Abstract
The study reports the transient expression of gusA gene in embryogenic cells using three banana derived fruit-specific promoters. Banana embryogenic cells were transformed with a pCAMBIA-1301 derived plasmid construct harboring gusA gene driven by either chitinase, glucanase or expansin promoters derived from banana. The transient expression of β-glucuronidase was studied 5 days after co-cultivation with Agrobacterium harboring the expression plasmids. The transformed embryogenic cells were treated with different inducers of ethylene such as ethephon, methyl jasmonate, methyl salicylate, abscisic acid and indole acetic acid. The maximum expression of 64099.78 pmoles 4-MU/h/mg total protein was noted with expansin promoter when the cells were treated with the combination of ethephon (0.25 mM) and MJ (10 mM). The results suggest that these promoters can be used to achieve fruit-specific expression of useful transgenes in banana. The results should prove to be an important guide for short term expression studies for promoter validation and gene screening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Banana (Musa spp.) is one of the most important food crops after rice, maize and wheat in tropical and subtropical countries. Indian banana industry is dominated by Cavendish cultivars specifically Robusta due to its high yield, ability to withstand strong winds; production of large bunches which satisfies the export standards, early maturity and fruits with consistent pulp having pleasant flavor and attractive color. Conventional techniques of crop improvement have achieved rather limited success in banana due to its complicated life cycle. This makes biotechnological approaches, in particular genetic engineering, especially attractive for banana improvement. Genetic transformation techniques can be used for incorporation of commercially important characters in proven elite cultivars without compromising their fundamental genetic integrity. There have been several reports on genetic transformation of banana. Transgenic banana plants have been obtained in different cultivars using biolistics (Sagi et al. 1995; Becker et al. 2000), Agrobacterium-mediated genetic transformation (May et al. 1995; Ganapathi et al. 2001; Ghosh et al. 2009), Agrobacterium-mediated transient expression in immature fruits (Matsumoto et al. 2009) and electroporation (Sagi et al. 1994). Stable genetic transformation techniques become very tedious and time consuming when the final aim is to carry out short term expression studies for promoter validation and gene screening. Hence, there is a need for an efficient transient genetic transformation system in banana.
Beaudry et al. (1987) reported about climacteric nature and short post-harvest life of banana fruit. Ripening in banana is a complex developmental process that involves changes in gene expression and enzyme activity (Brady 1987; Fischer and Bennett 1991). A wide range of hydrolases that catalyze the process of fruit softening are known to be activated at transcriptional and translational level. However, various molecular and genetic studies suggested that these hydrolases may not be individually responsible for softening (Giovannoni et al. 1989; Smith et al. 1998; Brummell et al. 1999; Brummell and Harpster 2001). In this study, we have selected promoters of three such hydrolases, expansin, chitinase and glucanase that are known to be active at various stages of banana fruit ripening (Trivedi and Nath 2004; Peumans et al. 2002; Kesari et al. 2007). Earlier work recommended that expansins act as mediators of cell wall softening and increase in fruit size during fruit development in various species such as tomato, pear, and banana (Hiwasa et al. 2003; Kitagawa et al. 1995; Rose et al. 1997; Trivedi and Nath 2004). Expansin promoter used in this study has previously been isolated and characterized (Trivedi and Nath 2004). It is strongly up-regulated by ethylene and is expressed only during ripening.
Isolation and characterization of chitinase from unripe banana pulp has been reported by Clendennen et al. (1997). While chitinases are basically associated with pathogenesis related functions, research has shown that in banana pulp this abundant protein is closely associated with fruit ripening and is potentially a transient storage protein (Peumans et al. 2002). Another cell wall hydrolase upregulated during banana ripening is β-1,3- glucanase (Kesari et al. 2007; MedinaSuarez et al. 1997; Clendennen and May 1997 and Peumans et al. 2000). Although the basic function of this hydrolase is defense related but there are evidences to prove its accumulation and involvement in banana fruit ripening.
The promoters reported in this study are strongly upregulated during banana ripening. While glucanase is more predominant in ripe banana pulp, chitinase accumulation is reported to be more in unripe fruit. Expansin expression increases with the progression of ripening. We believe that this report should be a guide in choosing a suitable promoter for the expression of recombinanat proteins and edible vaccines in bananas. The report aims at the optimization of a transient gene expression system in banana that would direct rapid gene screening and promoter validation.
Materials and methods
Cloning of chitinase, expansin and glucanase promoter in place of CaMV 35S promoter in pCAMBIA-1301 binary vector
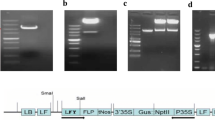
Three banana fruit-specific promoters namely chitinase (BAC) promoter (Genbank accession no. AY525367), expansin (EXP) promoter (Genbank accession no. AF539640) and glucanase (GLU) promoter (US patent application 20030226175A1) were amplified from banana cv. Rasthali derived genomic DNA using the following primers. BAC: Fw 5′ ATTACTGCAGTCAAAGTTAGAAAAATCTTTACCAAGAGC 3′, Rv 5′ TGATAGATCTACCATAGTGAATGAGGAGGCTATCG 3′; EXP: Fw 5′ AGCTAAGCTTAAACTCCACCGATAGCCATCTTC 3′, Rv 5′ AGCTAGATCTACCATTGGAATTATTCCCACTAAAGAAAGATTGGA 3′; GLU: Fw 5′ ATTACTGCAGGTATTTAGCCTAACATTCCCGGACTC 3′, Rv 5′ AGCTAGATCTACCATGACAACAACTCTGTCAAAAGG 3′. Subsequently, the CaMV 35S promoter driving β-glucuronidase in pCAMBIA 1301 binary vector (CAMBIA, Canberra, Australia) was replaced by these promoters using appropriate restriction enzymes to obtain three binary vectors called pBAC-1301, pEXP-1301 and pGLU-1301 respectively. These binary vectors were mobilized into the Agrobacterium tumefaciens strain EHA105 (Hood et al. 1993) by electroporation. The T-DNA portion of these vectors is depicted in Fig. 1.
T-DNA region of (A) pCAMBIA-1301, (B) pBAC-1301, (C) pEXP-1301 and (D) pGLU-1301. RB, right border; LB, left border; hpt, hygromycin phosphotransferase coding sequence; pD35S, double enhanced 35S promoter; pCaMV35S, Cauliflower Mosaic Virus 35S promoter; pBAC, chitinase promoter; pEXP, Expansin promoter; pGLU, Glucanase promoter
Agrobacterium strain, binary vector and preparation of Agrobacterium suspension
For transformation of banana cultivar Robusta ECS, the A. tumefaciens strain EHA105 harboring pCAMBIA-1301, pBAC-1301, pEXP-1301 or a pGLU-1301 binary vector was used. A single Agrobacterium colony was grown overnight in liquid YENB (0.75% w/v yeast extract, 0.8% w/v nutrient broth) medium supplemented with 50 mgL-1 kanamycin at 27°C with an orbital shaking of 150 rpm. Subsequently, the culture was resuspended in the same medium at an OD600 nm of 0.1 and grown under same conditions until an OD600 nm of 0.8–1.0 was reached. The suspension was centrifuged at 6000 g for 10 min and the pellet was subsequently resuspended at a final OD600 nm of 0.1 in M2 medium (Cote et al. 1996) supplemented with 100 μM acetosyringone.
Transformation of ECS and optimization of transient expression of β-glucuronidase
The initiation and maintenance of ECS was carried out as reported previously (Ghosh et al. 2009). Seven days post sub-cultured cells were sieved through an 85-μm sieve to remove large cell clumps. Approximately, 0.05 g settled cell volume of sieved ECS was co-cultivated with Agrobacterium (prepared as above) in liquid M2 medium supplemented with 100 μM acetosyringone. ECS were aspirated onto glass fiber discs with a Buchner apparatus and transferred to semi-solid M2 medium containing 100 μM acetosyringone and maintained in dark for 6 days. Post-coculture ECS were scraped from the glass fiber filters and induced with the respective agent. Additionally, 200 mg fresh weight of banana ECS transformed with pCAMBIA-1301 were put for GUS histochemical staining on the 4th, 5th and 6th day post infection to ascertain the day at which the transient expression is maximum.
Induction of co-cultivated ECS
Five days post co-cultivation, banana ECS transformed with different constructs were induced with ethephon at different concentrations (0.25 mM, 0.5 mM, 1 mM and 2 mM). ECS were scraped from the glass fiber disc and suspended in liquid M2 medium to which ethephon was added. The cells were kept on a gyratory shaker at 100 rpm in dark and at a temperature of 25 ± 2°C for 15 h. Subsequently, cells were harvested and β-glucuronidase specific activity was measured using the MUG- based fluorometric assay (Jefferson 1987). Total protein extracts obtained using GUS extraction buffer (50 mM NaHPO4 pH 7.0, 10 mM β-mercaptoethanol, 10 mM EDTA, 0.1% (w/v) sodium lauryl sarcosine, 0.1% (w/v) Triton X-100) were used for GUS specific activity using 2 mM`1xd MUG assay buffer. Fluorescence was measured using a fluorimeter with emission and excitation filters set at 465 nm and 360 nm respectively. Total protein concentration in samples was determined as described previously (Bradford 1976).
After ascertaining the ethephon concentration at which maximum β-glucuronidase expression was obtained for different constructs, effect of other elicitors on β glucuronidase expression was determined. Elicitors used for above experiments were MJ, MS, ABA and IAA. For ABA treatment, 5 days post transformed ECS were cultured in liquid M2 medium supplemented with 5 mM, 10 mM and 15 mM ABA for 15 h under the conditions mentioned above. For studying the synergistic action of ethephon and ABA on promoter activity, ABA treatment was followed by ethephon induction as described above. Similarly for studying the combinatorial effect of ethephon with IAA, ethephon with MJ and ethephon with MS, transformed ECS were cultured in liquid M2 medium supplemented with 5 mM, 10 mM and 15 mM IAA, MJ or MS respectively for 15 h under the conditions mentioned above. This was followed by overnight ethephon induction after which fluorometric assay was performed. Three replicates were used for each experiment.
Results
Optimization of transient expression of β-glucuronidase
Actively growing banana ECS maintained in liquid M2 medium was used for transformation with pCAMBIA-1301 for determining the maximum transient expression of β-glucuronidase (Fig. 2A, B). The gusA gene present in the T-DNA of pCAMBIA- 1301 is interrupted by a modified castor bean catalase intron that ensures the expression of β-glucuronidase only from eukaryotic expression machinery. Hence the banana ECS stained at 4th, 5th and 6th day post infection was successfully used to ascertain the efficiency of transient expression of β-glucuronidase. The expression of β-glucuronidase was found to be highest at the 5th day post infection (Fig. 2C, D). Therefore, all the further elicitor treatments were subsequently carried out at 5th day post infection.
Transient expression of promoters in cell cultures. A: Non-transformed embryogenic cells in liquid M2 medium B: Seven days post sub-cultured non-transformed embryogenic cells (sieved through a 85-μm sieve to remove large cell clumps) as observed in suspension cultures C, D: Transient expression of β-glucuronidase in transformed embryogenic cells (Note the maximum expression in 5 days post infection)
β-glucuronidase expression with chitinase promoter under different elicitors
Chitinase promoter responded well to ethylene induction with the maximum expression level of 21,545.17 pmoles 4-MU/h/mg total protein being observed at a concentration of 0.25 mM ethephon. This was approximately five times higher than uninduced cells. MJ in combination with ethephon (0.25 mM) further enhanced the expression level which reached up to 30613.77 pmoles 4-MU/h/mg total protein. ABA in combination with ethephon was not very effective with chitinase promoter as expression level dropped at all ABA concentrations. However, ABA alone could give an expression level that was at par with ethephon. At an ABA concentration of 5 mM, an expression level of 20,555 pmoles 4-MU/h/mg total protein was recorded. Very significant induction was not observed with MS in combination with ethephon. At all concentrations of methyl salicylate the expression level was lower than that with ethephon alone. IAA led to a significant reduction in expression level at all concentrations. Expression as low as 5848.16 pmoles 4-MU/h/mg total protein was recorded with IAA 15 mM. The expression pattern obtained using chitinase promoter in response to different treatments is depicted in Fig. 3.
Effect of different elicitors on β-glucuronidase expression by chitinase promoter (A) Transformed embryogenic cells treated with different ethephon concentrations (0, 0.25, 0.5, 1, 2 mM). Maximum transient gene expression was obtained with 0.25 mM ethephon, thus this concentration was used for further combination studies. Control stands for untransformed, untreated cells. (B) Transformed embryogenic cells treated with different ABA concentration (0, 5, 10, 15 mM). (C) Transformed embryogenic cells treated with combination of ethephon (0.25 mM) and different concentrations of ABA (5, 10, 15 mM). (D) Transformed embryogenic cells treated with combination of ethephon (0.25 mM) and different concentrations of methyl jasmonate (5, 10, 15 mM). (E) Transformed embryogenic cells treated with combination of ethephon (0.25 mM) and different concentrations of methyl salicylate (5, 10, 15 mM). (F) Transformed embryogenic cells treated with combination of ethephon (0.25 mM) and different concentrations of IAA (5, 10, 15 mM). Data represents means ± SD of three independent experiments
β-glucuronidase expression with glucanase promoter under different elicitors
The response of glucanase promoter with ethephon was better than chitinase promoter. Expression levels showed more than two fold increase with ethephon treatment. A maximum expression level of 30173.95 pmoles 4-MU/h/mg total protein was recorded at 1 mM ethephon concentration. For further combinatorial induction studies 1 mM ethephon was choosen. A further two fold increase in expression level was achieved when the transformed cells were treated with a combination of MJ and ethephon. Maximum expression with this combination was 49222.97 pmoles 4-MU/h/mg total protein at 10 mM MJ concentration. The combination of ethephon and ABA (5 mM) further enhanced the expression level to 54743.16 pmoles 4-MU/h/mg total protein. ABA alone could not enhance transgene expression which remained as low as 19293.49 pmoles 4-MU/h/mg total protein at 10 mM ABA. MS (5 mM) could induce glucanase promoter as the transgene expression level was 39043.77 pmoles 4-MU/h/mg total protein, which is slightly higher than the value recorded with ethephon alone. IAA led to a significant reduction in expression level at all concentrations. Expression as low as 13011.99 pmoles 4-MU/h/mg total protein was recorded with IAA 15 mM. The expression pattern obtained using glucanase promoter in response to different treatments is depicted in Fig. 4.
Effect of different elicitors on β-glucuronidase expression by glucanase promoter (A) Transformed embryogenic cells treated with different ethephon concentrations (0, 0.25, 0.5, 1, 2 mM). Maximum transient gene expression was obtained with 1 mM ethephon, thus this concentration was used for further combination studies. Control stands for untransformed, untreated cells. (B) Transformed embryogenic cells treated with different ABA concentration (0, 5, 10, 15 mM). (C) Transformed embryogenic cells treated with combination of ethephon (1 mM) and different concentrations of ABA (5, 10, 15 mM). (D) Transformed embryogenic cells treated with combination of ethephon (1 mM) and different concentrations of methyl jasmonate (5, 10, 15 mM). (E) Transformed embryogenic cells treated with combination of ethephon (1 mM) and different concentrations of methyl salicylate (5, 10, 15 mM). (F) Transformed embryogenic cells treated with combination of ethephon (1 mM) and different concentrations of IAA (5, 10, 15 mM). Data represents means ± SD of three independent experiments
β-glucuronidase expression with expansin promoter under different elicitors
Expansin promoter on induction with ethephon showed almost three times increase in transgene expression at 0.25 mM as compared to untreated transformed cells with a maximum expression of 31483.7 pmoles 4-MU/h/mg total protein. For further combination induction studies 0.25 mM ethephon was choosen. A further two fold increase in gene expression was recorded at all concentrations of MJ in combination with ethephon with maximum expression of 64099.78 pmoles 4-MU/h/mg total protein at MJ 10 mM. With ABA any further enhancement of gene expression was not observed. At all ABA and ethephon combinations expression level remained approximately 20,000 pmoles 4-MU/h/mg total protein. With ABA alone, maximum expression of 15,987 pmoles 4-MU/h/mg total protein was recorded at ABA 5 mM. MS also could not produce any further enhancement in gene expression as highest expression was with ethephon alone. Gene expression showed a significant two fold reduction at all concentrations of IAA. The expression pattern obtained using expansin promoter in response to different treatments is depicted in Fig. 5.
Effect of different elicitors on β-glucuronidase expression by expansin promoter (A) Transformed embryogenic cells treated with different ethephon concentrations (0, 0.25, 0.5, 1, 2 mM). Maximum transient gene expression was obtained with 0.25 mM ethephon, thus this concentration was used for further combination studies. Control stands for untransformed, untreated cells. (B) Transformed embryogenic cells treated with different ABA concentration (0, 5, 10, 15 mM). (C) Transformed embryogenic cells treated with combination of ethephon (0.25 mM) and different concentrations of ABA (5, 10, 15 mM). (D) Transformed embryogenic cells treated with combination of ethephon (0.25 mM) and different concentrations of methyl jasmonate (5, 10, 15 mM). (E) Transformed embryogenic cells treated with combination of ethephon (0.25 mM) and different concentrations of methyl salicylate (5, 10, 15 mM). (F) Transformed embryogenic cells treated with combination of ethephon (0.25 mM) and different concentrations of IAA (5, 10, 15 mM). Data represents means ± SD of three independent experiments
Discussion
The main objective of this study was to study three banana derived fruit specific promoters using an efficient transient expression of GUS reporter gene in ECS of an important banana cultivar Robusta. Ethylene has long been regarded as the main regulator of ripening in climacteric fruits. Earlier studies on the physiology of banana ripening have indicated that ABA and IAA could modulate ripening (Pathak and Sanwal 1999; Jiang et al. 2000). Recently some reports have come up which indicate the effect of MJ and MS on ripening related genes (Kesari et al. 2010; Ankala et al. 2009). In this study, we have tried to study the expression pattern of three ripening related promoters from banana. We believe that the transient gene expression system reported herein could be very useful for gene screening and promoter validation studies for a difficult—to transform species like banana.
Matsumoto et al. (2009) reported a transient expression of GUS in banana immature fruits using vacuum infiltration of Agrobacterium. Further the authors stated that the GUS-expressed areas differed from one repetition to the other. Thus, in promoter gene validation, for example, two or more promoter genes similarly expressing may still be difficult to be compared by this system. However, in our studies using transient expression in embryogenic cell suspension cultures we have compared the relative transient expression levels of three promoters and the system can be employed for validation of different constitutive or tissue specific promoters.
ABA plays a significant role in ripening of climacteric and non-climacteric fruits through interaction with ethylene. Increase in respiration and other ripening related changes was observed for ABA treated McIntosh apples (Vendrell and Lara 2003). Further Zhang et al. (2009) observed in tomato that ABA accumulation in seeds and flesh of fruits preceded ethylene production. Differential regulation of alcohol dehydrogenase gene during mango ripening by ABA was observed by Singh et al. (2010). Inducing effects of ABA on cell wall hydrolases during banana ripening has been reported by Lohani et al. (2004). In our study, ABA in combination with ethephon enhanced the expression of glucanase promoter by two fold as compared to ethephon alone. ABA alone could induce expansin promoter which was comparable to ethephon induction.
There are multiple factors in addition to ethylene which can modulate ripening. The characterization of tomato mutants like rin (Vrebalov et al. 2002), nor (Adams-Phillips et al. 2004) and cnr (Manning et al. 2006) is particularly relevant because it demonstrates that other factors, generally named ‘developmental factors’, act upstream of ethylene and their control of the ripening process is no less important than that played by ethylene (Giovannoni 2004). Further Jones et al. (2002), reported the expression of auxin/IAA encoding genes in tomato fruits. This suggests that IAA might also be a part of the mechanisms that control the ripening of climacteric fruits. Role of IAA in ripening is rather ambiguous. Though increased synthesis of IAA during ripening has been reported, but this does not establish any stimulatory effect of IAA on cell wall hydrolases. Bottcher et al. (2010), reported that IAA levels decline at the onset of ripening. This study suggested that application of IAA to fleshy fruits can delay ripening. Lohani et al. (2004) reported the suppressive effects of IAA on cell wall hydrolases. Our findings are in accordance with the above reports as IAA has suppressed the ethylene effects on all the three promoters tested.
Along with ethylene, methyl jasmonate has been considered as a safe agent for inducing fruit ripening. There are reports that showed inducing effects of jasmonate and salicylate on expression of pathogenesis-related proteins and chitinase during ripening (Kesari et al. 2010; Ankala et al. 2009). But there is not much evidence to prove the exact effect of jasmonate and salicylate on cell wall hydrolases. These elicitors might act upstream of ethylene and control the ripening process either directly or indirectly (Ankala et al. 2009). This is the reason for which we have treated the cells with MJ and MS prior to ethylene treatment. We observed inducing effects of MJ with all the three promoters, highest in expansin followed by glucanase and chitinase. Chitinase and expansin did not show much response to MS treatment. Slight induction in glucanase was observed with MS. A double pronged approach has been applied in this study. To begin with, we have optimized a transient expression system that can be applied for rapid expression studies in banana. All the stable transformation protocols reported so far are very tedious and time consuming. Generation of transgenic plants suitable for biochemical and molecular analysis takes anywhere between 9 and 18 months depending upon the genotype and cultivar. The present protocol makes it possible to validate genes and promoters in just 5 days. The quantification studies of the expression level of a reporter gene like β-glucuronidase gives a fairly good idea about the strength of the different promoters under different ripening conditions. In our studies, among the three promoters tested glucanase promoter showed a better response to ethylene and ABA treatments, indicating the usefulness of this promoter for fruit-specific expression of target genes. The information generated is helpful in choosing suitable promoters not only for the production of vaccines but also for the expression of other commercially important recombinant proteins in transgenic banana fruits.
Abbreviations
- ABA:
-
Abscisic acid
- ECS:
-
Embryogenic cell suspensions
- IAA:
-
Indole acetic acid
- MJ:
-
Methyl jasmonate
- MS:
-
Methyl salicylate
- MUG:
-
4-methylumbelliferyl-β - D-glucuronide
References
Adams-Phillips L, Barry C, Giovannoni J (2004) Signal transduction systems regulating fruit ripening. Trends Plant Sci 9:331–338
Ankala A, Luthe DS, Williams WP, Wilkinson JR (2009) Integration of ethylene and jasmonic acid signaling pathways in the expression of maize defense protein Mir1-CP. Mol Plant Microbe Interact 22:1555–1564
Beaudry RM, Paz N, Black CC, Kays SJ (1987) Banana ripening: implications of changes in internal ethylene and CO2 concentrations, pulp fructose 2,6-bisphosphate concentration, and activity of some glycolytic enzymes. Plant Physiol 85:277–282
Becker DK, Dugdale B, Smith MK, Harding RM, Dale JL (2000) Genetic transformation of Cavendish banana (Musa spp. AAA group) cv ‘Grand Nain’ via microprojectile bombardment. Plant Cell Rep 19:229–234
Bottcher C, Keyzers RA, Boss PK, Davies C (2010) Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J Exp Bot 61:3615–3625
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brady CJ (1987) Fruit ripening. Annu Rev Plant Phys 38:155–178
Brummell DA, Harpster MH (2001) Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol 47:311–340
Brummell DA, Hall BD, Bennett AB (1999) Antisense suppression of tomato endo-1,4-beta-glucanase Cel2 mRNA accumulation increases the force required to break fruit abscission zones but does not affect fruit softening. Plant Mol Biol 40:615–622
Clendennen SK, May GD (1997) Differential gene expression in ripening banana fruit. Plant Physiol 115:463–469
Clendennen SK, Kipp PB, May GD (1997) The role of ethylene in banana fruit ripening. In: Kanellis et al. (ed) Biology and biotechnology of the plant hormone ethylene. Kluwer, Dordrecht, The Netherlands, pp 141–148
Cote FX, Domergue R, Monmarson S, Schwendiman J, Teisson C, Escalant JV (1996) Embryogenic cell suspensions from the male flower of Musa AAA cv. Grand Nain. Physiol Plant 97:285–290
Fischer RL, Bennett AB (1991) Role of cell-wall hydrolases in fruit ripening. Annu Rev Plant Phys 42:675–703
Ganapathi TR, Higgs NS, Balint-Kurti PJ, Arntzen CJ, May GD, Van Eck JM (2001) Agrobacterium-mediated transformation of embryogenic cell suspensions of the banana cultivar Rasthali (AAB). Plant Cell Rep 20:157–162
Ghosh A, Ganapathi TR, Nath P, Bapat VA (2009) Establishment of embryogenic cell suspension cultures and Agrobacterium-mediated transformation in an important Cavendish banana cv. Robusta (AAA). Plant Cell Tiss Org 97:131–139
Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell 16:S170–S180
Giovannoni JJ, Dellapenna D, Bennett AB, Fischer RL (1989) Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell 1:53–63
Hiwasa K, Rose JKC, Nakano R, Inaba A, Kubo Y (2003) Differential expression of seven alpha-expansin genes during growth and ripening of pear fruit. Physiol Plantarum 117:564–572
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene-transfer to plants. Transgenic Res 2:208–218
Jefferson RA (1987) Assaying chimeric plant genes: the GUS fusion system. Plant Mol Biol Rep 5:387–405
Jiang Y, Joyce DC, Macnish AJ (2000) Effect of abscisic acid on banana fruit ripening in relation to the role of ethylene. J Plant Growth Regul 19:106–111
Jones B, Frasse P, Olmos E, Zegzouti H, Li ZG, Latche A, Pech JC, Bouzayen M (2002) Down-regulation of DR12, an auxin-response-factor homolog, in the tomato results in a pleiotropic phenotype including dark green and blotchy ripening fruit. Plant J 32:603–613
Kesari R, Trivedi PK, Nath P (2007) Ethylene-induced ripening in banana evokes expression of defense and stress related genes in fruit tissue. Postharvest Biol Technol 56:64–70
Kesari R, Trivedi PK, Nath P (2010) Gene expression of pathogenesis-related protein during banana ripening and after treatment with 1-MCP. Postharvest Biol Technol 6:136–143
Kitagawa Y, Kanayama Y, Yamaki S (1995) Isolation of beta-galactosidase fractions from japanese pear—activity against native cell-wall polysaccharides. Physiol Plantarum 93:545–550
Lohani S, Trivedi PK, Nath P (2004) Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: effect of 1-MCP, ABA and IAA. Postharvest Biol Technol 31:119–126
Manning K, Tor M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, Seymour GB (2006) A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 38:948–952
Matsumoto K, Cabral GB, Teixeira JB, Monte DC (2009) Agrobacterium-mediated transient expression system in banana immature fruits. African J Biotech 8:4039–4042
May GD, Afza R, Mason HS, Wiecko A, Novak FJ, Arntzen CJ (1995) Generation of transgenic banana (Musa acuminata) plants via agrobacterium-mediated transformation. Bio-Technol 13:486–492
MedinaSuarez R, Manning K, Fletcher J, Aked J, Bird CR, Seymour GB (1997) Gene expression in the pulp of ripening bananas—two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis of in vitro translation products and cDNA cloning of 25 different ripening-related mRNAs. Plant Physiol 115:453–461
Pathak N, Sanwal GG (1999) Regulation of the ripening of banana (Musa acuminata) fruits by chemicals. Indian J Agr Sci 69:17–20
Peumans WJ, Barre A, Derycke V, Rouge P, Zhang WL, May GD, Delcour JA, Van Leuven F, Van Damme EJM (2000) Purification, characterization and structural analysis of an abundant beta-1,3-glucanase from banana fruit. Eur J Biochem 267:1188–1195
Peumans WJ, Proost P, Swennen RL, Van Damme EJM (2002) The abundant class III chitinase homolog in young developing banana fruits behaves as a transient vegetative storage protein and most probably serves as an important supply of amino acids for the synthesis of ripening-associated proteins. Plant Physiol 130:1063–1072
Rose JKC, Lee HH, Bennett AB (1997) Expression of a divergent expansin gene is fruit-specific and ripening-regulated. Proc Natl Acad Sci USA 94:5955–5960
Sagi L, Remy S, Panis B, Swennen R, Volckaert G (1994) Transient gene-expression in electroporated banana (Musa Spp., cv. ‘Bluggoe’, Abb group) protoplasts isolated from regenerable embryogenetic cell-suspensions. Plant Cell Rep 13:262–266
Sagi L, Panis B, Remy S, Schoofs H, Desmet K, Swennen R, Cammue BPA (1995) Genetic-transformation of banana and plantain (Musa Spp) via particle bombardment. Bio-Technol 13:481–485
Singh RK, Sane VA, Misra A, Ali SA, Nath P (2010) Differential expression of the mango alcohol dehydrogenase gene family during ripening. Phytochemistry 71:1485–1494
Smith DL, Starrett DA, Gross KC (1998) A gene coding for tomato fruit beta-galactosidase II is expressed during fruit ripening. Cloning, characterization, and expression pattern. Plant Physiol 117:417–423
Trivedi PK, Nath P (2004) MaExp1, an ethylene-induced expansin from ripening banana fruit. Plant Sci 167:1351–1358
Vendrell M, Lara I (2003) Relationship between ABA and ethylene in the induction of fruit ripening. In: Vendrell et al. (ed) Biology and biotechnology of plant hormone ethylene III IOS Press, Amsterdam, pp 278–283
Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J (2002) A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (Rin) locus. Science 296:343–346
Zhang M, Yuan B, Leng P (2009) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60:1579–1588
Acknowledgement
Antara Ghosh and V.A. Bapat thank Council of Scientific and Industrial Research (CSIR), Government of India, for Senior Research Fellowship (SRF) and Emeritus Scientist Fellowship (ESF) respectively. Board of Research in Nuclear Sciences (BRNS) Department of Atomic Energy (DAE), Government of India is duly acknowledged for financial funding of the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, A., Shekhawat, U.K.S., Ganapathi, T.R. et al. Analysis of banana fruit-specific promoters using transient expression in embryogenic cells of banana cultivar Robusta (AAA Group). J. Plant Biochem. Biotechnol. 21, 189–197 (2012). https://doi.org/10.1007/s13562-011-0070-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-011-0070-5