Abstract
Background
Doxorubicin is an effective antineoplastic agent but has limited clinical application because of its cumulative toxicities, including cardiotoxicity. Cardiotoxicity causes lipid peroxidation, genetic impairment, oxidative stress, inhibition of autophagy, and disruption of calcium homeostasis. Doxorubicin-induced cardiotoxicity is frequently tried to be mitigated by phytochemicals, which are derived from plants and possess antioxidant, anti-inflammatory, and anti-apoptotic properties. Arbutin, a natural antioxidant found in the leaves of the bearberry plant, has numerous pharmacological benefits, including antioxidant, anti-bacterial, anti-hyperglycemic, anti-inflammatory, and anti-tumor activity.
Methods and results
The study involved male Wistar rats divided into three groups: a control group, a group treated with doxorubicin (20 mg/kg) to induce cardiac toxicity, a group treated with arbutin (100 mg/kg) daily for two weeks before doxorubicin administration. After treatment, plasma and heart tissue samples were collected for analysis. The samples were evaluated for oxidative stress parameters, including superoxide dismutase, malondialdehyde, and catalase, as well as for cardiac biomarkers, including CK, CK-MB, and LDH. The heart tissues were also analyzed using molecular (TNF-α, IL-1β and Caspase 3), histopathological and immunohistochemical methods (8-OHDG, 4 Hydroxynonenal, and dityrosine). The results showed that arbutin treatment was protective against doxorubicin-induced oxidative damage by increasing SOD and CAT activity and decreasing MDA level. Arbutin treatment was similarly able to reverse the inflammatory response caused by doxorubicin by reducing TNF-α and IL-1β levels and also reverse the apoptosis by decreasing caspase-3 levels. It was able to prevent doxorubicin-induced cardiac damage by reducing cardiac biomarkers CK, CK-MB and LDH levels. In addition to all these results, histopathological analyzes also show that arbutin may be beneficial against the damage caused by doxorubicin on heart tissue.
Conclusion
The study suggests that arbutin has the potential to be used to mitigate doxorubicin-induced cardiotoxicity in cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Doxorubicin (DX) is a potent antitumor agent that has been effectively used to treat a variety of cancers, such as breast, ovarian, lung, and uterine cancers, as well as soft-tissue sarcomas [1,2,3]. Unfortunately, its clinical use is restricted because of its cumulative toxicities, especially its cardiotoxicity, which has been extensively studied [4, 5]. The harmful effects of DX-induced cardiotoxicity comprise lipid peroxidation, oxidative stress, genetic impairment, inhibition of autophagy, apoptosis, and disruption of calcium homeostasis [6]. NADPH-cytochrome P-450 enzymes metabolize DX, producing superoxide anions and hydroxyl radicals that damage cellular membranes [7]. Additionally, excessive DX exposure has been linked to cardiac inflammation [8, 9].
DX is an effective chemotherapeutic drug, preventing and treating its associated cardiotoxicity remains a major research focus. Dexrazoxane, an efficacious agent utilized in combating the cardiotoxic effects induced by DX, stands as a cornerstone in oncological care [10]. However, its utilization comes with inherent limitations and drawbacks. Notably, its administration is tethered to specific patient demographics, such as pediatric and geriatric populations, and necessitates judicious dosing regimens. Moreover, while dexrazoxane mitigates cardiotoxicity, its employment may introduce rare adverse effects and pose risks of drug interactions [11]. The common side effects that culminate from dexrazoxane use include dose-limiting myelotoxicity (neutropenia, leukopenia, granulocytopenia, and thrombocytopenia). Thus, despite its therapeutic utility, the imperative for novel drug development persists. The quest for innovative pharmacotherapeutics arises from the ambition to devise agents that surpass the constraints of existing options, striving for enhanced efficacy, broader applicability across patient cohorts, and minimized adverse effects. In essence, while dexrazoxane remains a stalwart in cardioprotection, the exigency for pioneering pharmaceutical endeavors remains paramount in elevating the standards of oncologic care [10, 12].
One promising approach is the use of phytochemicals, which are small molecules derived from plants that possess antioxidant, anti-inflammatory, and anti-apoptotic properties [13]. Natural compounds have demonstrated their potential in the treatment of cardiovascular diseases. As a result, many phytoconstituents have been studied and have been shown to effectively mitigate DX-induced cardiotoxicity [14].
Arbutin (ARB) is a natural antioxidant that is primarily found in bearberry plant leaves [15]. It is well-known for its numerous pharmacological benefits, such as antioxidant, anti-bacterial, anti-hyperglycemic, anti-inflammatory, and anti-tumor activity [16, 17]. In addition to these benefits, ARB has also demonstrated its positive effects in treating isoproterenol-induced cardiac hypertrophy in animals [18]. In light of these findings, we conducted a study to investigate the potential beneficial impact of ARB in mitigating DX-induced cardiotoxicity in animals.
Materials and methods
Chemicals
We obtained DX (CAS No.: 25316-40-9) and ARB (CAS No.: 497-76-7) from Sigma-Aldrich, Germany.
Animals
In compliance with the “Guide for the Care and Use of Laboratory Animals” and the approval of the Ataturk University-Ethical Committee, all tests were conducted (2200370605). The study subjects consisted of male Wistar rats that were pathogen-free and were seven weeks old. The rats were housed at the Animal Experimental Center of Ataturk University and had access to food and water ad libitum. The facility was maintained at a temperature of 25 °C, and the rats were exposed to a 12-hour light and 12-hour dark cycle.
Methodology for conducting experiments
After being fed a specific diet for one week, the rats were separated randomly into three groups, each containing six rats. Group I: The control group was given sterile saline solution in the same amount as the experimental groups. Group II: In accordance with previous studies, rats in Group II (DX) were treated with a single intraperitoneal dose of 20 mg/kg DX that was dissolved in 0.9% normal saline to induce acute cardiac toxicity [19, 20]. In Group III (DX + ARB), the rats were given 100 mg/kg ARB [18, 21, 22] daily for two weeks and then administered a single dose of 20 mg/kg DX. Following two days of DX treatment, the rats were given sodium thiopental anesthesia, then euthanized and subjected to various experimental procedures.
Collection of plasma and heart tissue samples for analysis
Once the serum was centrifuged at 3000 rpm for 15 min, it was collected for the evaluation of Creatine Kinase (CK), Creatine Kinase-Myocardial Band (CK-MB), and Lactate Dehydrogenase (LDH) using ELISA kits based on the instructions provided by the manufacturer. Measurements of LDH, CK and CK-MB, were conducted using the standard protocol provided by the commercially available kits (Cat No: E-EL-R2547, E-EL-R0274, and E-EL-R1327 respectively, Elabscience, USA) The heart tissue was immediately dissected and was removed and washed in cold saline. Next, the heart was carefully dissected into two sections. The one half of the heart was mixed and homogenized in PBS solution and then immediately stored at a temperature of − 20 °C for later analysis of oxidative stress parameters including superoxide dismutase (SOD), malondialdehyde (MDA), and catalase (CAT) (Cat No: E-EL-R1424, E-EL-0060, and E-BC- K031-M respectively, Elabscience, USA) and molecular analysis including TNF-α, IL-1β and caspase 3. All absorbance measurements were conducted at 450 nm with a spectrophotometer (BIOTEK Instruments, USA) [23, 24]. The other half of the heart tissue were placed in a solution of 10% formal saline for future investigation using histopathological and immunohistochemical techniques as previously described [24, 25].
Real-time quantitative PCR analysis
RT-PCR analysis was conducted following the methods outlined in previous literature [26]. The relative mRNA expressions of TNF-𝛼, IL-1β, and caspase-3 mRNA in heart tissue were assessed by real time polymerase chain reaction (RT-PCR) system (QIAGEN-Rotor-Gene Q) (Hilden-Germany). Quadruplicate determinations were performed for each tissue sample, utilizing a 96-well optical plate. Each reaction included 2.5 µl of cDNA (100 ng), 1 µl of TaqMan gene expression assay, 10 µl of TaqMan PCR MasterMix (supplied by Applied Biosystems), and 6.5 µl of RNase-free water, totaling 20 µl per reaction. The plates were subjected to initial heating at 50 °C for 2 min followed by 10 min at 95 °C. Subsequently, 40 cycles were run, consisting of 15 s at 95 °C and 60 s at 60 °C for each cycle [27]. The obtained target gene expression levels were normalized to the housekeeping gene 𝛽-actin. The PCR primers utilized are listed in Table 1. The results were obtained with the 2−ΔΔCt method.
Tissue analysis of heart using histopathological methods
Samples of heart tissues were taken from all rats in each group, and then preserved in a solution called neutral buffered formalin. They were then processed according to a standard protocol. Thin sections of the tissues, about 5 micrometers in thickness, were cut and stained with Hematoxylin and Eosin (H&E) dye. The stained sections were then examined under a light microscope. A pathologist who did not know which treatment the rats had received, looked at the samples to see if there were any abnormal changes in the tissues [23].
Immunohistochemical analysis
The heart sections were exposed to primary antibodies against 8-OHDG, 4 Hydroxynonenal (4-HNE), and dityrosine (DT), which were diluted 1:200. Diaminobenzidine tetrachloride (DAB) was used to visualize the immune reaction. The staining was graded as negative, weak, moderate, or strong depending on the intensity of the staining [26]. The percentage of area expressing 8-OHDG, 4-HNE, and DT was estimated by measuring the stained areas of each section and calculating an average using imaging software called Image J. The person analyzing the images was not aware of the treatments given to the animals. The individual examining the images was blinded to the treatments administered to the animals.
Statistical analysis
The data collected from the experiments were analyzed using the SPSS STATISTIC software version 23, and the results were presented as mean ± standard deviation (mean ± SD). The ELISA and RT-PCR results were subjected to a one-way ANOVA followed by post hoc Tukey’s test, while the histopathological and immunohistochemical evaluations were analyzed using the Kruskal-Walls test followed by post hoc Mann-Whitney U test. A p-value of less than 0,001 was considered statistically significant.
Results
Cardiac biomarkers
The concentrations of serum cardiac biomarkers, including CK-MB, CK, and LDH, were assessed to ascertain the occurrence of DX-induced cardiotoxicity and to evaluate any potential protective effects conferred by ARB. In the DX group, there was a significant increase in CK-MB, CK, and LDH (p < 0.001) compared to the normal control group. However, in the treatment group, there was a significant decrease in CK-MB and CK compared to the DX group. Additionally, ARB significantly reduced CK-MB (p < 0.001) compared to the DX group. The increase in CK-MB, CK, and LDH levels indicate damage to the cardiomyocytes. These findings showed that DX induced cardiotoxicity in rats, while treatment with ARB protected against it by reducing CK-MB (Fig. 1A), CK (Fig. 1B) and LDH (Fig. 1C) levels.
Reduced oxidative damage, inflammation and apoptosis in cardiac tissues with ARB treatment against DX-induced injury
We found that giving DX to rats markedly decreased the activity of enzymes like SOD (Fig. 2A) and CAT (Fig. 2B) compared to control rats. However, when the rats were given ARB before DX, marked improvement was seen in the activity of these enzymes, compared to rats that were only given DX (shown in Fig. 2). In addition, giving DX to rats caused an elevation in the amount of MDA (Fig. 2C), a biomarker of oxidative stress, in their heart tissues, and a decrease in the activity of CAT and SOD enzymes in their hearts, compared to control rats. But when rats were given ARB before DX, there was a significant reduction in MDA levels and an increase in the activity of these enzymes in their heart tissues, compared to rats that were only given DX (Fig. 2).
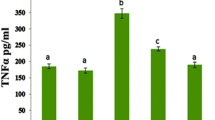
DX treated rats showed marked increase in mRNA expressions of TNF-α (Fig. 3A) and IL-1β (Fig. 3B) in the cardiac tissues compared to the control group. Despite that, in the ARB group, the expression levels of TNF-α and IL-1β were reduced compared to the DX group (Fig. 3). Furthermore, caspase-3 levels in the cardiac tissues were markedly elevated in the DX group compared to the control group. ARB treatment resulted in a significant reduction in caspase-3 levels (Fig. 3C).
Histopathological analysis
The slides of heart muscle tissue from different groups of rats were examined. The control group had healthy muscle tissue with visible muscle striations and central nuclei. However, the groups treated with DX had damaged tissue with loss of striations and vascular congestion. The group treated with ARB showed a significant improvement in the tissue, with no notable damage or necrosis. These findings suggest that ARB may have a protective effect on the heart tissue (Fig. 4).
Histopathological microphotograph of control and treatment groups stained with H&E. (A) Control group: Normal architecture of myocardium, (B) DX treated group: Severe mononuclear cell infiltrates (arrow) and severe hemorrhage (arrowhead), (C) DX + ARB treated group: Mild mononuclear cell infiltrates (arrowhead) and mild hemorrhage (arrowhead)
Immunohistochemical analysis
The analysis showed that 8-OHDG, 4-HNE, and DT expression was significantly higher in the DX group in comparison with the control group. However, the ARB pretreatment group had significantly lower 8-OHDG, 4-HNE, and DT immunoreactivity score in comparison with the DX group (Fig. 5). The control group showed negative expression (Fig. 5).
Discussion
The detrimental effects of DX-induced cardiotoxicity are well established and associated with the initiation of inflammation [8] and an overexpression of reactive oxygen species with DX [28, 29]. The myocardium is particularly susceptible to DX related oxidative stress due to decreased activity of antioxidant enzymes in the myocardium [30]. Furthermore, the cardiac muscle contains cardiolipin-rich mitochondria that have a high affinity for DX, leading to its accumulation in the cardiac mitochondria. This accumulation impairs the respiratory chain and triggers apoptotic death [31]. Our study’s biochemical, molecular, histopathological, and immunohistochemical analyses provided evidence of oxidative damage, apoptosis, and inflammation in an animal model of DX-induced cardiotoxicity, in line with previous studies.
Our current study provides compelling evidence that DX administration in rats results in a notable increase in the levels of serum cardiac injury markers such as CK, CK-MB, and LDH, thereby indicating significant cardiac damage. Interestingly, pretreatment with ARB was found to significantly decrease the levels of these injury markers, pointing towards its potential as a cardioprotective agent. Importantly, our results are consistent with earlier findings, which demonstrated that ARB treatment led to a decrease in the levels of CK-MB and LDH in rodent models of septic cardiomyopathy and isoproterenol-induced cardiac hypertrophy [18, 32]. These findings highlight the potential of ARB as a therapeutic agent for the prevention and management of DX-induced cardiotoxicity.
Results of the current study showed a significant decrease in cardiac SOD and CAT activities, important antioxidant enzymes that break down superoxide anions and hydrogen peroxide, respectively, following DX administration in rats. These results were consistent with other studies that also found DX caused a reduction in antioxidant mechanisms [33, 34]. We also found a significant increase in cardiac tissue levels of MDA and 8-OHdG in the DX group, indicating DX caused an increase in lipid peroxidation and DNA damage [35, 36]. 4-HNE is formed as a result of oxidative stress and is a product of lipid peroxidation. It has been shown to be highly reactive and can form adducts with proteins and DNA. Studies have suggested that 4-HNE plays a role in the pathogenesis of several diseases, including Alzheimer’s disease, Parkinson’s disease, and atherosclerosis [37]. The measurement of 4-HNE levels has been used as a marker of oxidative stress in various biological systems, including in animal models. Its accumulation has been associated with the activation of signaling pathways involved in apoptosis and inflammation. 3,3’-dityrosine has emerged as a key marker of protein oxidation due to its specific formation from the reaction of tyrosine residues with reactive oxygen species. The tyrosine radical, which is generated by the attack of various reactive oxygen species such as peroxynitrite and hydroxyl radicals, undergoes cross-linking with neighboring tyrosine residues to produce 3,3’-dityrosine [7]. These findings and previous studies suggest that DX causes oxidative damage in cardiac tissue by inhibiting antioxidant mechanisms.
Previous studies have shown that pretreatment with ARB can mitigate the DX-induced lipid peroxidation [38], and significantly reduce the levels of antioxidant enzymes. Hence, it is hypothesized that ARB could efficiently scavenge the uncontrolled production of reactive oxygen species caused by DX and safeguard the myocardium from DX-induced damage. The current results are supported by earlier findings that have highlighted the antioxidant properties of ARB both in vitro and in vivo [16, 39].
The observed association between DX-induced cardiotoxicity and inflammation is well-documented, primarily attributed to the release of pro-inflammatory cytokines [40]. Notably, two pivotal cytokines, TNF-α and IL-1β, have been consistently shown to exhibit elevated levels in response to cardiac injury induced by DX [41]. In this context, ARB, characterized by its notable anti-inflammatory properties, emerges as a compelling candidate for intervention. Studies across various experimental models have consistently demonstrated ARB’s ability to mitigate the levels of TNF-α and IL-1β, as also evidenced by the results of the present investigation [42, 43]. These findings collectively suggest a promising role for ARB as a therapeutic avenue in both preventing and managing DX-induced cardiotoxicity through its potent anti-inflammatory effects. By modulating the inflammatory response, ARB holds potential not only in attenuating the adverse effects of DX on the heart but also in preserving cardiac function during chemotherapy. Furthermore, the extensive body of evidence supporting ARB’s anti-inflammatory properties underscores its broader utility beyond DX-induced cardiotoxicity. Its efficacy as an adjunctive therapy in chemotherapy-induced cardiotoxicity is particularly noteworthy, suggesting a multifaceted approach to mitigating cardiac complications associated with cancer treatment [42, 43].
The harmful effects of DX on the heart are not limited to oxidative stress and inflammation; it can also induce apoptosis, which is the programmed cell death mechanism that occurs naturally in cells. DX-induced apoptosis is mediated by the overproduction of caspase-3 enzyme. According to a previous study, DX has been shown to induce the release of cytochrome C into the cytoplasm and increase the expression of caspase-3 and caspase-9 in cardiomyocytes [44]. Furthermore, DX can disturb calcium homeostasis, resulting in cellular and mitochondrial calcium overload. This can disturb cellular metabolism, increase the production of free radicals, and initiate apoptosis. The opening likelihood of sarcoplasmic reticulum Ca channels increases with DX, while Na+–Ca2 + exchanger membrane proteins are inhibited [45]. In line with previous studies that showed the anti-apoptotic effects of ARB, our study also found that ARB reduces apoptosis by decreasing caspase-3 levels [46]. ARB can help protect the myocardium from DX-induced damage by reducing the expression of caspase-3 and preventing the occurrence of apoptosis. Our results are consistent with other reports that demonstrated the anti-apoptotic effect of ARB in vitro and in vivo [16, 39].
Conclusion
In the present study, our investigation demonstrated that ARB exerted significant ameliorative effects against DX-induced cardiotoxicity in animal subjects. The administration of ARB effectively attenuated DX-induced oxidative stress, inflammation, and apoptosis, thus indicating its potential as a protective agent against cardiac damage induced by DX. Furthermore, our histopathological analyses revealed noteworthy improvements in the myocardial tissue profile, reinforcing the cardioprotective properties of ARB. The findings from this preclinical study provide valuable evidence supporting the therapeutic potential of ARB as a promising intervention to mitigate DX-induced cardiotoxicity in cancer patients. Despite the encouraging outcomes presented in this study, it is essential to acknowledge the need for future clinical trials to validate the safety and efficacy of ARB in human subjects.
Data availability
Data and materials are available from the authors upon request.
References
Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, Moreira PI (2009) Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem 16:3267–3285
Erfu C, Li L, Weiting Q, Tao C, Liwei M, Hemin Y, Junkun L (2024) Matrine attenuating cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity through improved mitochondrial membrane potential and activation of mitochondrial respiratory chain Complex I pathway. Biomed Pharmacother 173:116464
Xu S, Zheng S, Ma N, Zhang H, Shi J, Huang J, Luo N, Wang M, Xiong Y (2024) Rhein potentiates doxorubicin in treating triple negative breast cancer by inhibiting cancer-associated fibroblasts. Biochem Pharmacol 223:116139
Hao G, Yu Y, Gu B, Xing Y, Xue M (2015) Protective effects of berberine against doxorubicin-induced cardiotoxicity in rats by inhibiting metabolism of doxorubicin. Xenobiotica 45:1024–1029
Olson RD, Boerth RC, Gerber JG, Nies AS (1981) Mechanism of adriamycin cardiotoxicity: evidence for oxidative stress. Life Sci 29:1393–1401
Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL (2012) Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52:1213–1225
Benzer F, Kandemir FM, Ozkaraca M, Kucukler S, Caglayan C (2018) Curcumin ameliorates doxorubicin-induced cardiotoxicity by abrogation of inflammation, apoptosis, oxidative DNA damage, and protein oxidation in rats. J Biochem Mol Toxicol, 32
Deepa PR, Varalakshmi P (2005) Biochemical evaluation of the inflammatory changes in cardiac, hepatic and renal tissues of adriamycin-administered rats and the modulatory role of exogenous heparin-derivative treatment. Chem Biol Interact 156:93–100
Wang T, Xing G, Fu T, Ma Y, Wang Q, Zhang S, Chang X, Tong Y (2024) Role of mitochondria in doxorubicin-mediated cardiotoxicity: from molecular mechanisms to therapeutic strategies. Cell Stress Chaperones
QuanJun Y, GenJin Y, LiLi W, YongLong H, Yan H, Jie L, JinLu H, Jin L, Run G, Cheng G (2017) Protective effects of Dexrazoxane against Doxorubicin-Induced cardiotoxicity: a metabolomic study. PLoS ONE 12:e0169567
Bosman M, Kruger DN, Favere K, De Meyer GRY, Franssen C, Van Craenenbroeck EM, Guns PJ (2023) Dexrazoxane does not mitigate early vascular toxicity induced by doxorubicin in mice. PLoS ONE 18:e0294848
Yu X, Ruan Y, Huang X, Dou L, Lan M, Cui J, Chen B, Gong H, Wang Q, Yan M, Sun S, Qiu Q, Zhang X, Man Y, Tang W, Li J, Shen T (2020) Dexrazoxane ameliorates doxorubicin-induced cardiotoxicity by inhibiting both apoptosis and necroptosis in cardiomyocytes. Biochem Biophys Res Commun 523:140–146
Abushouk AI, Ismail A, Salem AMA, Afifi AM, Abdel-Daim MM (2017) Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed Pharmacother 90:935–946
Abdel-Daim MM, Kilany OE, Khalifa HA, Ahmed AAM (2017) Allicin ameliorates doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Cancer Chemother Pharmacol 80:745–753
Sugier P, Seczyk L, Sugier D, Krawczyk R, Wojcik M, Czarnecka J, Okon S, Plak A (2021) Chemical characteristics and antioxidant activity of Arctostaphylos uva-ursi L. Spreng. At the Southern Border of the geographical range of the species in Europe. Molecules, 26
Migas P, Krauze-Baranowska M (2015) The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem Lett 13:35–40
Ahmadian SR, Ghasemi-Kasman M, Pouramir M, Sadeghi F (2019) Arbutin attenuates cognitive impairment and inflammatory response in pentylenetetrazol-induced kindling model of epilepsy. Neuropharmacology 146:117–127
Nalban N, Sangaraju R, Alavala S, Mir SM, Jerald MK, Sistla R (2020) Arbutin attenuates Isoproterenol-Induced Cardiac Hypertrophy by inhibiting TLR-4/NF-kappaB pathway in mice. Cardiovasc Toxicol 20:235–248
Lv X, Zhu Y, Deng Y, Zhang S, Zhang Q, Zhao B, Li G (2020) Glycyrrhizin improved autophagy flux via HMGB1-dependent Akt/mTOR signaling pathway to prevent doxorubicin-induced cardiotoxicity. Toxicology 441:152508
Seyedan AA, Dezfoulian O, Alirezaei M (2020) Satureja Khuzistanica Jamzad essential oil prevents doxorubicin-induced apoptosis via extrinsic and intrinsic mitochondrial pathways. Res Pharm Sci 15:481–490
Khadir F, Pouramir M, Joorsaraee SG, Feizi F, Sorkhi H, Yousefi F (2015) The effect of arbutin on lipid peroxidation and antioxidant capacity in the serum of cyclosporine-treated rats. Casp J Intern Med 6:196–200
Demir EA, Demir S, Kazaz IO, Kucuk H, Alemdar NT, Buyuk A, Mentese A, Aliyazicioglu Y (2023) Arbutin abrogates testicular ischemia/reperfusion injury in rats through repression of inflammation and ER stress. Tissue Cell 82:102056
Ahiskali I, Ferah Okkay I, Mammadov R, Okkay U, Keskin Cimen F, Kurt N, Suleyman H (2021) Effect of taxifolin on cisplatin-associated oxidative optic nerve damage in rats. Cutan Ocul Toxicol 40:1–6
Okkay U, Ferah Okkay I, Aydin IC, Bayram C, Ertugrul MS, Gezer A, Hacimuftuoglu A (2021) Effects of Achillea millefolium on cisplatin induced ocular toxicity: an experimental study. Cutan Ocul Toxicol 40:214–220
Ferah Okkay I, Okkay U, Cicek B, Yilmaz A, Yesilyurt F, Mendil AS, Hacimuftuoglu A (2021) Neuroprotective effect of bromelain in 6-hydroxydopamine induced in vitro model of Parkinson’s disease. Mol Biol Rep 48:7711–7717
Ferah Okkay I, Okkay U, Gundogdu OL, Bayram C, Mendil AS, Ertugrul MS, Hacimuftuoglu A (2022) Syringic acid protects against thioacetamide-induced hepatic encephalopathy: behavioral, biochemical, and molecular evidence. Neurosci Lett 769:136385
Okkay U, Ferah Okkay I, Cicek B, Aydin IC, Ozkaraca M (2022) Hepatoprotective and neuroprotective effect of taxifolin on hepatic encephalopathy in rats. Metab Brain Dis 37:1541–1556
Berthiaume JM, Wallace KB (2007) Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol 23:15–25
Ahmed HH, Mannaa F, Elmegeed GA, Doss SH (2005) Cardioprotective activity of melatonin and its novel synthesized derivatives on doxorubicin-induced cardiotoxicity. Bioorg Med Chem 13:1847–1857
De Beer EL, Bottone AE, Voest EE (2001) Doxorubicin and mechanical performance of cardiac trabeculae after acute and chronic treatment: a review. Eur J Pharmacol 415:1–11
Ascensao A, Magalhaes J, Soares JM, Ferreira R, Neuparth MJ, Marques F, Oliveira PJ, Duarte JA (2005) Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am J Physiol Heart Circ Physiol 289:H722–731
Zhou N, Zeng MN, Li K, Yang YY, Bai ZY, Zheng XK, Feng WS (2018) An integrated metabolomic strategy for the characterization of the effects of Chinese yam and its three active components on septic cardiomyopathy. Food Funct 9:4989–4997
Dong Q, Chen L, Lu Q, Sharma S, Li L, Morimoto S, Wang G (2014) Quercetin attenuates doxorubicin cardiotoxicity by modulating Bmi-1 expression. Br J Pharmacol 171:4440–4454
Raskovic A, Stilinovic N, Kolarovic J, Vasovic V, Vukmirovic S, Mikov M (2011) The protective effects of silymarin against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. Molecules 16:8601–8613
Yilmaz S, Atessahin A, Sahna E, Karahan I, Ozer S (2006) Protective effect of lycopene on adriamycin-induced cardiotoxicity and nephrotoxicity. Toxicology 218:164–171
Hwang S, Kim SH, Yoo KH, Chung MH, Lee JW, Son KH (2022) Exogenous 8-hydroxydeoxyguanosine attenuates doxorubicin-induced cardiotoxicity by decreasing pyroptosis in H9c2 cardiomyocytes. BMC Mol Cell Biol 23:55
Uchida K (2003) 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res 42:318–343
Dadgar M, Pouramir M, Dastan Z, Ghasemi-Kasman M, Ashrafpour M, Moghadamnia AA, Khafri S, Pourghasem M (2018) Arbutin attenuates behavioral impairment and oxidative stress in an animal model of Parkinson’s disease. Avicenna J Phytomed 8:533–542
Takebayashi J, Ishii R, Chen J, Matsumoto T, Ishimi Y, Tai A (2010) Reassessment of antioxidant activity of arbutin: multifaceted evaluation using five antioxidant assay systems. Free Radic Res 44:473–478
Mohajeri M, Sahebkar A (2018) Protective effects of curcumin against doxorubicin-induced toxicity and resistance: a review. Crit Rev Oncol Hematol 122:30–51
Zhang J, Cui L, Han X, Zhang Y, Zhang X, Chu X, Zhang F, Zhang Y, Chu L (2017) Protective effects of tannic acid on acute doxorubicin-induced cardiotoxicity: involvement of suppression in oxidative stress, inflammation, and apoptosis. Biomed Pharmacother 93:1253–1260
Safari H, Zabihi E, Pouramir M, Morakabati P, Abedian Z, Karkhah A, Nouri HR (2020) Decrease of intracellular ROS by arbutin is associated with apoptosis induction and downregulation of IL-1 beta and TNF-alpha in LNCaP; prostate cancer. J Food Biochem, 44
Lee HJ, Kim KW (2012) Anti-inflammatory effects of arbutin in lipopolysaccharide-stimulated BV2 microglial cells. Inflamm Res 61:817–825
Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C (2002) Doxorubicin treatment in vivo causes cytochrome c release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2: Bax ratio. Cancer Res 62:4592–4598
Asensio-Lopez MC, Soler F, Sanchez-Mas J, Pascual-Figal D, Fernandez-Belda F, Lax A (2016) Early oxidative damage induced by doxorubicin: source of production, protection by GKT137831 and effect on ca(2+) transporters in HL-1 cardiomyocytes. Arch Biochem Biophys 594:26–36
Ma C, Zhang D, Ma Q, Liu Y, Yang Y (2021) Arbutin inhibits inflammation and apoptosis by enhancing autophagy via SIRT1. Adv Clin Exp Med 30:535–544
Acknowledgements
We thank Levent PAY for comments on the manuscript.
Funding
The authors did not receive any financial support.
Author information
Authors and Affiliations
Contributions
O.B., I.F.O., U.O. designed the study. O.B., I.F.O., U.O., C.B., M.S.E and B.M. carried out the experiments. O.B., I.F.O., U.O., A.H., H.S. and E.A. wrote the main manuscript. All authors provided critical feedback and helped shape the research.
Corresponding authors
Ethics declarations
Ethical approval
All experiments were conducted in compliance with the “Guide for the Care and Use of Laboratory Animals” and the approval of the Ataturk University-Ethical Committee (2200370605).
Consent to participate
All individuals have signed consent forms before sample acquisition. Information on potential publications is included in consent forms signed by all individuals.
Consent for publication
The participants were informed about publishing the results and they all agreed.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Birdal, O., Ferah Okkay, I., Okkay, U. et al. Protective effects of arbutin against doxorubicin-induced cardiac damage. Mol Biol Rep 51, 532 (2024). https://doi.org/10.1007/s11033-024-09488-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09488-4