Abstract
Objective
Doxorubicin (DOX) is a widely used antineoplastic drug with several toxic effects. We investigated the protective effect of co-administration of baicalein (BL; a flavonoid) and losartan (LT; angiotensin receptor blocker) on DOX-induced hepatotoxicity.
Methods
Male Wistar albino rats were divided into these seven groups (n = 6): (1) Control group; (2) DOX group; (3) DOX + LT group (LT, 7 mg/kg/day orally); (4) DOX + BL low-dose group (BL, 5 mg/kg/day orally); (5) DOX + BL high-dose group (BL, 10 mg/kg/day orally); (6) DOX + LT + BL(5) low-dose group; and (7) DOX + LT + BL(10) high-dose group. After 2 weeks of LT and BL treatment, a dose of DOX (15 mg/kg, intraperitoneal) was administered to the rats in groups 2 to 6 and continued for seven more days.
Results
The use of serum levels of liver enzymes, tumor necrosis factor-α, interleukin (IL)-6, and IL1β and estimated the activity of thiobarbituric acid-reactive substances, glutathione, superoxide dismutase, catalase, and glutathione peroxidase in liver homogenates. In addition, we measured the activity of caspase-3, nitric oxide, inducible nitric oxide synthase, endothelial nitric oxide synthase, and nuclear factor kappa-B (NF-κB) p65 in hepatic cells and subjected the liver sections to histological examination. While the DOX-induced increase in serum liver enzymes, pro-inflammatory cytokines, and biomarkers was alleviated by BL and/or LT treatment, the BL + LT groups showed the most potent protective effects.
Conclusions
Our study demonstrates remarkable anti-oxidative and anti-inflammatory effects of BL and LT in rodents challenged with DOX. Concurrent administration of BL and LT showed a marked synergistic effect in restoration of histological features and alleviation of hepatic oxidative injury via inhibition of reactive oxygen species and anti-inflammatory mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthracycline antibiotics are a group of chemotherapeutic agents extensively used in the management of different types of malignancies. Doxorubicin (DOX) is an important member of this group; however, regular use is limited due to its associated toxicity (both acute and chronic). Reported adverse effects of DOX include pericarditis, transient arrhythmias, hepatotoxicity, renal damage, and progressive left ventricular dysfunction [1]. Several studies have investigated the pathways that mediate these adverse effects. Overproduction of reactive oxygen species (ROS) and subsequent oxidative damage are considered major contributing factors [2]. DOX-induced hepatotoxicity may also be mediated by oxidative stress and the production of ROS compounds, particularly in response to the semiquinone form of DOX [3]. In addition, some studies have suggested that DOX is subject to the reduction of one electron in the presence of NADPH–cytochrome P450 reductase, resulting in increased serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and bilirubin [4]. This was attributed to a decrease in the level of antioxidant enzymes such as glutathione (GSH), glutathione peroxidase (GPx), and glutathione reductase (GR), as well as to superoxide dismutase (SOD) and catalase (CAT) activity and high levels of MDA [4, 5].
A single dose of DOX administered to rats resulted in reduced cytochrome P-450 and GSH content in the liver [6]. Further clinical studies have shown DOX-induced liver toxicity, manifesting as excessive liver lipid peroxidation, following a single dose of DOX [7, 8]. Hepatic cells showed increased cell membrane permeability resulting in leakage of the cell contents into the serum, including liver enzymes. Serum transaminase levels are one of the most sensitive indicators of liver toxicity [8]. In addition, increased exposure to DOX has been shown to significantly increase the serum LDH level in experimental animals [9, 10]. Animals administered the DOX compound showed histopathological evidence of vascular blockage, mononuclear cell infiltration, sinusoidal dilatation, and hepatocyte degeneration. These findings suggest that DOX-induced hepatotoxicity may be mediated by impairment of the cell barrier [3]. The generation of free radicals, as seen during DOX therapy, may overwhelm the antioxidant defense system. This was validated in experimental animals by histopathological evidence of blood vessel congestion, expansion of the sinuses, infiltration of mononuclear cells, and degeneration of liver cells.
The involvement of the renin–angiotensin system (RAS) in DOX-mediated physiological toxicity has been reported in several studies, particularly in relation to cardiotoxicity. The octapeptide angiotensin II (Ang II) is a major pathophysiological factor in DOX-induced cardiomyopathy as well as several other cardiovascular diseases including hypertensive and ischemic heart disease [11]. Furthermore, Ang II has been shown to trigger the production of inflammatory biomarkers. Thus, medicines shown to inhibit the RAS cascade were evaluated in their ability to ameliorate DOX-induced toxic effects. Losartan (LT) is an Ang II receptor blocker shown to alleviate DOX-induced oxidative damage (Fig. 1A) [12]. Moreover, in other studies, LT was found to enhance the therapeutic efficacy of DOX [13].
A large number of studies have proven the therapeutic effect of flavonoids. The anti-inflammatory, antihistaminic, antiviral, and antioxidant effects of flavonoids are well documented. Flavonoids have been shown to prevent various diseases such as cancer, cardiovascular disease, and diabetes mellitus [14,15,16]. Baicalein (5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one; BL) is a common flavonoid usually isolated from the roots of Scutellaria baicalensis (Fig. 1B). Numerous studies have confirmed the anti-inflammatory, antidiabetic, antibacterial, antimicrobial, and anticarcinogenic effects of BL [17,18,19,20,21]. BL is one of the most potent antioxidants with protective effects on endothelial function and cardiovascular health. In vivo studies have shown that BL therapy leads to low blood pressure, which in turn inhibits the enzyme lipoxygenase. This has been shown to reduce the synthesis and release of arachidonic acid metabolites from blood vessels [22]. BL has also been shown to ameliorate diabetic neuropathy [23].
Research on combination therapies or poly-therapies has evoked considerable interest in recent years. For instance, concomitant treatment with quercetin, LT, p-coumaric acid, or NG was found to ameliorate DOX-induced cardiotoxicity [12, 24]. In the present study, we investigate the effect of co-administration with LT and BL on DOX-induced hepatic oxidative damage and inflammation in rodents.
Results and discussion
The mean body weight of DOX-injected rats was significantly (p < 0.001) reduced compared with the control group. The animals treated with LT, LT + BL (5), and LT + BL (10) for 3 weeks and subsequently challenged with a single dose of DOX had markedly (p < 0.05) elevated body weight compared to the DOX group. However, organ weights (g/100 g body weight) including liver, heart, and kidneys showed no significant changes statistically when compared: Control versus DOX or DOX versus DOX + LT or DOX versus DOX + BL (5) or DOX + BL (10) or DOX + LT + BL (5) or DOX + LT + BL (10) (Table 1).
The level of liver enzymes AST and ALT in the serum was significantly (p < 0.001) increased in the DOX compared with normal group (Fig. 2). The AST levels were significantly decreased in the DOX + BL (10; p < 0.01), DOX + LT + BL (5; p < 0.001), and DOX + LT + BL (10; p < 0.001) groups compared to DOX group. Similarly, ALT levels in these groups were also markedly decreased compared to the DOX group.
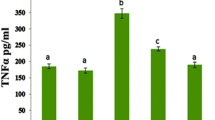
Pro-inflammatory cytokines in serum, including TNF-α, IL-6, and IL-1β, were significantly (p < 0.001) increased compared to the control group (Fig. 3). The TNF-α levels were markedly decreased in the DOX + BL (10; p < 0.05), DOX + LT + BL (5; p < 0.01), and DOX + LT + BL (10; p < 0.001) groups compared to the DOX group. The serum IL-6 levels were significantly decreased in the DOX + BL (5; p < 0.05), DOX + BL (10; p < 0.01), DOX + LT + BL (5; p < 0.01), and DOX + LT + BL (10; p < 0.01) groups compared to the DOX group. Serum IL-1β levels were significantly decreased in the DOX + BL (10; p < 0.01), DOX + LT + BL (5; p < 0.001), and DOX + LT + BL (10; p < 0.001) groups compared to the DOX group.
Effect of baicalein (BL) and/or losartan (LT) on doxorubicin (DOX)-induced changes in serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Where ‘A’ control, ‘B’ DOX, ‘C’ DOX + LT, ‘D’ DOX + BL (5), ‘E’ DOX + BL (10), ‘F’ DOX + LT + BL (5), and ‘G’ DOX + LT + BL (10). Data were expressed as mean ± SD (n = 6) and analyzed using one-way ANOVA followed by Student–Newman–Keuls as post-hoc test. aControl versus DOX group; bDOX versus DOX + LT or DOX versus DOX + BL (5) or DOX + BL (10) or DOX + LT + BL (5) or DOX + LT + BL (10). p values were considered significant when *p < 0.05, **p < 0.01, and ***p < 0.001
The inflammatory activities of caspase-3, NF-κB p65, NO, iNOS, and eNOS were markedly increased in the hepatic cells of DOX-challenged rats compared with the control group of animals (Fig. 4). Caspase-3 activity significantly enhanced the DOX + BL (10; p < 0.05), DOX + LT + BL (5; p < 0.001), and DOX + LT + BL (10; p < 0.001) groups compared to DOX-challenged rats. Also, NF + κB p65 activity was enhanced in DOX + LT (p < 0.05), DOX + BL (5; p < 0.05), DOX + BL (10; p < 0.01), DOX + LT + BL (5; p < 0.001), and DOX + LT + BL (10; p < 0.001) groups compared to the DOX group of rats. NO, iNOS, and eNOS activities in the hepatic cells of DOX + BL (10; p < 0.05), DOX + LT + BL (5; p < 0.01), and DOX + LT + BL (10; p < 0.001) rats were significantly enhanced compared to the DOX group of animals.
Effect of baicalein (BL) and/or losartan (LT) on doxorubicin (DOX)-induced changes in serum levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β). Where ‘A’ control, ‘B’ DOX, ‘C’ DOX + LT, ‘D’ DOX + BL (5), ‘E’ DOX + BL (10), ‘F’ DOX + LT + BL (5), and ‘G’ DOX + LT + BL (10). Results are expressed as mean ± SEM n = 6. The statistically significant difference between groups was considered. aControl versus DOX group; bDOX versus DOX + LT or DOX versus DOX + BL (5) or DOX + BL (10) or DOX + LT + BL (5) or DOX + LT + BL (10). *p < 0.05, **p < 0.01, and ***p < 0.001
Some oxidative stress markers in hepatic cells of DOX-challenged rats such as TBARS were significantly (p < 0.001) increased compared with the control group whereas others such as GSH were significantly decreased. The TBARS levels were markedly enhanced in the DOX + BL (10; p < 0.01), DOX + LT + BL (5; p < 0.001), and DOX + LT + BL (10; p < 0.001) groups compared to the DOX group. Hepatic GSH levels were markedly enhanced in the DOX + BL (10; p < 0.05), DOX + LT + BL (5; p < 0.01), and DOX + LT + BL (10; p < 0.001) groups compared to the DOX group (Fig. 5).
Effect of baicalein (BL) and/or losartan (LT) on doxorubicin (DOX)-induced changes in liver activities of caspase-3, NF-ĸB p65, NO, iNOS, and eNOS. Where ‘A’ control, ‘B’ DOX, ‘C’ DOX + LT, ‘D’ DOX + BL (5), ‘E’ DOX + BL (10), ‘F’ DOX + LT + BL (5), and ‘G’ DOX + LT + BL (10). Results are expressed as mean ± SEM n = 6. The statistically significant difference between groups was considered. aControl versus DOX group; bDOX versus DOX + LT or DOX versus DOX + BL (5) or DOX + BL (10) or DOX + LT + BL (5) or DOX + LT + BL (10). *p < 0.05, **p < 0.01, and ***p < 0.001
The enzymatic activities of CAT, SOD, GST, and GPx in hepatic cells were significantly (p < 0.001) decreased in DOX-challenged rats compared to controls. Liver SOD activity was markedly enhanced in the DOX + LT (p < 0.05), DOX + BL (5; p < 0.05), DOX + BL (10; p < 0.05), DOX + LT + BL (5; p < 0.001), and DOX + LT + BL (10; p < 0.0001) groups compared to those rats treated with DOX alone. The CAT activity, however, was only enhanced in the DOX + LT + BL (5; p < 0.05) and DOX + LT + BL (10; p < 0.01) groups compared to the DOX group. GPx activity in hepatic cells was significantly enhanced in DOX + BL (5; p < 0.005), DOX + BL (10; p < 0.01), DOX + LT + BL (5; p < 0.001), and DOX + LT + BL (10; p < 0.001) groups compared to the DOX group. The enzymatic activity of GST in hepatic cells was markedly enhanced in the DOX + BL (10; p < 0.005), DOX + LT + BL (5; p < 0.01), and DOX + LT + BL (10; p < 0.001) groups compared to DOX-challenged rats (Fig. 6).
Effect of baicalein (BL) and/or losartan (LT) on doxorubicin (DOX)-induced changes in thiobarbituric acid-reactive substances (TBARS) and glutathione (GST) levels in hepatic cells. Where ‘A’ control, ‘B’ DOX, ‘C’ DOX + LT, ‘D’ DOX + BL (5), ‘E’ DOX + BL (10), ‘F’ DOX + LT + BL (5), and ‘G’ DOX + LT + BL (10). Results are expressed as mean ± SEM n = 6. The statistically significant difference between groups was considered. aControl versus DOX group; bDOX versus DOX + LT or DOX versus DOX + BL (5) or DOX + BL (10) or DOX + LT + BL (5) or DOX + LT + BL (10). *p < 0.05, **p < 0.01, and ***p < 0.001
Histopathological changes are displayed in Fig. 7. The effect of baicalein (BL) or/and losartan (LT) on doxorubicin (DOX) is caused by histopathological changes in liver tissue. Hepatic cells appear in the control group normally (Fig. 7A) and when hepatocytes are treated by DOX (Fig. 7B), changes occur in cells that consist of congestion, central venous thrombosis, degeneration and polymorphism in liver cells, cytoplasmic eosinophils, platelet necrosis, bile duct diffusion, focal necrosis, and inflammation in the portal area compared to the control group that showed normal architecture. When hepatocytes are treated by LT (Fig. 7C), partial liver regeneration is observed. When treated with BL (Fig. 7D), hepatocytes show mild improvement and liver regeneration cells partially. However, when the liver cells are treated with the combination of both LT and BL, the results of the liver cells show complete regeneration (Fig. 7E).
Effect of baicalein (BL) and/or losartan (LT) on doxorubicin (DOX)-induced changes in superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione-S-transferase (GST) activities in hepatic cells. Where ‘A’ control, ‘B’ DOX, ‘C’ DOX + LT, ‘D’ DOX + BL (5), ‘E’ DOX + BL (10), ‘F’ DOX + LT + BL (5), and ‘G’ DOX + LT + BL (10). Results are expressed as mean ± SEM n = 6. The statistically significant difference between groups was considered. a Control versus DOX group; b DOX versus DOX + LT or DOX versus DOX + BL (5) or DOX + BL (10) or DOX + LT + BL (5) or DOX + LT + BL (10). *p < 0.05, **p < 0.01, and ***p < 0.001
DOX is an anthracycline cytotoxic agent used for the treatment of several tumor types. Its clinical use is associated with several adverse effects, particularly cardiovascular toxicity. Several studies have investigated the underlying mechanisms for these side effects. Studies have demonstrated extensive accumulation of ROS following DOX therapy [25]. In addition, DOX has been found to inhibit endogenous antioxidant capability through depletion of antioxidant molecules and suppression of antioxidant enzymes [26]. Consistent with previous studies, we found that DOX significantly reduced the activities of SOD, CAT, GPx, and GST, which are mitochondrial enzymes involved in detoxification of free radicals. Furthermore, DOX altered the structure of hepatic tissue considerably. This was associated with elevated levels of AST and ALT in the serum, which also indicates hepatic damage. These toxic effects are likely due to ROS-induced oxidative damage which impairs the integrity of cellular membranes. Previous studies have shown peroxidation of lipids in the cellular membranes induced by compounds such as hydroxyl radical, superoxide anion radical, and hydrogen peroxide, leading to augmented production of TBARS in DOX-treated rats [27]. Inflammation and oxidative stress are correlated. Oxidative stress induces the expression of multiple transcription factors including NF-κB and results in the production of inflammatory cytokines [28]. We observed a similar inflammatory response in this study, as DOX groups exhibited higher levels of TNF-α, IL-6, IL-1β, caspase-3, NO, iNOS, eNOS, and NF-κB compared to LT- and/or BL-treated groups.
Effect of baicalein (BL) and/or losartan (LT) on doxorubicin (DOX)-induced histopathological changes in hepatic tissue. a Section from control showing normal structure. b Section from DOX showing fatty degeneration, infiltration of inflammatory cells, vacuoles (arrow), and necrotic cells (head arrow). c Section from LT showing partially regenerating hepatocytes with small individual vacuoles (arrow). d Section from BL showing mild degenerative hepatic cells and partially regenerating hepatocytes. e Section from LT and BL (10) showing reversed tissue with complete regenerating hepatocytes. Scale bar = 50 µm
LT therapy alleviated the signs of oxidative damage and lipid peroxidation and preserved cardiac, hepatic, and renal function as well as histological structures in DOX-treated animals in this study. LT also lowered the DOX-induced increase in serum inflammatory cytokines. Numerous other studies have shown a similar protective efficacy of AT1 receptor antagonists against cellular oxidative and inflammatory damage induced by cytotoxic medications. In a study by Kabel et al. [29], BALB/c mice treated with AT1 receptor blocker, irbesartan, showed dose-dependent attenuation of DOX-induced hepatotoxicity via inhibition of oxidative stress, apoptosis, and inflammation. Another AT1 receptor blocker, fimasartan, was shown to prevent DOX-associated cardiotoxicity in rats [30]. In addition, olmesartan has been shown to ameliorate the nephrotoxic effects of DOX in rats [31]. Studies have suggested that Ang II may provoke cellular oxidative stress through stimulation of its AT1 receptor [32]. Therefore, blockage of this receptor by LT enhances the endogenous oxidative defensive cascades, exerting a protective biological effect.
Several studies have evaluated the medicinal value of natural chemical compounds (including flavonoids) against the cytotoxic effects of chemotherapy. BL markedly reduced DOX-induced hepatic oxidative damage in this study as demonstrated by the histological findings and lower serum aminotransferase levels. These protective effects are likely the result of the protective effects of BL against oxidative stress. BL restored antioxidant enzyme activity and GSH and lowered the level of lipid peroxidation products. Previous studies have demonstrated the anti-inflammatory [17], antidiabetic [18], antibacterial [19], antimicrobial [20], and anticarcinogenic [21] effects of BL. BL is a powerful antioxidant with vasculoprotective properties against oxidative stress-induced damage [22, 33]. The in vivo hypotensive effect of BL may be partially due to its inhibitory effect on lipoxygenase protein. These results may lead to reduced biosynthesis, which helps to release arachidonic acid-derived barrier materials [22]. Therapy with BL was shown in a previous study to alleviate diabetic neuropathy [23].
The treatment of animals with both LT and BL showed a protective effect against DOX-induced oxidative stress, inflammation, and histological injury. We observed a marked synergistic effect of the two therapies in this study. This may be due to the similar mechanism of action of the two drugs, especially in terms of their ROS scavenging capabilities. Other studies have also demonstrated the benefits of combining anti-RAS medications and natural compounds. Hadi et al. [34], found that co-administration of vitamin E (well-known antioxidant) and telmisartan ameliorated the DOX-induced cardiac inflammatory and oxidative response in rats. In a study by Matouk et al. [12], quercetin (another natural flavones) augmented the protective effect of LT in DOX-treated rodents through restoration of TNF-α, CK-MB, LDH, MDA, and nitric oxide levels and re-activation of SOD and CAT. In a study by Sahu et al. [35], BL therapy was shown to ameliorate DOX-induced cardiotoxicity in mice. The cardioprotective effect of BL is likely due to both myocardial non-enzymatic and enzymatic cancer prevention agents and the down-guideline of natural and NF-κB-controlled apoptotic pathway related to doxorubicin-instigated cardiomyocyte cell.
Our study has two main limitations. First, the experiment included male rats only. Gender differences and sex hormones may however influence the metabolic and physiological response to drug toxicity. Secondly, we did not evaluate the expression of inflammatory cytokines in hepatic tissue. We did however, determine the systemic levels of TNF-α, IL-6, and IL-1β. Furthermore, the distribution of inflammatory infiltrates was considered an indicator of tissue inflammation on histological assessment.
Materials and methods
Animals
Wistar white rats (Male albino) were obtained (aged 8–10 weeks, weight 240–300 g) from King Saud University, College of Pharmacy, Center and House of Animal Experiments, Riyadh, Saudi Arabia. The animals were kept in cages under controlled conditions. The temperature was set at 22 °C (± 1), humidity levels ranged from 50 to 55%, and a light–dark cycle (with continuous lighting for 12 h) was maintained. Prior to the study start, rats were kept under excellent laboratory conditions for 1 week. They were fed quality food from Purina rat chow (Grain Silos and Flour Mills Organization, Riyadh, Saudi Arabia) and given drinking water. Experimental tests, including euthanasia, were conducted in accordance with the National Institutes of Health (NIH Publications No. 80-23; 1996) guidelines.
Chemicals and kits
LT was obtained from Toronto Research Chemicals Inc, Toronto, Canada. BL was obtained from Aldrich Chemicals, UK. DOX was received from EBEWE Pharma, Austria, while phosphate buffered saline (PBS) was received from Hoefer Inc, San Francisco, USA. The diagnostic kits ALT and AST, creatinine, and urea were purchased from Human Diagnostics, Wiesbaden, Germany. Pro-inflammatory cytokines and biomarkers of oxidative stress such as NO, iNOS, eNOS, NF-κB (p65), caspase-3, TNF-α, IL-1β, IL-6, SOD, CAT, Gpx, and GST were detected using ELISA kits purchased from R&D Systems, Minneapolis, MN, USA. Thiobarbituric acid-reactive substance (TBARS) and glutathione (GSH) were measured using ELISA kits obtained from Cayman Chemical, Ann Arbor, MI, USA.
Study design
Following an acclimatization week, rats were randomized into seven groups as follows: (1) Control group (vehicle), (2) DOX group (vehicle), (3) DOX + LT group (LT, 7 mg/kg/day orally), (4) DOX + BL low-dose group (BL, 5 mg/kg/day orally), (5) DOX + BL high-dose group (BL, 10 mg/kg/day orally), (6) DOX + LT + BL(5) low-dose group, and (7) DOX + LT + BL(10) high-dose group. In control group, only 6 rats were used whereas in DOX-injected groups, 12 rats were used because of the reported high mortality rate upon sole administration of DOX [36]. After 2 weeks of treatment with LT and BL, a single dose of DOX (15 mg/kg, intraperitoneal) was injected into rats in groups 2, 3, 4, 5, and 6. Treatment with LT and BL continued for seven more days. Rats were weighed and then anesthetized with isoflurane. Blood was collected from a heart puncture into a plain tube and centrifuged at 4,000 rpm for 10 min to obtain serum. The liver and kidney were dissected, weighed, and stored. With regards to storage, one piece of each tissue was preserved in aluminum foil and kept in a deep freezer at − 80 °C for later biochemical examination and another piece of each tissue was kept in 10% formalin for histopathological studies.
Serum analysis
Diagnostic kits were used to measure ALT, AST, creatine, and urea levels in serum. ELISA kits for rats were used to estimate levels of inflammatory biomarkers in serum including TNF-α, IL-1β, and IL-6.
Tissue analysis
ELISA kits were used to measure NO, iNOS, eNOS, NF-κB p65, caspase-3, TBARS, and GSH levels in liver homogenates. SOD, CAT, GPx, and GST activity in post-mitochondrial supernatant (PMS) of hepatic cells were measured using ELISA kits for rats.
Histological study
Cross sections of liver samples from controls and rats from each treatment group were preserved in 10% buffered formalin and embedded in paraffin blocks. Sections of 5 µm thickness were sliced with an American Optical rotary microtome (Leica Camera AG, Wetzlar, Germany). The sections were stained with hematoxylin and eosin and examined under a microscope to observe any histological changes.
Statistical analysis
Measurements were expressed as the mean ± standard deviation (SD; n = 8). All estimations were analyzed using ANOVA pursued by the Student–Newman–Keuls t test for comparisons using Prism 5 software (GraphPad, La Jolla, CA, USA). A p value < 0.05 was considered statistically significant.
The DOX group was compared to the control group, and the LT- and BL-treated groups were compared to the DOX group. The significance levels reported included *p < 0.05, **p < 0.01, and ***p < 0.001. Statistical analyses were conducted using Graph Pad Prism (v. 5) software.
Conclusions
In summary, our study demonstrates the remarkable therapeutic value of BL and LT in terms of their anti-oxidative and anti-inflammatory properties in rodents challenged with DOX. The protective effects of both compounds were markedly enhanced by their concurrent administration. The synergistic protective efficacy of combination therapy restored histological features and alleviated hepatic oxidative injury via inhibition of ROS formation, amelioration of antioxidant enzymes dysfunction, and anti-inflammatory mechanisms.
References
Upadhyay KK, Bhatt AN, Mishra AK et al (2010) The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly(gamma-benzyl l-glutamate)-b-hyaluronan polymersomes. Biomaterials 31:2882–2892
Aluise CD, Miriyala S, Noel T et al (2011) 2- Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-alpha release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic Biol Med 50:1630–1638
Rashid S, Ali N, Nafees S et al (2013) Alleviation of doxorubicin-induced nephrotoxicity and hepatotoxicity by chrysin in Wistar rats. Toxicol Mech Methods 23(5):337–345
Kalender Y, Yel M, Kalender S (2005) Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats: the effects of vitamin E and catechin. Toxicology 209(1):39–45
Lee V, Randhawa AK, Singal PK (1991) Adriamycin-induced myocardial dysfunction in vitro is mediated by free radicals. Am J Physiol Heart Circ Physiol 261(4):989–995
Marchand DJ, Renton KW (1981) Depression of cytochrome P-450-dependent drug biotransformation by adriamycin. Toxicol Appl Pharmacol 58(1):83–88
Damodara RV, Saayi KG, Padmavathi P et al (2007) Effect of Emblica officinalis against alcohol-induced biochemical changes in plasma and red blood cells of rats. Afr J Biochem Res 1(6):101–105
Mohan M, Kamble S, Gadhi P et al (2010) Protective effect of Solanum torvum on doxorubicin-induced nephrotoxicity in rats. Food Chem Toxicol 48(1):436–440
Rašković A, Stilinović N, Kolarović J et al (2011) The protective effects of silymarin against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. Molecules 16(10):8601–8613
Saad SY, Najjar TA, Al-Rikabi AC (2001) The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol Res 43(3):211–218
Toko H, Oka T, Zou Y et al (2002) Angiotensin II type 1a receptor mediates doxorubicin-induced cardiomyopathy. Hypertens Res 25:597–603
Matouk AI, Taye A, Heeba GH et al (2013) Quercetin augments the protective effect of losartan against chronic doxorubicin cardiotoxicity in rats. Environ Toxicol Pharmacol 36:443–450
Xiao L, Hu SQ, Wang LY et al (2015) Losartan improves the distribution and efficacy of doxorubicin in CT26 tumor. Eur Rev Med Pharmacol Sci 19:3763–3769
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:e47
Ginwala R, Bhavsar R, Chigbu DI et al (2019) Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants (Basel) 8(2):35
Al-Oanzi ZH (2019) Erectile dysfunction attenuation by naringenin in streptozotocin-induced diabetic rats. J Food Biochem 43(7):e12885
Patwardhan RS, Sharma D, Thoh M et al (2016) Baicalein exhibits anti-inflammatory effects via inhibition of NF-κB transactivation. Biochem Pharmacol 15(108):75–89
Fu Y, Luo J, Jia Z et al (2014) Baicalein protects against type 2 diabetes via promoting islet β-cell function in obese diabetic mice. Int J Endocrinol 2014:846742
Yun BY, Zhou L, Xie KP et al (2012) Antibacterial activity and mechanism of baicalein. Yao Xue Xue Bao 47(12):1587–1592
Lu Y, Joerger R, Wu C (2011) Study of the chemical composition and antimicrobial activities of ethanolic extracts from roots of Scutellaria baicalensis Georgi. J Agric Food Chem 59(20):10934–10942
Palko-Labuz A, Sroda-Pomianek K, Uryga A et al (2017) Anticancer activity of baicalein and luteolin studied in colorectal adenocarcinoma LoVo cells and in drug-resistant LoVo/Dx cells. Biomed Pharmacother 88:232–241
Huang Y, Tsang SY, Yao X et al (2005) Biological properties of baicaleinin cardiovascular system. Curr Drug Targets Cardiovasc Haematol Disord 5:177–184
Stavniichuk R, Drel VR, Shevalye H et al (2011) Baicalein alleviates diabetic peripheral neuropathy through inhibition of oxidative-nitrosative stress and p38 MAPK activation. Exp Neurol 230:106–113
Shiromwar SS, Chidrawar VR (2011) Combined effects of p-coumaric acid and naringenin against doxorubicin-induced cardiotoxicity in rats. Pharmacogn Res 3:214–219
Cappetta D, De Angelis A, Sapio L et al (2017) Oxidative stress and cellular response to doxorubicin: a common factor in the complex milieu of anthracycline cardiotoxicity. Oxid Med Cell Longev 2017:13
Akolkar G, da Silva DD, Ayyappan P et al (2017) Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 313:795–809
Al-Harthi SE, Alarabi OM, Ramadan WS et al (2014) Amelioration of doxorubicin-induced cardiotoxicity by resveratrol. Mol Med Rep 10(3):1455–1460
Imam F, Al-Harbi NO, Al-Harbi MM et al (2018) Apremilast prevent doxorubicin-induced apoptosis and inflammation in heart through inhibition of oxidative stress mediated activation of NF-kappaB signaling pathways. Pharmacol Rep 70:993–1000
Kabel AM, Alzahrani AA, Bawazir NM et al (2018) Targeting the proinflammatory cytokines, oxidative stress, apoptosis and TGF-beta1/STAT-3 signaling by irbesartan to ameliorate doxorubicin-induced hepatotoxicity. J Infect Chemother 24:623–631
Chang SA, Lim BK, Lee YJ et al (2015) A novel angiotensin type I receptor antagonist, fimasartan, prevents doxorubicin-induced cardiotoxicity in rats. J Korean Med Sci 30:559–568
Hrenak J, Arendasova K, Rajkovicova R et al (2013) Protective effect of captopril, olmesartan, melatonin and compound 21 on doxorubicin-induced nephrotoxicity in rats. Physiol Res 62(Suppl 1):S181
Koba S, Angiotensin II (2018) Oxidative stress, and sympathetic nervous system hyperactivity in heart failure. Yonago Acta Med 61:103–109
Li XX, He GR, Mu X et al (2012) Protective effects of baicalein against rotenone-induced neurotoxicity in PC12 cells and isolated rat brain mitochondria. Eur J Pharmacol 674:227–233
Hadi N, Yousif NG, Al-amran FG et al (2012) Vitamin E and telmisartan attenuates doxorubicin induced cardiac injury in rat through down regulation of inflammatory response. BMC Cardiovasc Disord 12:63
Sahu BD, Kumar JM, Kuncha M et al (2016) Baicalein alleviates doxorubicin-induced cardiotoxicity via suppression of myocardial oxidative stress and apoptosis in mice. Life Sci 1(144):8–18
Siveski-Iliskovic N, Kaul N, Singal PK (1994) Probucol promotes endogenous antioxidants and provides protection against adriamycin-induced cardiomyopathy in rats. Circulation 89:2829–2835
Acknowledgements
This study has been supported by Jouf University under the following Grant: Project No. 39/647.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ziad H. Al-Oanzi, Abdelbaset M. Elasbali, Nabil K. Alruwaili, Nasser Hadal Alotaibi, Khalid S. Alharbi, Abdulaziz I. Alzarea, Bader H. Alsuwayt, and Maher M. Al-Enazi declare that we have no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee at the Experimental Animal Center and House, Faculty of Pharmacy, King Saud University, Riyadh, Saudi Arabia (621-EACC-2016 dated 02-03-2016).
Rights and permissions
About this article
Cite this article
Al-Oanzi, Z.H., Elasbali, A.M., Alruwaili, N.K. et al. Protective effect of baicalein alone and losartan–baicalein combination therapy on doxorubicin-induced hepatotoxicity in rats. Toxicol. Environ. Health Sci. 12, 45–54 (2020). https://doi.org/10.1007/s13530-020-00037-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-020-00037-7