calli of Withania somnifera (Sabir et al. 2012).
The first response of cells during salt stress is the imbalance of osmotic potential due to excess salt. To adjust the osmotic potential of the cell, a suitable osmolyte or osmoprotectant molecules are required, which accumulate in cytosol. In the present study, a progressive increase in free proline content, glycine betaine and TSS were observed (Figs. 3, 4, and 5). Proline serves as a storage sink for carbon and nitrogen and as a free-radical scavenger (Chinnusamy et al. 2005; Flors et al. 2007). It stabilizes sub-cellular structures (membranes and proteins) and buffers cellular redox potential under stress (Kavi Kishor et al. 2005; Chinnusamy et al. 2005). Glycine betaine and trehalose act as osmoprotectants by stabilizing quaternary structures of proteins and highly ordered states of membrane. Hence, these organic osmolytes are known as osmoprotectants and thus may alleviate salt induced damages (Bohnert and Jensen 1996; Chen and Murata 2002). Sugar is also considered as an osmoprotectant and reported to accumulate during salinity stress (Kovacik et al. 2009). Soluble sugars that are altered by abiotic stresses may also act as signaling molecules (Chaves and Oliveira 2004) and also interact with hormones as part of the sugar-sensing and signaling network in plants (Sabir et al. 2012). Synthesis of proline, glycine betaine and other osmolytes is an energy dependent process and consumes large number of ATP molecules (Raven 1985), thus their synthesis affect the plant growth (Lokhande et al. 2011).
Salt stress is associated with complex traits, which include osmotic stress, specific ion effect, ion imbalances and nutrient deficiency. Accordingly, salt stress affects various physiological and biochemical mechanisms related to plant growth and development (Pitman and Lauchli 2002; Ahire et al. 2012b). In the present study, Na+ accumulation decreased with increasing concentrations of KCl and CaCl2 in the medium (Table 3). K+ concentration increased significantly in the KCl-stressed shoots as compared to the control up to 100 mM KCl level. High Ca2+ accumulation was noticed when cultures were treated with 150 mM CaCl2. At still higher concentrations of both KCl and CaCl2, K+ and Ca2+ ion accumulations declined. Thus, this study indicates that the shoots of Bacopa accumulate the K+ and Ca2+ at low concentrations of salt stress but may exclude them at higher KCl and CaCl2 concentrations in the medium. Different plant species develop different adaptive mechanisms either to exclude salt from their cells or to tolerate it within the cells by sequestering in the vacuoles (Kozlowski 1997; Munns 2002; Parida and Das 2005). The uptake of large amounts of salt by the plant leads to the increase of osmotic pressure in the cytosol.
Salt stress leads to oxidative stress through an increase in reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), superoxide (O2 •−) and hydroxyl (OH•) radicals. ROS can modulate normal cellular metabolism through oxidative damage to lipids, proteins and nucleic acids (Imlay 2003). Plants have developed defensive antioxidative system, including low-molecular mass antioxidants as well as antioxidative enzymes such as SOD, CAT, APX and GPX. In the present study, KCl and CaCl2 stresses (0–100 mM) significantly increased the antioxidant enzyme (SOD, CAT, APX and GPX) activities in shoots of Bacopa (Fig. 6a, b, c, d). Higher activity of SOD is an indication of efficient detoxification of superoxide radical (Abraham and Dhar 2010) and results in formation of H2O2. The excess H2O2 produced by SOD in response to salt stress is removed by both CAT, and APX (Parida et al. 2004; Lokhande et al. 2011). The removal of H2O2 in microbodies is mainly carried by the catalases (Scandalios et al. 1997) and catalyzes either the direct decomposition of H2O2 or the oxidation by H2O2 of substrates. APX catalyzes the dismutation of H2O2 through the ascorbate-glutathione cycle (Shigeoka et al. 2002; Jithesh et al. 2006). Ascorbate is the most important reducing substrate for H2O2 detoxification in the plant cells and APX uses ascorbate to reduce H2O2 to water (Noctor and Foyer 1998; Sergio et al. 2012). SOD generated H2O2 is eliminated by GPX (Rios-Gonzales et al. 2002). Similar increase in the antioxidative enzyme activities under increased salt stress was reported in several other plants like rice seedlings (Kumar et al. 2009), Sesuvium protulacastrum shoot cultures (Lokhande et al. 2011), brinjal seedlings (Ahire et al. 2012b); shoot and callus culture of Withania somnifera (Sabir et al. 2012). The study indicates that Bacopa has efficient antioxidative defensive mechanism to ameliorate the salt stress induced damages at lower salt concentrations.
Accumulation of bacoside A was enhanced with an increase in KCl and CaCl2 concentrations in the medium up to 100 mM (Fig. 7). Treatment of 100 mM CaCl2 resulted in higher accumulation of bacoside A. But, KCl and CaCl2 at higher concentrations suppressed its accumulation (Fig. 7). Salt stress exerts an unfavorable effect on the growth (Jaleel et al. 2007), but increases secondary metabolite accumulation during biotic and abiotic stress conditions (Wahid and Ghazanfar 2006; Cheng et al. 2007; Ghorpade et al. 2011). Increase in bacoside A content might be due to increased water stress which can regulate large number of transcripts including phenylpropanoid metabolic pathway genes. Calcium may act as a second messenger and thus trigger the expression of several genes associated with the biosynthetic pathway of bacoside A. Bacopa might synthesize the triterpene saponin to prevent the membrane damage. The low amount of saponins at higher concentrations of Cu provided an intrinsic defense to resist Cu-induced oxidative damage in P. ginseng plants (Ali et al. 2006). Similarly, the decrease in bacoside A accumulation at higher concentrations of salts might be due to inhibition of the metabolic pathway enzymes associated with its biosynthesis.
In conclusion, the findings presented here demonstrate that KCl and CaCl2 induced salt stresses affected the number of shoots per culture, shoot length, shoot fresh and dry weights, tissue water content and lipid peroxidation (MDA content). Increased K+ and Ca2+ ions were observed under KCl and CaCl2 stresses respectively. Antioxidant enzyme activities (SOD, CAT, APX and GPX) increased in shoots of Bacopa with enhanced salt levels (up to 100 mM). Bacoside A content increased but only under moderate stress (100 mM KCl and CaCl2). The studies presented in this investigation provide an impetus for conducting growth adaptability responses of Bacopa monnieri in field conditions under different salt stress conditions and also to enhance bacoside A concentration.
Abbreviations
- APX:
-
Ascorbate peroxidase
- BA:
-
6-Benzyladenine
- CAT:
-
Catalase
- GPX:
-
Guaiacol peroxidase
- MDA:
-
Malondialdehyde
- MS:
-
Murashige and Skoog
- NBT:
-
Nitroblue tetrazolium chloride
- SOD:
-
Superoxide dismutase
- TSS:
-
Total soluble sugars
- TWC:
-
Tissue water content
References
Abraham G, Dhar DW (2010) Induction of salt tolerance in Azolla microphylla Kaulf through modulation of antioxidant enzymes and ion transport. Protoplasma 245:105–111
Agastian P, Kingsley SJ, Vivekanandan M (2000) Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes. Photosynthetica 38:287–290
Aghaleh M, Niknam V, Ebrahimzadeh H, Razavi K (2009) Salt stress effects on growth, pigments, proteins and lipid peroxidation in Salicornia persica and S. europaea. Biol Plant 53:243–248
Ahire ML, Lokhande VH, Kavi Kishor PB, Nikam TD (2012a) Brinjal (Solanum melongena Linn.) varieties accumulate both Na+ and K+ under low NaCl stress, but excludes Na+ and accumulate K+ under high salt levels. Asian Australian J Plant Sci Biotechnol 6:1–6
Ahire ML, Patil PP, Kavi Kishor PB, Nikam TD (2012b) Micropropagation and assessment of antibiotic selection in vitro of Bacopa monniera (L.) Pennell. Int J Plant Dev Biol 6:34–39
Ali G, Srivastava PS, Iqbal M (1998a) Effect of cadmium and copper on growth of Bacopa monniera regenerants. Biol Plant 41:635–639
Ali G, Srivastava PS, Iqbal M (1998b) Morphogenic response and proline content in Bacopa monniera cultures grown under copper stress. Plant Sci 138:191–195
Ali G, Srivastava PS, Iqbal M (1999) Proline accumulation, protein pattern and photosynthesis in Bacopa monniera regenerants grown under NaCl stress. Biol Plant 42:89–95
Ali G, Srivastava PS, Iqbal M (2000) Influence of cadmium and zinc on growth and photosynthesis of Bacopa monniera cultivated in vitro. Biol Plant 43:599–601
Ali MB, Hahn EJ, Paek KY (2006) Copper-induced changes in the growth, oxidative metabolism and saponin production in suspension culture roots of Panax ginseng in bioreactors. Plant Cell Rep 25:1122–1132
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Baatour O, Kaddour R, Wannes WA, Lachaâl M, Marzouk B (2010) Salt effects on the growth, mineral nutrition, essential oil yield and composition of marjoram (Origanum majorana). Acta Physiol Plant 32:45–51
Bates L, Waldren RP, Teare JD (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Becana M, Moran JF, Iturbe-Ormaetxe I (1986) Iron-dependent oxygen free radical generation in plants subjected to environmental stress: toxicity and antioxidant protection. Plant Soil 201:137–147
Belkheiri O, Mulas M (2013) The effects of salt stress on growth, water relations and ion accumulation in two halophyte Atriplex species. Environ Exp Bot 86:17–28
Benavente LM, Teixeira FK, Kamei CLA, Pinheiro MM (2004) Salt stress induces altered expression of genes encoding antioxidant enzymes in seedlings of a Brazilian indica rice (Oryza sativa L.). Plant Sci 166:323–331
Binzel ML, Reuveni M (1994) Cellular mechanisms of salt tolerance in plant cells. Hort Rev 16:33–69
Bohnert HJ, Jensen RG (1996) Strategies for engineering water-stress tolerance in plants. Trends Biotechnol 14:89–97
Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7:1099–1111
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55:2365–2384
Chen THH, Murata N (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5:250–257
Cheng AX, Lou YG, Mao YB, Lu S, Wang LJ, Chen XY (2007) Plants terpenoids: biosynthesis and ecological functions. J Integr Plant Biol 49:179–186
Chinnusamy V, Jagendorf A, Zhu JK (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448
Cuin TA, Shabala S (2005) Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant cell Physiol 46:1924–1933
Debnath M (2008) Responses of Bacopa monniera to salinity and drought stress in vitro. J Med Plants Res 2:347–351
Dhindsa RS, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J Bot 32:79–91
Elkahoui S, Hernandez JA, Abdelly C, Ghrir R, Limam F (2005) Effects of salt on lipid peroxidation and antioxidant enzyme activities of Catharanthus roseus suspension cells. Plant Sci 168:607–613
Erturk U, Sivritepe N, Yerlikaya C, Bor M, Ozdemir F, Turkan I (2007) Responses of the cherry rootstock to salinity in vitro. Biol Plant 51:597–600
Flors V, Paradís M, García-Andrade J, Cerezo M, González-Bosch C (2007) A tolerant behavior in salt-sensitive tomato plants can be mimicked by chemical stimuli. Plant Signal Behav 2:50–57
Ghorpade RP, Chopra A, Nikam TD (2011) Influence of biotic and abiotic elicitors on four major isomers of boswellic acid in callus culture of Boswellia serrata Roxb. Plant Omics J 4:169–176
Gratten SR, Grieve CM (1994) Mineral element acquisition and response of plants grown in saline environments. In: Pessaraki M (ed) Handbook of plant and crop stress. Marcel Dekker, Inc., New York, pp 203–227
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Heath RL, Packer L (1968) Photooxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hemeda HM, Klein BP (1990) Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci 55:184–185
Hepler PK (2005) Calcium: a central regulator of plant growth and development. Plant Cell 17:2142–2155
Hernández JA, Jiménez A, Mullineaux P, Sevilla F (2000) Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ 23:853–862
Imlay JA (2003) Pathways of oxidative damage. Ann Rev Microbiol 57:395–418
Irigoyen J, Emerich D, Sanchez-Diaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalafa (Medicago sativa) plants. Physiol Plant 84:55–60
Jaleel CA, Manivannan P, Lakshmanan GMA, Sridharan R, Panneerselvam R (2007) NaCl as a physiological modulator of proline metabolism and antioxidant potential in Phyllanthus amarus. C R Biol 330:806–813
Jithesh MN, Prashanth SR, Sivaprakash KR, Parida AK (2006) Antioxidative response mechanisms in halophytes: their role in stress defence. J Genet 85:237–254
Jogeswar G, Pallela R, Jakka NM, Reddy PS, Rao JV, Sreeniwasulu N, Kavi Kishor PB (2006) Antioxidative response in different Sorghum species under short term salinity stress. Acta Physiol Plant 28:465–475
Kanashiro S, Ribeiro RCS, Gonçalves AN, Demétrio VA, Jocys T, Tavares AR (2009) Effect of calcium on the in vitro growth of Aechmea blanchetiana (Baker) L. B. Smith plantlets. J Plant Nutr 32:867–877
Kavi Kishor PB, Sangam S, Amrutha RN, Sri Laxmi P, Naidu KR, Rao KRS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu P (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Kovacik J, Klejdus B, Hedbavny J, Backor M (2009) Salicylic acid alleviates NaCl-induced changes in the metabolism of Matricaria chamomilla plants. Ecotoxicology 18:544–554
Kozlowski TT (1997) Responses of woody plants to flooding and salinity. Tree Physiology Monograph No. 1, Heron Publishing, Victoria, Canada, pp 1–29
Kumar V, Shriram V, Nikam TD, Jawali N, Shitole MG (2009) Antioxidant enzyme activities and protein profiling under salt stress in indica rice genotypes differing in salt tolerance. Arch Agron Soil Sci 55:379–394
Lin JN, Kao C (1998) Water stress, ammonium and leaf senescence in detached rice leaves. Plant Growth Regul 28:165–169
Lokhande VH, Nikam TD, Suprasanna P (2010) Biochemical, physiological and growth changes in response to salinity in callus cultures of Sesuvium portulacastrum L. Plant Cell Tissue Organ Cult 102:17–25
Lokhande VH, Nikam TD, Patade VY, Ahire ML, Suprasanna P (2011) Effects of optimal and supra-optimal salinity stress on antioxidative defence, osmolytes and in vitro growth responses in Sesuvium portulacastrum L. Plant Cell Tissue Organ Cult 104:41–49
Lowry OH, Roenbrough NJ, Farr AL, Randal EJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lutts S, Kinet J, Bouharmont J (1996) NaCl induce senescence in leaves of rice (Oryza sativa) cultivars differing in salinity resistance. Ann Bot 78:389–398
Lv S, Nie LL, Fan PX, Wang XH, Jiang D, Chen XY, Li YX (2012) Sodium plays a more important role than potassium and chloride in growth of Salicornia europaea. Acta Physiol Plant 34:503–513
Maathuis FJ, Amtmann A (1999) K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann Bot 84:123–133
Maathuis FJM, Sanders D (1996) Mechanism of potassium absorption by higher plant roots. Physiol Plant 96:158–168
Marschner H (1995) Mineral nutrition of higher plants. Academic, London, p 889
Martinez JP, Stanley L, Schanck A (2004) Is osmotic adjustment required for water stress resistance in the Mediterranean shrub Atriplex halimus L.? J Plant Physiol 161:1041–1051
Meloni DA, Martínez CA (2009) Glycine betaine improves salt tolerance in vinal (Prosopis ruscifolia Griesbach) seedling. Braz J Plant Physiol 21:233–241
Molassiotis AN, Sotiropoulos T, Tanou G, Kofidis G, Diamantidis G, Therios I (2006) Antioxidant and anatomical responses in shoot culture of the apple rootstock MM 106 treated with NaCl, KCl, mannitol or sorbitol. Biol Plant 50:331–338
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–497
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol Plant Mol Biol 49:249–279
Parale A, Barmukh R, Nikam T (2010) Influence of organic supplements on production of shoots and callus biomass and accumulation of bacoside in Bacopa monniera (L.) Pennell. Physiol Mol Biol Plants 16:167–175
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plant: a review. Ecotoxicol Environ Safe 60:324–349
Parida AK, Das AB, Mohanty P (2004) Defense potentials to NaCl in a mangrove, Bruguiera parviflora: differential changes of isoforms of some antioxidative enzymes. J Plant Physiol 161:531–542
Pitman MG, Lauchli A (2002) Global impact of salinity and agricultural ecosystems. In: Lauchli A, Luttge V (eds) Salinity: Environment - plants molecules. Kluwer, The Netherlands, pp 3–20
Rascio A, Russo M, Mazzucco L, Platani C, Nicastro G, Fonzo ND (2001) Enhanced osmotolerance of a wheat mutant selected for potassium accumulation. Plant Sci 160:441–448
Raven JA (1985) Regulation of pH and generation of osmolarity in vascular land plants: costs and benefits in relation to efficiency of use of water, energy and nitrogen. New Phytol 101:25–77
Rios-Gonzales K, Erdei L, Lips SH (2002) The activity of antioxidant enzymes in maize and sunflower seedlings as affected by salinity and different nitrogen sources. Plant Sci 162:923–930
Sabir F, Sangwan RS, Kumar R, Sangwan NS (2012) Salt stress-induced responses in growth and metabolism in callus cultures and differentiating in vitro shoots of Indian ginseng (Withania somnifera Dunal). J Plant Growth Regul 31:537–548
Salama S, Trivedi S, Busheva M, Arafa A, Garab G, Erdei L (1994) Effects of NaCl salinity on growth, cation accumulation, chloroplast structure and function in wheat cultivars differing in salt tolerance. J Plant Physiol 144:241–247
Sangwan NS, Farooqi AHA, Sangwan RS (1994) Effect of drought on growth and essential oil metabolism in lemongrass species. New Phytol 128:173–179
Santos CLV, Campos A, Azevedo H, Caldeira G (2001) In situ and in vitro senescence induced by KCl stress: nutritional imbalance, lipid peroxidation and antioxidant metabolism. J Exp Bot 52:351–360
Scandalios JG, Guan L, Polidoros AN (1997) Catalases in plants: gene structure, properties, regulation, and expression. In: Scandalios JG (ed) Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 343–406
Sekmen AH, Türkan I, Takio S (2007) Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritime L. and salt-sensitive Plantago media L. Physiol Plant 131:399–411
Sergio L, Paola AD, Cantore V, Pieralice M, Cascarano NA, Bianco VV, Venere DD (2012) Effect of salt stress on growth parameters, enzymatic antioxidant system, and lipid peroxidation in wild chicory (Cichorium intybus L.). Acta Physiol Plant 34:2349–2358
Shalata A, Tal M (1998) The effects of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol Plant 104:169–174
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319
Taiz L, Zeiger E (2010) Plant Physiology, 5th edn. Sinauer Associates Inc, Sunderland, p 115
Tarchoune I, Degl’Innocenti E, Kaddour R, Guidi L, Lachaâl M, Navari-Izzo F, Ouerghi Z (2012) Effects of NaCl or Na2SO4 salinity on plant growth, ion content and photosynthetic activity in Ocimum basilicum L. Acta Physiol Plant 34:607–615
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Vaidyanathan H, Sivakumar P, Chakrabarty R, Thomas G (2003) Scavenging of reactive oxygen species in NaCl stressed rice (Oryza sativa L.) – differential response in salt tolerant and sensitive cultivars. Plant Sci 165:1411–1418
Wahid A, Ghazanfar A (2006) Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J Plant Physiol 163:723–730
Watoo P, Waraporn P, Hiroyuki T, Kanchalee J, Sakchai W, Kornkanok I (2007) Comparison of various extraction methods of Bacopa monniera. Naresuan Univ J 15:29–34
Acknowledgments
The authors wish to acknowledge the financial support from the Department of Botany, University of Pune, under UGC, SAP-DRS III program sanctioned by UGC, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahire, M.L., Laxmi, S., Walunj, P.R. et al. Effect of potassium chloride and calcium chloride induced stress on in vitro cultures of Bacopa monnieri (L.) Pennell and accumulation of medicinally important bacoside A. J. Plant Biochem. Biotechnol. 23, 366–378 (2014). https://doi.org/10.1007/s13562-013-0220-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-013-0220-z