Abstract
Lifespans are both shorter and more variable for blacks than for whites in the United States. Because their lifespans are more variable, there is greater inequality in length of life—and thus greater uncertainty about the future—among blacks. This study is the first to decompose the black-white difference in lifespan variability in America. Are lifespans more variable for blacks because they are more likely to die of causes that disproportionately strike the young and middle-aged, or because age at death varies more for blacks than for whites among those who succumb to the same cause? We find that it is primarily the latter. For almost all causes of death, age at death is more variable for blacks than it is for whites, especially among women. Although some youthful causes of death, such as homicide and HIV/AIDS, contribute to the black-white disparity in variance, those contributions are largely offset by the higher rates of suicide and drug poisoning deaths for whites. As a result, differences in the causes of death for blacks and whites account, on net, for only about one-eighth of the difference in lifespan variance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Demographers have a long-standing interest in the variability of adult lifespans. The classic reason is to determine whether variance in the age at death among adults has been shrinking in low-mortality societies, possibly indicating a ceiling for human longevity (Fries 1980, 1984; Gillespie et al. 2013; Kannisto 2000; Lynch and Brown 2001; Manton and Singer 1994; Myers and Manton 1984; van Raalte and Caswell 2013; Wilmoth and Horiuchi 1999). Results have been mixed, with most studies finding evidence of mortality compression, especially until the 1960s (e.g., Edwards and Tuljapurkar 2005), and others finding no evidence of compression (e.g., Lynch and Brown 2001).
More recently, researchers have focused on national or group differences in the variability of adult lifespans because those differences have important implications for group members (Edwards 2011; Edwards and Tuljapurkar 2005; Engelman et al. 2010; Nau and Firebaugh 2012; Smits and Monden 2009; Tuljapurkar 2010; van Raalte et al. 2014). Greater lifespan variability means greater uncertainty about age at death, which can induce individuals to discount their future more. In fact, it can affect decisions about lifestyle, reduce the value of investments in education (Edwards 2013) and in health, and undermine incentives for retirement planning. Moreover, an individual who lives twice as long has twice the time to enjoy her consumption stream (Becker et al. 2005). Thus “[a]ny account of social inequality may be seriously incomplete if it ignores differences in longevity within a society” (Peltzman 2009:175).
Although variability in age at death historically has been inversely associated with life expectancy, two populations with similar mean age at death can still exhibit very different dispersion around that mean (Smits and Monden 2009). To fully characterize the mortality experiences of populations, then, we must examine lifespan variance (the second moment of the age-at-death distribution) as well as life expectancy (the first moment). The current study is the first to decompose the second-moment differences for blacks and whites. A deeper understanding of racial differences in lifespan variability is particularly pertinent for the United States, where eliminating racial health disparities has been singled out as one of the principal goals of public health policy (U.S. Department of Health and Human Services 2011). Because the stated goal is to eliminate racial health disparities, we compare the variability of the lifespans of non-Hispanic black adults versus non-Hispanic white adults. We decompose the difference in that variability by using the new spread-allocation-timing decomposition method (Nau and Firebaugh 2012), a method that reveals which causes of death and which variance components of those causes are most important for producing differences in lifespan variance between two populations. To gain further insights on what generates the racial differences in lifespan variability, we extend the method to look specifically at the differences between black women and white women as well as between black men and white men. This more refined analysis can help better inform policy-makers because some of the mechanisms that generate racial disparities in lifespan variability might work differently for men and women.
The article is organized as follows. We begin by noting that prior research on racial disparities in mortality has focused on average age at death—life expectancy—rather than on variability in age at death, which leaves significant gaps in our understanding of why lifespans vary more for blacks. After describing our methods, data, and cause-of-death categories, we report the findings in two parts. In the first part, we describe the fundamental sources of racial differences in lifespan variance. The goal is to determine whether lifespans are more variable for blacks because they die of different causes than whites (allocation effect), because age at death varies more for blacks than for whites who die of the same cause (spread effect), or because the average age at death varies more across causes for blacks than for whites (timing effect). In the second part, we break the results down further by sex. We conclude by discussing the implications of our results for policy and for future research.
Mortality Differences Between Blacks and Whites
Although we know from prior studies that lifespans are more variable for blacks than for whites (Edwards and Tuljapurkar 2005; Lynch et al. 2003; Tuljapurkar and Edwards 2011), the sources of this difference remain unexplored. Studies of the black-white disparity in mortality have focused instead on life expectancy. Recent data from the National Center for Health Statistics (Kochanek et al. 2013) indicate that the black-white gap in life expectancy is now approximately 3.8 years, which is an historic low. To account for a gap in life expectancy, researchers generally begin by identifying the causes of death that contribute the most to it (Arriaga 1984; Pollard 1982, 1988; Beltrán-Sánchez et al. 2008). Based on such a decomposition, Kochanek et al. (2013) concluded that the 3.8-year gap is attributable largely to higher death rates in the black population from heart disease, cancers, homicide, diabetes, and perintatal conditions. Heart disease alone accounts for 26 % of the racial gap in longevity (Kochanek et al. 2013: figure 3). Other decompositions of the black-white gap in life expectancy report similar results. Harper et al. (2012), for example, found that heart disease accounts for 22 % of the gap for men and 29 % of the gap for women in 2008.
In this study, we focus on adult lifespan variability rather than on life expectancy. The black-white difference in life expectancy is the smaller of the two disparities: roughly 5 %, as opposed to the roughly 20 % difference in adult lifespan variance. Using the same mortality data as Kochanek et al. (2013), we find that the variance in age at death among those who survive to age 10 is 244.0 for blacks and 199.1 for whites.Footnote 1 Our goal is to decompose that difference. Methods for decomposing life expectancy do not apply to lifespan variance because lifespan variance is a function of variance in age at death within causes of death, whereas life expectancy is not. Suppose, for example, that we held constant the proportion of whites and blacks who die of each cause and the average age at which they die, while we increased the within-cause variance in age at death for blacks but not whites. The racial gap in lifespan variance would increase, but the racial gap in life expectancy would not.
The important implication is that the results in this article cannot be inferred from prior studies of the life expectancy gap because the factors accounting for differences in lifespan variability differ from those accounting for differences in life expectancy. This is easy to demonstrate with the 2010 mortality data. Based on their decomposition of the black-white gap in life expectancy, Kochanek et al. (2013:3) found that 17 % of the gap in life expectancy is due to higher cancer death rates among blacks. Yet, as we show subsequently, cancer accounts for only about 8 % of the black-white gap in lifespan variance. Of that 8 %, most is due to greater within-cause variability for blacks who succumb to cancer, a factor that accounts for none of the gap in life expectancy. Furthermore, only 0.6 % of the gap in lifespan variance is due to higher cancer death rates for blacks, in sharp contrast to the 17 % reported by Kochanek et al. (2013) for the life expectancy gap.Footnote 2
In short, our findings are new, and they contribute in significant ways to our understanding of black-white mortality differences. First, we discover what portion of the greater variance in the lifespans of blacks is attributable to greater cause-specific variance in age at death among blacks: the “spread component.” The spread component cannot be deduced from gaps in life expectancy because, as just noted, life expectancy is not affected by cause-specific variability per se. Second, we discover what portion of the greater variance in the lifespans of blacks is attributable to differences in cause-specific death rates: the “allocation component.” Racial differences in lifespan variability are boosted to the extent that blacks disproportionately die of causes that largely affect the young, such as homicide. We also add to our understanding of black-white mortality differences by discovering what portion of the racial disparity in lifespan variance is attributable to greater variance among blacks in the average age at death across causes: the “timing component.” Finally, we decompose further by gender so that we can assess whether the racial gap in lifespan variance is due to different causes for men and women.
We now describe the Nau-Firebaugh decomposition method (Nau and Firebaugh 2012) and show how it can be extended to determine the separate contributions of men and women to black-white differences in lifespan variance.
Decomposition Method
Nau-Firebaugh Decomposition
Following Nau and Firebaugh (2012), we present a stylized representation of the spread, allocation, and timing (SAT) components (Fig. 1). To simplify the figure, we assume just four causes of death. The x-axis is age at death, and the y-axis is number of life table deaths. The top panel depicts pure spread effects, where lifespans are more variable for blacks because of blacks’ greater within-cause dispersion in age at death. In this case, there is no allocation component because blacks and whites die of the same causes at the same rates; and there is no timing component because black and white victims of each cause die on average at the same age (as indicated by the vertical dashed lines). The second panel depicts a pure allocation effect. Again there is no timing effect: blacks and white victims of each cause die at the same age, on average; but, unlike the top panel, the within-cause variance is the same for blacks and whites. Lifespan variability nonetheless is greater for blacks because they are more likely than whites to die of causes that disproportionately strike the young (causes that disproportionately strike the very elderly would have the same effect). Finally, for a pure timing effect (bottom panel), blacks and whites die of the same causes at the same rates, with the same cause-specific variances, but the means of the distributions differ. Lifespan variability could be greater for blacks because of blacks’ greater variability in the average age at death across causes of death.
We can derive equations for the spread, allocation, and timing components from standard analysis of variance (ANOVA), with age at death as the dependent variable and cause of death as the categorical independent variable. We first write an ANOVA equation for the difference in variance, and then use algebra to separate the spread, allocation, and timing components. To begin, denote the age at death for the ith victim of cause c as X ic , the overall mean age at death in the population as \( \overline{X} \), and the mean age at death for victims of cause c as \( {\overline{X}}_c \). Observe that the difference between X ic and \( \overline{X} \) can be expressed as the sum of two differences: \( {x}_{ic}={X}_{ic}-{\overline{X}}_c \) and \( {\overline{x}}_c={\overline{X}}_c-\overline{X} \) (a within-cause part and a between-cause part). Based on this notation, the standard ANOVA equation for the total variance in age at death, by cause, is
where N is the total number of deaths, and the c = 1, 2, . . . , C causes of death are mutually exclusive and exhaustive. Note that \( {x}_{ic}^2={\left({X}_{ic}-{\overline{X}}_c\right)}^2 \) and that \( {\overline{x}}_c^2={\left({\overline{X}}_c-\overline{X}\right)}^2 \).
The first term in Eq. (1) is the incidence-weighted sum of the within-cause variances, σ 2 c (Appendix):
where p c = N c / N, the proportion of deaths from cause c. The second term in Eq. (1) is likewise an incidence-weighted sum of dispersion—in this case, an incidence-weighted sum of \( {\overline{x}}_c^2 \), the dispersion of the cause-specific means:
From Eqs. (2) and (3), it follows that the difference in lifespan variance for blacks and whites is the sum of the black-white differences in ∑ C c = 1 p c σ 2 c and in \( {\displaystyle {\sum}_{c=1}^C{p}_c{\overline{x}}_c^2} \).
where ∑ c p cB = ∑ c p cW = 1.0. Note that Eq. (4) uses whites as the reference category, so σ 2 B − σ 2 W is positive when σ 2 B > σ 2 W and negative when σ 2 B < σ 2 W . Using blacks as the reference category would reverse the signs.
By manipulating Eq. (4), we can partition the black-white difference in lifespan variance into its spread, allocation, timing, and joint components (Nau and Firebaugh 2012):
The joint component captures the part of the racial difference in lifespan variance that is attributable to simultaneous racial differences in incidence and in cause-specific variances. We can eliminate the joint component by weighting the differences in Eqs. (5a)–(5c) by average values for blacks and whites—that is, by changing the reference from whites to the average for whites and blacks. However, because our research question implies the use of whites as the reference point (we want to know why there is greater variance among blacks than among whites), we include the joint component in our decompositions.
The components sum exactly to the total difference in variance, so we can express the components as proportions of the total difference in variance by dividing them by the total difference in variance. This property of Nau-Firebaugh decomposition also means that the components for one cause of death are not affected by how finely or coarsely the other causes are classified. The magnitude of a component indicates how large the disparity between blacks and whites would have been if blacks had the same values as whites on all other components, and had differed only with respect to the particular factor under investigation (for example, the spread effect for heart disease). Because σ 2 B − σ 2 W is a positive number, a positive value in our results indicates that the component in question contributes to the greater variance in ages of death for blacks, whereas a negative value indicates that the component operates in the opposite direction, that is, it compresses the difference in the variance for blacks and whites.
Decomposing the Black-White Difference in Lifespan Variance by Sex
In this section, we extend the Nau-Firebaugh equations to decompose spread and allocation components by sex. To anticipate a major result of this study, we find that the spread component accounts for about 87 % of the greater lifespan variance for blacks, and the allocation component accounts for most of the remainder. The timing and joint components are relatively small. The small joint component indicates that most of the disparity in lifespan variance is caused either by spread effects or by allocation effects but rarely by a combination of the two. Because the spread and allocation components account for virtually all the disparity in variance, we probe further to determine whether those two components arise primarily from differences between black men and white men or from differences between black women and white women.
The spread and allocation components can be broken down by subpopulations—in our case, by sex—as follows. For the allocation component, we begin with the identity p cB = p cBWomen + p cBMen (and similarly for p cW ). Hence, the allocation component for cause c (from Eq. 5b) can be rewritten as follows:
The first term in Eq. (6) is the part of the allocation component for cause c attributable to differences between black women and white women, and the second term is the part attributable to differences between black men and white men. The two parts sum exactly to the allocation component for a particular cause; thus, by summing over all causes, we obtain the part of the all-cause allocation component that is attributable to differences between white women and black women versus the part that is attributable to differences between white men and black men.
Now consider the spread component. With whites as the reference population, the formula for the cth cause of death is (σ 2 cB − σ 2 cW )p cW (Eq. (5a)). Because the sum of squares for blacks is the sum for women (SSBlackWomen) plus the sum for men (SSBlackMen), the numerator of σ 2 cB can be partitioned by sex:
where \( SSBlackWomen={{\displaystyle {\sum}_{i=1}^{N_{cBlackWomen}}\left({X}_{iBWomen}-{\overline{X}}_{Blacks}\right)}}^2 \) and similarly for black men. N cBlacks is the number of black victims of cause c, and \( {\overline{X}}_{Blacks} \) is the mean age of those victims.
The variance for whites is partitioned in the same way, so the difference in the within-cause variance for white and black victims is
By weighting the differences in Eq. (8) by p cW , the proportion of all white deaths due to the cth cause of death, we determine the part of the spread component for cause c attributable to differences between black women and white women versus the part attributable to differences between black men and white men:
The gender-specific components in Eq. (9) sum exactly to the spread component for a particular cause; thus, by summing over all causes of death, we obtain the part of the all-cause spread component that is attributable to differences between white women and black women versus the part that is attributable to differences between white men and black men.
Data
We compute multidecrement life tables using information on the number of deaths by cause, age, sex, race, and Hispanic origin from death records of the entire U.S. resident population in a given calendar year from the 2010 Multiple Cause of Death data archive (National Center for Health Statistics (NCHS) 2012). Causes of death are coded according to the 10th revision of the International Classification of Diseases (ICD-10) based on the recorded underlying cause of death, defined as “the disease or injury which initiated the train of morbid events leading directly to death, or the circumstances of the accident or violence which produced the fatal injury” (World Health Organization 2010).
To reduce the more than 10,000 ICD-10 codes to a manageable number of causes of death, we grouped them into four general categories—chronic diseases, communicable diseases, external causes of death, and a residual category of minor causes not elsewhere classified—further broken down into more specific causes, as follows (see Table S1 in Online Resource 1 for more details):
-
Chronic diseases: heart diseases, cancers, cerebrovascular diseases, chronic lower respiratory diseases, Alzheimer’s, diabetes, and nephritis.
-
Communicable diseases: influenza and pneumonia, septicemia, HIV/AIDS, and other communicable diseases.
-
External causes: homicide, suicide, traffic accidents, accidental poisoning, and other external causes.
-
A residual category (“minor causes, not elsewhere classified”) consisting of all ill-defined causes of death as well as those causes that have too few deaths to be considered independently, or that are not of primary interest in this analysis.
This coding scheme is based on a template employed to identify the 15 leading causes of death in the National Vital Statistics Report for 2006 (Heron et al. 2009). In particular, from the 10 leading causes of death in the United States, we separated HIV/AIDS, homicide, accidental poisoning, and suicide as stand-alone categories because they are highly associated with racial mortality differences in the United States (Harper et al. 2007; Warner et al. 2011). We also separated traffic accidents and accidental poisoning from the more general category “accidents” because they are two of the few causes of death where age at death varies more for whites than for blacks (thus reducing the overall black-white disparity). We grouped the remaining external causes under “other external causes.” We also added a category called “other communicable diseases” to capture the effect of communicable diseases not already listed in our classification scheme. Similarly, to separate the effect of all external causes, we added a remainder category for external causes that includes causes other than those already on our list (homicide, suicide, traffic accidents, and accidental poisoning). About 90 % of all deaths fall under the two general categories of “chronic diseases” (70 %) and “minor causes, not elsewhere classified” (20 %). Communicable diseases and external causes each account for about 5 % of all deaths.
The NCHS files also contain population counts from the U.S. Census Bureau’s April 1 modified race census counts for 2010 (NCHS 2012). These counts serve as population denominators for calculating age-, race-, and sex-specific mortality rates by cause category. We compute multidecrement life tables for non-Hispanic blacks and non-Hispanic whites to eliminate the effects of differences in population size and age composition (Das Gupta 1993). Ages are aggregated into five-year intervals, with the open-ended age category being 85 or older.Footnote 3 The life table deaths occurring after age 10 are then used to calculate cause-specific means, variances, and proportions of deaths for blacks and for whites, which are the statistics needed for decomposing the black-white difference in lifespan variance.
Results
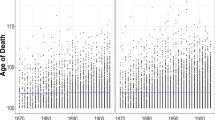
There are excess deaths for blacks relative to whites from about age 20 to about age 75 (Fig. 2).Footnote 4 Because overmortality for blacks occurs below the mean age at death for whites, it accounts for the lower life expectancy of blacks. However, a lower mean does not necessarily imply greater variance, so we cannot determine the sources of the black-white disparity in variance from Fig. 2. To determine those sources, we now employ the Nau-Firebaugh method to decompose the racial difference in variance into its spread, allocation, timing, and joint components.
Decomposition of the Racial Disparity in Lifespan Variance
As noted earlier, the black-white difference in adult lifespan variance (σ 2 B − σ 2 W ) was 244.0 – 199.1 = 44.9 in 2010. Figure 3 displays the all-cause components of that disparity, where “all-cause” is the sum of the cause-specific components. The all-cause spread component accounts for about 87 % of the disparity, indicating that lifespans are more variable for blacks largely because age at death varies more for blacks than for whites among those who succumb to the same cause. The all-cause allocation component is about 12 %, indicating that only about 12 % of the disparity in lifespan variance would persist if blacks and whites differed only with regard to cause-specific death rates. The all-cause allocation component is small because of offsetting cause-specific allocation effects, as we show later. The all-cause timing component is even smaller and is negative (–4.7 %), indicating that lifespans would vary less for blacks than for whites if blacks and whites differed only with respect to variance in the average age at death across causes. The all-cause joint component is also small (about 5 %) and is due largely to an allocation-timing interaction effect for homicides: blacks are more likely to die of homicides, and black homicide victims tend to be younger than white homicide victims (see Table S2 in Online Resource 1 for cause-specific proportion of deaths, and mean age at death, for blacks versus whites).
The cause-specific spread components show that age at death in the United States varies more for blacks than for whites for almost all causes of death (Fig. 4). As one would expect, the most common causes of death contribute the most to the spread component. Heart disease, for example, is the biggest contributor, with a spread component of 29 %. The next most common causes of adult deaths in the United States—cancers, chronic lower respiratory diseases, and cerebrovascular diseases—each contribute about 8 % to the black-white disparity in variance. Likewise, among the minor causes of death that we were unable to place in one of our other cause categories, there typically is greater variance in the age at which blacks succumb than in the age at which whites succumb. As a result, greater within-cause variance for blacks is the main driver of the black-white disparity in lifespan variance, accounting for nearly nine-tenths of the disparity.Footnote 5
The profile is very different for the cause-specific allocation components (Fig. 5). Relatively rare causes of death—HIV/AIDS, homicide, suicide, and accidental poisoning—contribute much more to the allocation component than do much more common causes of death, such as heart disease, cancer, cerebrovascular diseases, and minor causes not elsewhere classified. Moreover, unlike the cause-specific spread components (all of which are either positive or negligibly negative), the cause-specific allocation components largely offset one another. Homicide’s large allocation effect (38 %), for example, is almost entirely offset by the combined allocation effects of suicide (–22 %) and accidental poisoning (–15 %). The results for homicide indicate that 38 % of the black-white difference in lifespan variance would persist if higher homicide rates for blacks were the only difference in the mortality regimes of blacks and whites. On the other hand, if higher suicide rates for whites were the only difference in the mortality regimes of blacks and whites, the direction of the gap would reverse, and lifespan variance would be greater for whites than for blacks. Because of offsetting effects (Fig. 5), only about 12 % of the black-white difference in lifespan variance is attributable to differences in cause-specific death rates for blacks and whites. It is important to emphasize that the all-cause allocation component is not small because the cause-specific components are uniformly small; rather, the all-cause component is small because significant cause-specific components largely offset one another.
The timing and joint components contribute very little to the disparity in lifespan variance. Their contributions are minimal because virtually all of their cause-specific components are negligible (Table S3 in Online Resource 1). In the case of timing, accidental poisoning (mainly narcotics-related: Warner et al. 2011) has the largest effect by far (–14.9 %). Accidental poisoning also has a negative allocation effect (Fig. 5). Figure 6 helps us understand why both the allocation and the timing components are negative for accidental poisoning (thus narrowing the overall black-white disparity). From Fig. 6, we see that whites are more likely to die of accidental poisoning and that victims of accidental poisoning are disproportionately young. This explains the negative allocation effect: whites are more likely to die of a cause with a distinctively youthful profile. In addition, we see from Fig. 6 that whites who die of accidental poisoning are, on average, younger than blacks who die of accidental poisoning, thus increasing the between component of the variance in the mean age at death more for whites than for blacks. This accounts for the negative timing component for accidental poisoning.
Decomposition of the Racial Disparity in Lifespan Variance, by Sex
Figures 7 and 8 display the cause-specific spread and allocation components broken down by sex. We focus on spread and allocation because the spread and allocation components together account for more than 99 % of the black-white disparity in 2010.
Overall, women contribute twice as much to the racial disparity in lifespan variance as men do. Women’s spread component alone accounts for 62.5 % of the racial disparity in lifespan variance. For every cause of death, the female spread component is either positive or barely below zero (Fig. 7), indicating that regardless of the cause of death, age at death tends to be more variable for black women than for white women. The female differences in variance are most significant for heart disease and minor causes not elsewhere classified, each amounting to about 21 % of the racial disparity.
We find the same cause-specific dynamic for the spread component for men. That is, for every cause of death, age at death is more variable for black men than for white men or—if the variance is greater for white men—the differences are negligible. Compared with disparities for women, however, the differences in variance between black men and white men are much smaller for heart disease and for minor causes. In the case of heart disease, for example, the spread component for women is three times larger than the component for men. This finding raises an important question for future research: Why is the difference in the cause-specific variance in age at death (spread component) greater for black women versus white women than it is for black men versus white men?
The all-cause allocation component is relatively small for both women and men. For women, the all-cause allocation component accounts for less than 4 % of the overall racial disparity in lifespan variance. This result, however, masks several race- and sex-specific differences in cause-specific mortality (Fig. 8). For instance, although differences in death rates for diabetes, HIV/AIDS, and homicide for black women versus white women add to the difference in lifespan variance for blacks and whites, differences in death rates for chronic lower respiratory diseases, suicide, and accidental poisoning compress the black-white difference in lifespan variance. These contrasting patterns offset, producing the small overall contribution of allocation for women.
The allocation component for men accounts for less than 9 % of the total racial difference in the variance of age at death, with most causes of death contributing only marginally. There are, however, four causes for which the allocation component among males is notable. The first is homicide. Although deaths due to homicide represent less than 0.3 % of all white deaths and less than 1.3 % of all black deaths, the allocation component for men constitutes one-third of the overall black-white disparity in lifespan variance. This result is even more striking when we consider that homicides’ allocation component for men greatly exceeds heart diseases’ allocation component for men, even though men are 36 times more likely to die of heart disease.
HIV/AIDS also contributes to the allocation effect for men, accounting for about 10 % of the overall racial disparity in variance. Allocation components for suicide (–17.1 %) and accidental poisoning (–9.2 %) work in the opposite direction, reducing the disparity. Because suicide and accidental poisoning victims tend to be relatively young, the greater incidence of suicide and accidental poisoning among white men compresses the disparity. As a result, the all-cause allocation component for men is not as large as one might expect in light of the magnitude of racial differences in homicide and in HIV/AIDS death rates.
Sensitivity Analyses
Studies of adult lifespan variance have used various age thresholds to distinguish adult mortality from infant and child mortality. Edwards (2011), Edwards and Tuljapurkar (2005), Engelman et al. (2010), Nau and Firebaugh (2012), Tuljapurkar (2010), Tuljapurkar and Edwards (2011), and van Raalte and Caswell (2013) used age 10. Gillespie et al. (2013) and Smits and Monden (2009) used age 15, but Wilmoth and Horiuchi (1999) used both age 15 and age 30. Lynch and Brown (2001) used age 20.
As a sensitivity check, we reestimated our results with age 20 as the threshold. The findings are virtually the same. Most components are within one percentage point whether we use age 10 or age 20, and only one component—the allocation component for homicide—changes by more than 2 percentage points. Changing the threshold to age 20 reduces the allocation component for homicide from 38 % to 32 %, indicating that a significant number of homicide victims are adolescents.
We also checked the sensitivity of our results to the choice of whites as the reference group. For this test, we replaced the values of whites with the midpoint values for blacks and whites. This eliminates the joint component by dividing it evenly among blacks and whites. Because the joint components are small, the findings are essentially the same. The all-cause spread component declines slightly but remains by far the dominant component, accounting for 85 % of the greater lifespan variance among blacks. The all-cause allocation component increases somewhat, from 12.4 % to 14.9 %. Timing remains a nonfactor, accounting for only 0.1 % of the greater variance for blacks.
Discussion and Conclusion
The lifespans of blacks in the United States are both shorter and more variable than the lifespans of whites. Most prior studies examine the shorter duration of blacks’ lifespans; this study, by contrast, examines the greater uncertainty of blacks’ lifespans. By using the spread-allocation-timing decomposition method (Nau and Firebaugh 2012), we are able to determine the proximate sources of the black-white disparity in lifespan variability in America. The method reveals which causes of death and which variance components of those causes are most important for producing differences in lifespan variance between two populations.
For every major cause of death, we find that the variation in age at death is greater for blacks than it is for whites. In fact, 87 % of the racial disparity in lifespan variance can be attributed to spread effects. This means that 87 % of the overall difference in variance would persist if blacks and whites differed only with respect to cause-specific variability in age at death. Furthermore, the racial difference in variation in age at death is driven largely by differences between black women and white women. If the mortality regimes of black and white men did not differ at all, most of the overall disparity (62.5 %) in lifespan variance would remain if black and white women differed only with respect to cause-specific variability in age at death. On the other hand, if the mortality regimes of black and white women did not differ at all, about 25 % of the disparity would remain if black and white men differed only with respect to cause-specific variability in age at death.
Because timing and joint effects are small and offsetting, the remainder of the racial disparity in lifespan variance is due almost entirely to differences in the incidence of causes of deaths for blacks and whites. We might expect a much larger allocation component because of blacks’ higher rates of premature deaths from homicide and HIV/AIDS. By decomposing simultaneously by cause of death as well as by spread-allocation-timing, we find that the notable racial disparity in lifespan variance attributable to higher rates of homicide and HIV/AIDS among blacks is largely offset by whites’ higher rates of premature deaths from suicide and drug poisoning. In other words, the black-white disparity in lifespan variance would not narrow very markedly if blacks had the same cause-specific mortality rates as whites for all causes of death, but it would narrow significantly if blacks’ death rates for homicide and HIV/AIDS alone were reduced to the rates for whites.
In addition to presenting these new findings, this study demonstrates the power of the spread-allocation-timing decomposition method for gauging the effects of premature deaths on the variability of lifespans. One benefit of the spread-allocation-timing method is that it enables researchers to quantify the contribution of group differences in death rates to group differences in lifespan variance. This quantification is particularly important for relatively uncommon causes of death, such as homicide, that “overcontribute” to lifespan variance by increasing the incidence of premature deaths. Among the causes of death that disproportionately affect the young, some (e.g., homicide) disadvantage blacks, while others (e.g., suicide) disadvantage whites. By quantifying the contribution of group differences in cause-specific death rates to group differences in lifespan variance, researchers now have an elegant way to disentangle the offsetting effects of premature deaths.
In interpreting our results, readers should consider the limitations of studies based on the underlying cause of death. The underlying cause may be hard to determine, particularly among the elderly, for whom multiple diseases at the time of death are more likely (Israel et al. 1986). Where cause of death is miscoded, the error has been shown to be correlated with race (Noymer et al. 2011). In addition, mortality estimates could be biased, especially for minority populations, by census undercounts, age misreporting, and race misclassification.
Finally, information is lost due to the aggregation of causes in our classification scheme. In collapsing the thousands of specific death codes in the ICD-10 classification scheme into a manageable number of cause categories, we tried to balance specificity with parsimony and relevance to black-white health disparities. As reported earlier, we focused on (1) the most common causes of death and (2) those causes—such as homicide, HIV/AIDS, accidental poisoning, and suicide—that are less common but disproportionately afflict blacks or whites. Because disagreement over cause of death is more likely when causes are grouped into narrow cause categories, we did not further disaggregate common causes of death unless they disproportionately affected blacks and whites. Importantly, due to the additivity property of the Nau-Firebaugh decomposition method, the results that we report for a particular cause of death would not change if we aggregated or disaggregated the other causes of death. This means, for example, that our results for chronic diseases are not dependent on the coarseness of our classification of other types of causes.
Our findings have implications for health policy and research. With regard to research, they demonstrate the value of decomposing mortality along a spread-allocation-timing axis as well as a cause-of-death axis. The spread-allocation-timing method enables researchers to describe and quantify how differences in the mortality regimes for two populations produce differences in the variability of lifespans in those populations. In the case of blacks and whites in the United States in 2010, the decomposition of lifespan data reveals that the greater variance among blacks is primarily the product of cause-specific spread effects. Because disparities in life expectancy are not a function of differences in cause-specific variance, studies that focused only on racial differences in life expectancy would miss this significant source of black-white differences in mortality.
We also find that spread effects are more critical for women than for men, whereas allocation effects are more critical for men than for women. Hence, our findings imply that the causal mechanisms that matter are not necessarily the same for women and men. The further implication is that observed sex differences could be strategic in the search for those causal mechanisms. Consider, for example, our finding that the spread effect for heart disease is much greater for women than for men. This finding means that the variance in age at death for heart disease victims is greater for black women than it is for white women, and that this difference is greater than the difference in variance between black men and white men. Important causal mechanisms should exhibit similar variance patterns. If behavioral factors largely account for the greater variance in the lifespans of black heart disease victims, for example, then we expect to find greater racial variance in those factors for women than for men. Hence, the results of this study provide a basis for more targeted future research on racial disparities in mortality.
With regard to policy, our results indicate the importance of sex-specific interventions to reduce racial disparities. In the case of HIV/AIDS, for example, there is greater potential for significant reductions of the racial gap when men are targeted; the opposite is true for heart disease and diabetes, where interventions focused on women are more likely to narrow the racial gap. Our findings also imply that the black-white disparity in lifespan variance could be narrowed substantially by the prevention of a relatively small proportion of deaths in the United States. About 2 % more blacks than whites die of homicide or HIV/AIDS. Eliminating that 2 % difference in deaths due to homicide and HIV/AIDS, other things equal, would cut the black-white disparity in lifespan variance by half (the allocation component is 38 % for homicides and 16 % for HIV/AIDS). Although eliminating that 2 % difference might not be easy, it is likely easier than the alternatives. Preventing fewer deaths should be easier than preventing more deaths, so resources devoted to reducing homicides and HIV/AIDS in the black population are likely to provide larger and more immediate narrowing of racial disparity in lifespan variance than the same level of resources spent on reducing the gap in more common causes of death.
Notes
Following Tuljapurkar and Edwards (2011), we use age 10 to separate adult mortality from infant and child mortality. We reach the same conclusions whether we use age 10 or age 20 as the cutoff point.
Other examples can be given. Higher rates of Alzheimer’s for blacks, for example, would reduce the black-white disparity in life expectancy but increase the black-white disparity in lifespan variance. Findings about racial differences in life expectancy do not necessarily apply to racial differences in lifespan variability.
For the open-ended age category of 85+, we used age 90 as the midpoint for our calculations because for both men and women and similarly for whites and blacks, age 90 is approximately the mean age at death for those who survived to age 85.
Age-specific death rates are higher for blacks than for whites until age 87 (Fenelon 2013). Because the black-white crossover at age 87 might be due in part to age misreporting for blacks (Fenelon 2013), we performed simulations based on downward adjustments of the age at death among older blacks. These adjustments had very little effect on the difference in the black-white variance.
This result is not an artifact of the decomposition method. Using the Nau-Firebaugh method, Lariscy et al. (2013) found that allocation effects—not spread effects—account for the majority of the difference in the age-at-death variation between Hispanics and whites.
References
Arriaga, E. (1984). Measuring and explaining the change in life expectancies. Demography, 21, 83–96.
Becker, G., Philipson, T., & Soares, R. (2005). The quantity and quality of life and the evolution of world inequality. American Economic Review, 95, 277–291.
Beltrán-Sánchez, H., Preston, S. H., & Canudas-Romo, V. (2008). An integrated approach to cause-of-death analysis: Cause-deleted life tables and decompositions of life expectancy. Demographic Research, 19(article 35), 1323–1350. doi:10.4054/DemRes.2008.19.35
Das Gupta, P. (1993). Standardization and decomposition of rates: A user’s manual. Washington, DC: U.S. Department of Commerce, Economics and Statistics Administration, Bureau of the Census.
Edwards, R. D. (2011). Changes in world inequality in length of life: 1970–2000. Population and Development Review, 37, 499–528.
Edwards, R. D. (2013). The cost of uncertain lifespan. Journal of Population Economics, 26, 1485–1522.
Edwards, R. D., & Tuljapurkar, S. (2005). Inequality in life spans and a new perspective on mortality convergence across industrialized countries. Population and Development Review, 31, 645–674.
Engelman, M., Canudas-Romo, V., & Agree, E. M. (2010). The implications of increased survivorship for mortality variation in aging populations. Population and Development Review, 36, 511–539.
Fenelon, A. (2013). An examination of black/white differences in the rate of age-related mortality increase. Demographic Research, 29(article 17), 441–472. doi:10.4054/DemRes.2013.29.17
Fries, J. F. (1980). Aging, natural death, and the compression of morbidity. New England Journal of Medicine, 303, 130–135.
Fries, J. F. (1984). The compression of morbidity: Miscellaneous comments about a theme. Gerontologist, 24, 354–359.
Gillespie, D. O. S., Trotter, M. E., & Tuljapurkar, S. D. (2013). Mortality change and lifespan inequality (Working Paper No. 127). Stanford, CA: Stanford University, Morrison Institute for Population and Resource Studies.
Harper, S., Lynch, J., Burris, S., & Davey Smith, G. (2007). Trends in the black-white life expectancy disparity in the United States, 1983–2003. Journal of the American Medical Association, 297, 1224–1232.
Harper, S., Rushani, D., & Kaufman, J. S. (2012). Trends in the black-white life expectancy disparity, 2003–2008. Journal of the American Medical Association, 307, 2257–2259.
Heron, M. P., Hoyert, D. L., Murphy, S. L., Xu, J. Q., Kochanek, K. D., & Tejada-Vera, B. (2009). Deaths: Final data for 2006 (National Vital Statistics Reports). Hyattsville, MD: National Center for Health Statistics.
Israel, R. A., Rosenberg, H. M., & Curtin, L. R. (1986). Analytical potential for multiple cause-of-death data. American Journal of Epidemiology, 124, 161–179.
Kannisto, V. (2000). Measuring the compression of mortality. Demographic Research, 3(article 6), 1–24. doi:10.4054/DemRes.2000.3.6
Kochanek, K. D., Arias, E., & Anderson, R. N. (2013). How did cause of death contribute to racial differences in life expectancy in the United States in 2010? (NCHS data brief). Hyattsville, MD: National Center for Health Statistics.
Lariscy, J. T., Nau, C., Firebaugh, G., & Hummer, R. A. (2013, April). Racial/ethnic inequality in adult survival: Decomposition of age at death variation among U.S. adults. Paper presented at the annual meeting of the Population Association of America, New Orleans, LA.
Lynch, S. M., & Brown, J. S. (2001). Reconsidering mortality compression and deceleration: An alternative model of mortality rates. Demography, 38, 79–95.
Lynch, S. M., Brown, J. S., & Harmsen, K. G. (2003). Black-white differences in mortality deceleration and compression and the mortality crossover reconsidered. Research on Aging, 25, 456–483.
Manton, K. G., & Singer, B. (1994). What’s the fuss about compression of mortality? Chance, 7, 21–30.
Myers, G. C., & Manton, K. G. (1984). Compression of mortality: Myth or reality? Gerontologist, 24, 346–353.
National Center for Health Statistics (NCHS). (2012). Multiple cause of death file 2010. Washington, DC: CDC.
Nau, C., & Firebaugh, G. (2012). A new method for determining why length of life is more unequal in some populations than in others. Demography, 49, 1207–1230.
Noymer, A., Penner, A. M., & Saperstein, A. (2011). Cause of death affects racial classification on death certificates. PloS One, 6(1), e15812. doi:10.1371/journal.pone.0015812
Peltzman, S. (2009). Mortality inequality. Journal of Economic Perspectives, 23, 175–190.
Pollard, J. H. (1982). The expectation of life and its relationship to mortality. Journal of Institute of Actuaries, 109, 225–240.
Pollard, J. H. (1988). On the decomposition of changes in expectation of life and differentials in life expectancy. Demography, 25, 265–276.
Smits, J., & Monden, C. (2009). Length of life inequality around the globe. Social Science & Medicine, 68, 1114–1123.
Tuljapurkar, S. (2010). The final inequality: Variance in age at death. In J. B. Shoven (Ed.), Demography and the economy (pp. 209–221). Chicago, IL: The University of Chicago Press.
Tuljapurkar, S., & Edwards, R. D. (2011). Variance in death and its implications for modeling and forecasting mortality. Demographic Research, 24(article 21), 497–526. doi:10.4054/DemRes.2011.24.21
U.S. Department of Health and Human Services. (2011). Healthy people 2020: Maternal, infant, and child health objectives. Washington, DC: U.S. Department of Health and Human Services.
van Raalte, A. A., & Caswell, H. (2013). Perturbation analysis of indices of lifespan variability. Demography, 50, 1615–1640.
van Raalte, A. A., Martikainen, P., & Myrskylä, M. (2014). Lifespan variation by occupational class: Compression or stagnation over time? Demography, 51, 73–95.
Warner, M., Chen, L. H., Makuc, D. M., Anderson, R. N., & Miniño, A. M. (2011). Drug poisoning deaths in the United States, 1980–2008 (NCHS Data Brief No. 81). Hyattsville, MD: National Center for Health Statistics.
Wilmoth, J. R., & Horiuchi, S. (1999). Rectangularization revisited: Variability of age at death within human populations. Demography, 36, 475–495.
World Health Organization. (2010). WHO mortality database documentation. Retrieved from http://www.who.int/healthinfo/statistics/mortality_rawdata/en/index1.html
Acknowledgments
This research was supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to the Population Research Institute at The Pennsylvania State University for Population Research Infrastructure (R24HD041025), and as well as from a Family Demography Training grant (T-32HD007514). We thank Jenny Van Hook for her comments and encouragement.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(DOCX 34.2 KB)
Appendix: Derivation of Equations for Spread, Allocation, and Timing Components
Appendix: Derivation of Equations for Spread, Allocation, and Timing Components
As noted in the text, the ANOVA equation for the total variance in age at death, by cause, is
where N is the total number of deaths and the c = 1, 2, . . . , C causes of death are mutually exclusive and exhaustive. The first term in Eq. (A1) is the within-category component of the variance, and the second term is the between-category component of the variance.
Among those who are victims of a given cause, the variance in age at death is
Thus:
Substituting Eq. (A3) into the ANOVA within-component in Eq. (A1), we see that the ANOVA within-cause component is the incidence-weighted sum of the within-cause variances, σ 2 c :
where p c = N c / N, the proportion of deaths due to cause c. The between-variance component in ANOVA is likewise an incidence-weighted sum of dispersion:
The difference in the variances for two populations is the difference in the ANOVA within- and between-variance components for the two populations. It follows that the difference in the overall variances of lifespans of two populations B and W, broken down by cause, is the sum of the within-component difference and the between-component difference in the two populations:
where ∑ c p cB = ∑ c p cW = 1.0. Because positive differences are more intuitive to interpret than negative differences, we designate the population with the greater variance as B, so σ 2 B − σ 2 W > 0.
Algebraic manipulation of Eq. (A6) separates the spread component, which captures the part of the total disparity in variance that is due to cause-specific differences in the variances of the two populations; the allocation component, which accounts for the contribution of differences in cause-specific incidence; and the timing component, which isolates the effect of differences between the two populations in the variability of the mean age at death across causes. Taking the population with the smaller variance as the reference, and summing over all C causes, the spread, allocation, timing, and joint components are as given in the text (also in Nau and Firebaugh 2012).
Rights and permissions
About this article
Cite this article
Firebaugh, G., Acciai, F., Noah, A.J. et al. Why Lifespans Are More Variable Among Blacks Than Among Whites in the United States. Demography 51, 2025–2045 (2014). https://doi.org/10.1007/s13524-014-0345-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13524-014-0345-2