Abstract
Objective
In this review, several interlinking issues related to cancer stem cells (CSCs) in malignant solid tumors are sequentially discussed.
Methods
A literature search was performed using PubMed, Web of Science and the Cochrane library, combining the words CSCs, solid tumor, isolation, identification, origination, therapy, target and epithelial–mesenchymal transition.
Results
Because a primary problem is the isolation of CSCs, we first analyzed the advantages and disadvantages of recently used methods, which were mostly based on the physical or immunochemical characteristics of CSCs. Once CSCs are isolated, they should be identified by their stem cell properties. Here, we suggest how to establish a standard identification strategy. We also focused on the origination hypotheses of CSCs. The supporting molecular mechanisms for each theory were thoroughly analyzed and integrated. Especially, epithelial– mesenchymal transition is an increasingly recognized mechanism to generate CSCs that are endowed with a more invasive and metastatic phenotype. Finally, we discuss putative strategies of eliminating CSCs as effective cancer therapies.
Conclusion
After several interlocking issues of CSCs are thoroughly clarified, these CSCs in solid malignant tumors may specifically be targeted, which raises a new hope for eliminating these tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recent evidence indicates that tumors contain a small subset of stem-like cells, named cancer stem cells (CSCs), which are responsible for tumor initiation, maintenance and proliferation [1]. CSCs are generally dormant or slowly cycling tumor cells that have the ability to reconstitute tumors [2]. They are thought to be involved in resistance to chemo-/radiation therapy and tumor relapse and progression. The American Association of Cancer Research Workshop adopted the consensus definition of CSCs as “cells within a tumor that possess the capacity for self-renewal and that can cause the heterogeneous lineages of cancer cells that constitute the tumor” [3]. The notion that CSCs actually may exist has created great excitement in the research community [2].
Although the existence of CSCs was first proposed over 36 years ago, only recently CSCs were identified in solid tumors, including breast [4], prostate [5], brain [6], colon [7] and liver [8]. Among these cancer types, hepatocellular carcinoma (HCC) is a highly malignant solid tumor with frequent metastases and a poor prognosis [9]. Hepatic CSCs (HCSCs) are a suitable in vitro model to study HCC-initiating cells and to develop therapeutic strategies aimed at eradicating the tumorigenic subpopulation within HCC. It is supposed that any drug capable of killing CSCs would, in theory, be curative. To make the above presumption come true, there are several questions to be answered. The primary question is how to isolate CSCs efficiently. In case CSCs were isolated by different methods, is there a standard criterion to identify them? Assuming that the criterion is established, a next question is from where CSCs originate. Although several theories of CSC origination have attracted much attention, there is little sound evidence to support any of them, and the underlying molecular mechanisms of CSC origination remain to be established. Only when the above issues are well resolved, researchers can begin to search for effective treatment strategies targeting CSCs. In this review, the above questions are sequentially discussed. The resulting information may be relevant for the development of specific therapies targeting CSCs.
2 The isolation of CSCs
CSC researchers are challenged by developing a simplified approach to purify these rare cells. A variety of separation techniques is available (Fig. 1).
2.1 The isolation of CSCs by surface markers
2.1.1 The feasibility and application of surface markers
Among the methods for isolating CSCs, fluorescence-activated cell sorting (FACS) or magnetic-activated cell sorting (MACS), which are based on cell surface markers, are most commonly used for either positive or negative selection [10]. Many types of CSCs have been isolated by their cell-surface markers, and the same markers can be used to isolate CSCs from different cancer types. In reverse, different markers can be used to isolate CSCs from the same cancer type [11]. Epithelial cell adhesion molecule (EpCAM) is over-expressed in most solid cancers and has recently been identified as a CSC marker [12]. CD133 is also among the most useful markers for the identification of solid cancer CSCs [13] although it is not specific for some cancer types. CD44, for example, was found to be more specific than CD133 to isolate gastric cancer-initiating cells from a panel of human gastric cancer cell lines [6]. In addition, it should be noted that there exist discrepancies in the identities of CSC markers among different reports [14–16]. Thus, a single marker may not be sufficient to isolate CSCs.
The primary obstacle in isolating pure HCSCs is the lack of a specific marker. Four years ago, a study by our group revealed that reactivated CD133+ cells were frequently present in HCC [17], which corresponded with higher-stage tumors. To date, although several possible markers for HCSCs, including CD133 [18], EpCAM [19], CD90/CD44 [20], DLK [21], CD13 [22] and OV6 [23], have been used to isolate putative CSCs from HCC, it is unknown which one serves as a uniform marker for HCSCs. Therefore, the presence of certain markers alone should be taken with caution when separating HCSCs. A combination of several markers may be a better choice. For example, CD133, a useful marker for identifying HCSCs, combined with other stem cell markers, can more specifically characterize the tumorigenic sub-population. Such markers may include ALDH [24] and CD44 [25]. EpCAM, another commonly used HCSC marker, combined with alpha-fetoprotein [26] or CK-19 [27], may also serve as better indicators to isolate HCSCs. As yet, however, no standard combination has been found. Further study is needed to clarify this issue.
2.1.2 The limitations of surface markers
The main weakness is that the markers used to purify CSCs are not specific enough, and in most cases CSCs have not been completely purified. Besides, immunochemical methods present the disadvantage of being time-consuming and expensive because of the use of monoclonal antibodies and advanced technologies.
Let's again take HCSCs as an example. First, there is no specific marker for HCSCs. Hepatic cancer cells with a CD133+ or EpCAM+ phenotype are believed to have stem-like properties, but they exhibit limited plasticity [19]. In fact, the expression level of CD133 on tumor cells might change during long-term culture in serum-containing conditions. Second, hepatic normal stem cells (HNSCs) and HCSCs share many surface markers, such as CD133 and EpCAM. This phenomenon has also been observed for other normal and cancer stem cells. Third, markers for HCSCs in primary cultured hepatic cancer cells and HCC cell lines are different. These differences can be divided into three aspects: before the isolation of HCSCs, primary HCC cells often need to be purified by other methods, such as centrifugation. When one marker is used for isolation, the marker for separation of HCSCs in primary HCC cells may be totally different from these in HCC cell lines (EpCAM and CD133 are more likely to be HCSC markers in primary HCC cells than in HCC cell lines [13, 26]). When several markers are used, the separation of HCSCs in primary tumor cells requires more markers than in HCC cell lines. The reasons mainly come from the distinct properties of these two kinds of cancer cells. Through in vitro selection, cancer cell lines are relatively homogeneous in view of cell category. In contrast, without purification primary cancer cells are considered to be more heterogeneous.
2.2 The isolation of CSCs by efflux of fluorescent dyes
2.2.1 The application of fluorescent dye efflux
To avoid the above limitations of surface markers, other methods for CSC separation include isolation based on the differential efflux of fluorescent dyes, such as rhodamine 123 (Rho) or Hoechst 33342 (Hoechst). After the cells are stained with Hoechst, the visualization of Hoechst fluorescence simultaneously at two emission wavelengths localizes a distinct, small, non-stained cell population that is designated as the side population (SP). The isolation of the SP is based on the expression of ATP-binding cassette (ABC) transporters [28]. There is mounting speculation that ABC transporters repress the maturation and differentiation of stem cells [29]. In the last decade, the isolation of SP cells has become a useful method for obtaining CSCs from various tumors originating from skeletal muscle, breast, liver, small intestine, and uterine [30–34]. In recent years, SP cell analysis and sorting have been successfully applied to many HCC cell lines to identify a minor cell population with CSC properties. Chiba and colleagues reported that among four HCC cell lines analyzed, SP cells were detected in Huh7 (0.25 %) and PLC/PRF/5 (0.80 %), but not in HepG2 or Huh6 [35]. Later, SP cells were sorted from four other HCC cell lines, and the SP proportions among HCCLM3, MHCC97-H, MHCC97-L and Hep3B were 28.7 ± 1.6 %, 14.5 ± 0.6 %, 4.2 ± 0.4 %, and 0.9 ± 0.1 %, respectively [36]. All of the SP cells showed similar characteristics to CSC-like cells. Recently, we isolated SP cells not only from the HCC line, but also from primary cultured diethylnitrosamine (DEN)-induced HCC cells [37, 38]. Based on in vitro and in vivo experiments, we demonstrated that these SP cells might serve as a reservoir of CSCs [39, 40].
2.2.2 The flaws of fluorescent dye efflux
Although SP may be an alternative method to isolate CSCs when specific markers are lacking, there are many questions about this method. First, the main criticism concerns the use of Hoechst dye as a mean to isolate stem-like cells because it can be toxic. Hoechst interferes with cell functions as long as the dye is present in the nucleus [38]. Second, the SP assay and its applications in stem cell biology pose technical challenges related to sample preparation, data acquisition, analysis, and interpretation. In addition, we should highlight the value of multicolor phenotyping, the impact of DNA ploidy, and the importance of distinguishing graft versus host cells for an appropriate SP discrimination [41]. Put simply, it takes much thought and effort and many resources to perform a standard SP isolation. Third, importantly, although SP cells are clearly enriched among stem cells, several reports caution that dye efflux is not a common property of all stem cell populations. It should be emphasized that, similar to normal tissues, not every cancer contains SP cells, such as HepG2 and Huh6 cells [35]. Therefore, SP cells may only represent one of the putative cancer stem cell populations. That is to say, non-SP cell populations may also harbor cancer stem-like cells. Lastly, the equipment required for SP isolation is expensive. SP isolation requires a specialized flow cytometer, in which the ultraviolet (UV) laser device must be installed for fluorescence activation [42]. Thus, it is very expensive to buy and operate. These limitations of using SPs to isolate CSCs need to be overcome for this approach to become widely used.
To avoid some of the above limitations of SP isolation, in our previous study, Hoechst dye was replaced by another fluorescent dye, Rho, to isolate CSC-like cells from MHCC97 cells. Rho is used as a substrate of the ABCB1/P-gp transporter to evaluate the toxicity of drugs and to examine the functional activity of P-gp in cultured cells [43]. Therefore, Rho is not toxic to cells, even at high doses (1–10 mM) [44]. Additionally, to perform Rho/FACS, a standard flow cytometer is sufficient, in which the fluorescence is activated by an argon-ion or helium-cadmium laser. A standard flow cytometer costs less (in terms of both purchase and maintenance) than cytometers offering UV excitation [45]. Although Rho may not be as effective as Hoechst for CSC sorting, given its low toxicity and cost it may be a useful method for CSC identification. However, there are still some limitations to this method, such as false-positive stem cell yields.
2.3 The isolation of CSCs by physical characteristics
2.3.1 The feasibility of physical separation
The last choice for isolation of CSCs is based on physical separation methods, such as density-gradient centrifugation (DGC), which remains a good alternative to immunochemical methods. Percoll is an ideal medium for DGC, as it is a well-known reagent that is relatively inexpensive and easy to acquire [46]. Its nearly ideal physical characteristics make it especially useful in the enrichment of cell populations [46]. Without difficulty, this medium can be diluted to cover a wide range of densities. Even by using continuous-gradient centrifugation, cancer cells can be separated into as many fractions as desired, and stem/progenitor cells are significantly enriched within some fractions. Thus, Percoll-based DGC is a convenient method for enriching and harvesting CSCs [47]. The brief protocol is as follows: to explore optimal continuous gradients, different starting Percoll solutions are centrifuged at high-speed (20,000 × g) during different time periods (30, 60, or 90 min) in an angle head rotor, separately. After the resulting tubes have been standing for 30 min, the tube with the best formation of gradients is selected. The cell suspension is gently layered on top of the gradients and centrifuged at 500 × g for 15 min in a swinging bucket rotor. As the densities increase continuously from top to bottom of the tube, the sizes of the cells rise correspondingly. Based on the monitor of Density Marker Beads, the cell fractions are numbered increasingly from top to bottom of the tube. Different cell fractions are obtained by upward displacement. The principle of separation is based on the specific cell densities of CSCs in mixed cancer cell populations.
Previously, we successfully isolated HNSCSs from primary cultures of fetal liver cells [48]. Following this method, with minor adjustments, HNCSCs were also isolated from primary cultured HCCs. Interestingly, the flow cytometry results showed a preferential distribution of the progenitor cell markers CD133 and EpCAM in a HCC population characterized by a density between 1.041 and 1.062 g/ml. These HCCs showed CSC characteristics [49].
2.3.2 The disadvantages of physical separation
The main shortcoming of physical separation is its low efficiency. Purified cell populations may harbor false CSCs and, therefore, not constitute pure CSCs. Apart from the isolation efficiency, the complexity of the procedure is another factor that should be considered. Moreover, a high-speed centrifuge must be used for DGC [46].
3 The identification of CSCs (Fig. 2)
3.1 The in vitro identification of CSCs
Based on the consensus of many different researchers, the in vitro identification of CSCs can be divided into two parts: their static characteristics and their dynamic characteristics. The static characteristics of CSCs include their specific morphology and high expression of stem cell markers [35]. Notably, the morphology of CSCs is different from ordinary non-CSCs, such as a high nucleus:cytoplasm volume ratio, few organelles, many cilia or pseudopodia, cellular pleomorphism, and hyperchromatic nuclei. Immunological assays are commonly used to detect the levels of stem cell markers, such as immunocytochemistry, immunofluorescence, western blotting and flow cytometry. Microarray analysis and polymerase chain reaction are frequently used to detect the mRNA levels of the above mentioned stem cells [36].
The tests of dynamic characteristics of CSCs mainly consist of the evaluation of self-renewal, the observation of clone formation, the identification of multi-lineage differentiation and the evaluation of their chemoresistance. First, self-renewal means that after growth daughter CSCs retain the same stem cell properties as parental CSCs. That is to say, the daughter CSCs should display the same static characteristics (described above) as their parental CSCs. Moreover, these CSCs proliferate much faster than non-CSCs, which is not dependent on CSCs having stronger anti-apoptotic properties compared to non-CSCs. Second, multi-potentiality refers to the ability to differentiate into heterogeneous cancer cells (including CSCs and non-CSCs). These cancer cells are different in morphology and express different markers. Third, CSCs can easily form cell clones, and these clones remain relatively tightly associated. Fourth, the chemo-resistance of CSCs is often evaluated. It is widely accepted that CSCs are more chemo-resistant than non-CSCs.
3.2 The in vivo identification of CSCs
The growth of a subset of tumor cells (typically fewer than 5 % of all of the tumor cells) in immunodeficient mice has become the gold standard for identifying CSCs [50]. To this end, different numbers of cancer cells from distinct populations are injected into immunodeficient mice. Approximately 8 weeks later, these mice are sacrificed, and the tumors are examined. It is believed that as few as 1× 103 CSCs are sufficient for tumor formation [36]. Furthermore, the tumor-forming ability is judged by serial transplantation. It has been proposed that only CSCs can generate tumors via serial transplantation [3, 51].
4 The origination theories of CSCs
The origin of CSCs has been debated for several decades, but it remains elusive and is likely to be cancer type specific [52]. There are two major nonexclusive hypotheses about the cellular origin of CSCs (Fig. 3). One is the maturation arrest theory, in which CSCs originate from normal stem/progenitor cells. The other is the dedifferentiation theory, in which mature cells dedifferentiate and acquire features of stem/progenitor cells that retain the ability to proliferate during carcinogenesis. In addition, another possible but under-appreciated mechanism for the generation of CSCs is the fusion of stem cells and differentiated cells [52]. The last proposed origin of CSCs represents a combination of the above three theories.
4.1 The differentiation arrest of normal stem cells (NSCs)
At present, it is widely accepted that cancer arises from stem cells [53, 54], because these are the only cells that persist sufficiently long to acquire the required number of genetic changes for neoplastic development. For example, via tamoxifen treatment, Schepers et al. successfully induced intestinal stem cells to generate tumors [55]. More and more studies demonstrate that malignant cells and NSCs share many features, such as surface markers. It is even proven that some types of CSCs originate from NSCs, including breast CSCs from mammary epithelial stem/progenitor cells [56]. As mentioned above, accumulating evidence suggests that CSCs originate from stem cells and the committed progenitor cells. Some HCSCs express both CK19 and AFP, indicating a possible origin from a bipotential HNSC or its predecessor. An analysis of surgical specimens also demonstrated biliary marker–positive HCC, which suggests the possibility that at least some hepatic cancer cells arise from subpopulations with bipotential stem/progenitor properties. HCSCs and HNSCs also share many properties, such as small size [27], self-renewal, and stem cell markers, including CD133 and EpCAM [53].
4.2 The dedifferentiation of mature cells
Since 1993, cancer has been widely speculated to arise from dedifferentiation of mature cells that retain the ability to proliferate [57]. During the next two decades, several types of cancers have indeed been found to arise from the dedifferentiation of mature cells, such as HCC [58], gastric carcinoma [59], pancreatic cancer [60] and colorectal cancer [61]. Next to the differentiation arrest of NSCs, these results provide indications for the genesis of CSCs via dedifferentiation of mature cells. Nevertheless, there is no strong evidence for this option at present.
4.3 The cell fusion theory
Another possible but under-appreciated mechanism for the generation of CSCs is the fusion of stem cells and differentiated cells. The cell fusion hypothesis of CSCs adds an important functional basis to the potential multifaceted roles of cell fusion in the initiation and progression of cancer. However, this concept remains largely speculative because experimental evidence directly supporting or refuting it, especially in humans, is lacking [52]. One argument against the cell fusion hypothesis is that there is no evidence that the CSCs found in human or animal models are tetraploid in nature.
4.4 The multiple origins of CSCs
A single tumor may contain multiple CSC clones that are genetically distinct. In other words, CSCs may form hierarchical populations that consist of precancerous stem cells, primary CSCs, migrating CSCs and chemoradioresistant CSCs, all of which play different roles in cancer initiation and progression [62]. CSC heterogeneity could be an indication for the dedifferentiation theory, because some researchers believe that when, CSCs are eliminated, the remaining cancer cells could gain stem properties and generate new tumors [62–64]. On one hand, if some cancers have several distinct subtypes, the origination of CSCs from each subtype may follow different mechanisms [19]. On the other hand, the origins of CSCs from primary tumors and metastatic tumors may be totally different [65]. A new concept of “horizontal hierarchy of CSCs” has been proposed to distinguish them from a vertical-hierarchy of CSCs. A better understanding of this distinction could open up novel therapeutic strategies that target CSCs. The heterogeneity of CSCs may be due to their different origins [41]. For example, the heterogeneity of HCSCs may be due to their derivation from endogenous stem/progenitor cells or dedifferentiation of a transformed cell. Poorly differentiated HCC originates from immature cells (stem cells), while the well-differentiated HCC originates from mature cells (hepatocytes) [9]. Specific markers for CSC isolation can be selected on the basis of a deeper understanding of the underlying stem cell biology of the pertinent tissue from which the cancer originates.
5 The origination mechanisms of CSCs
Whatever types of cells CSCs originate from, the core issue may reside in an abnormal self-renewal or impaired differentiation of the original cells, which include NSCs, mature tissue cells and common cancer cells.
5.1 The abnormal self-renewal of CSC origin cells
The initial event for the genesis of CSCs has been speculated to be the change in self-renewal ability of primitive cells. The malignant transformation mechanism from NSCs to CSCs remains unknown. Elucidation of the genes involved in the mechanism(s) underlying the malignant transformation of NSCs may provide a novel target for cancer prevention. Many pathways appear to play central roles in the self-renewal of somatic stem cells in a variety of tissues and organs and they seem to be implicated in tumor development [66], including transforming growth factor-beta signaling [2], the pathways involving microRNA-181, EpCAM and beta-catenin [67], and Myc-driven reprogramming of microRNA (miRNA) expression patterns [68]. Nevertheless, the exact mechanism of abnormal self-renewal is far from clear.
5.2 The impaired differentiation of CSC origin cells
Aside from abnormal self-renewal as the initial event of CSC genesis, impaired differentiation is considered to be the most important factor. On the one hand, some cumulative mutations of genes or miRNAs are vital for impaired differentiation of NSCs. Consistent with another study [69], we found that mutation of the Tg737 gene results in a phenotype in which HNSCs no longer respond to external differentiation signals and undergo uncontrolled stem cell proliferation without concomitant differentiation [70]. Alterations in the control of cellular proliferation and differentiation by Tg737 may be important during the genesis of HCSCs. During the process of CSC genesis, mature miRNAs engage in either degradation of target mRNAs or translational repression [71]. Overall, some miRNAs are common to both NSCs and CSCs and may be required to maintain stemness [72]. However, some miRNAs that are differentially expressed between NSCs and CSCs may contribute to the malignant transformation from NSCs to CSCs. To further clarify the mechanism of the malignant transformation from HNSCs to HCSCs, we compared the expression of several miRNAs between SP cells from hepatic normal cells (SP-HNCs) and SP cells from hepatic cancer cells (SP-HHCs) [39]. Similar to the findings from carcinomas of the lung [73] and liver [74], our data revealed a higher frequency of miRNA over-expression than under-expression in SP-HCCs compared with SP-HNCs. On the other hand, some mutations of genes or miRNAs are vital for the dedifferentiation of mature cells to form tumors, such as GSK-3beta, which regulates NF-kappaB activity in pancreatic cancer [60], transforming growth factor-beta, Wnt, and Hedgehog signals in colorectal cancer [61], and progesterone (Pg) in breast cancer [75].
6 The therapeutic strategies targeting CSCs
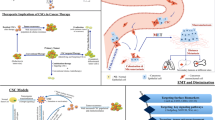
There are two main reasons why CSCs are resistant to therapy: first, because CSCs are quiescent they can escape the damage caused by therapy and second, because they highly express ABC transporters, they can become resistant to therapy by discharging cytotoxic materials [7]. Therefore, it is tempting to explain all therapeutic failures by the persistence of CSCs [76]. Consequently, it has been proposed that targeting CSCs is the only way to obtain durable cancer treatment responses [77]. Moreover, Dingli and Michor have designed a simple mathematical model to demonstrate the importance of eliminating CSCs [78] (Fig. 4a). Overall, at present the strategies of eliminating CSCs can mainly be divided into two types: directly eliminating CSCs by killing them and indirectly eliminating CSCs after they are modified to be easily targeted (Fig. 4b).
6.1 Directly eliminating CSCs by killing them
Among all the therapeutic strategies that target CSCs, the most thorough method may be to directly kill them. To achieve this aim, we should investigate the following three approaches. First, find the most effective and specific CSC-killing drugs. Vinorelbine in combination with parthenolide can be developed to eradicate CSCs [79]. Second, increase the sensitivity of CSCs to the above drugs. The reasons why CSCs become resistant to treatment are the following: CSCs express high levels of ABC drug transporters, providing a level of resistance; CSCs are relatively quiescent; CSCs exhibit higher levels of DNA repair and a reduced ability to enter apoptosis [80]. These traits need to be taken into account to develop molecularly targeted strategies to overcome the therapeutic resistance of CSCs [81]. Third, construct efficient drug carrier delivery systems. Targeted drug delivery systems (TDDSs) have attracted much attention in recent years. More and more drug/gene targeted delivery carriers, such as liposomes, magnetic nanoparticles, ligand-conjugated nanoparticles, and microbubbles, have been developed and are under investigation for clinical application [82]. In addition, knowledge of specific markers for CSCs may improve the effectiveness of TDDSs. For example, because CD13 is a marker for semiquiescent HCSCs, combining a CD13 inhibitor with a ROS-inducing chemo-/radiation therapy may improve the treatment of liver cancer [22]. Furthermore, mesenchymal stem cells (MSCs) are being used as tools for delivering therapeutic genes and proteins and as drug vectors to eliminate malignant cells [83]. MSCs can migrate towards and engraft into tumor sites, which make them a potential resource for efficient targeted-delivery vehicles in cancer gene therapy [82, 84]. Engineered with specific anticancer genes, MSCs are capable of constitutively producing specific anticancer agents locally [85]. For some cancer types, MSCs have been readily engineered to express anti-proliferative, pro-apoptotic, and anti-angiogenic agents that specifically target CSCs [86]. Through the above three classes of advancements, the most effective use of targeted molecular therapy drugs may effectively inhibit or at least retard CSC proliferation [87].
6.2 Indirectly eliminate CSCs by modifying them
6.2.1 The strategies of modifying CSCs
If CSCs cannot be killed directly, they can possibly be modified to be targeted more easily. The core issue for CSC genesis lies in impairing the proliferation and differentiation of primitive cells. Thus, CSCs could be modified in two main ways: inhibition of their proliferation and/or induction of their apoptosis, and induction of their proper differentiation. Through these modifications, CSCs may become normal cells. Early in 2009, Beug, et al. speculated that breast cancer stem cells reversal to breast somatic stem cells might offer a new therapy, which could stop the spread of breast cancer cells. The main strategy was to restore epithelial polarity [88]. Recently, it has been revealed that modification of genes by reprogramming factors such as nanog, sox-2, klf-4, oct-3/4, together with stem cell differentiation stage factors, might be important. Nevertheless, it is still a very preliminary investigation and the complexity of this therapy approach needs further research [89].
Thus far, only a limited number of studies have addressed the CSC-killing potential of apoptosis-targeted therapies. Apoptosis resistance may involve inherent cellular mechanisms that may change depending on the differentiation status of stem cells and, on the other hand, on extrinsic factors provided by the microenvironment, such as secreted survival factors, adhesion-mediated apoptosis resistance and hypoxic conditions [90]. Because the mechanisms of apoptosis resistance of CSCs are not clear, it may be disputed in relation to the efficacy of apoptosis therapies targeting CSCs, such as those involving TRAIL, BCL-2 family proteins and XIAP. The second strategy, inducing CSCs to properly differentiate, also holds promise. Regardless of the origins of CSCs, the consensus is that CSCs are immature cells. Thus, CSCs can be induced to differentiate, and as a result, these cells can be attacked by conventional radiation treatment or chemotherapy [91]. For example, oncostatin m can efficiently activate the differentiation and division of HCSCs, and the combination of oncostatin m and conventional chemotherapy with 5-FU can efficiently eliminate HCC by targeting both CSCs and non-CSCs [53].
6.2.2 The mechanisms for modifying CSCs
Regardless of the strategy used to modify CSCs, the targeted genes, molecules or miRNAs need to be identified, and the related mechanisms need to be clarified. Epigenetic alterations and mutations of genes or miRNAs involved in signal transduction may promote the formation of CSCs [92]. Therefore, monoclonal antibodies (mAbs) targeting specific antigens and related pathways that are altered in CSCs should facilitate CSC killing [93]. The signaling pathways active within various CSC types mainly include Nanog, Nestin, Notch1, Notch2, Oct3, Oct4, Wnt, Hedgehog, aldehyde dehydrogenase, epithelial to mesenchymal transition (EMT), miRNAs, and other epigenetic modifiers. They are all potential targets for therapeutic manipulation of CSCs [94]. In addition, the molecular targeting of key cytokine network axes of CSCs must also be considered, such as stem cell factor (SCF) and its receptor c-kit (CD117) in lung CSCs [95]. In our previous study, it was found that silencing of Tg737 can result in the malignant transformation of HNSCs [70]. Thus, targeting Tg737 may be an effective therapy for modifying HCSCs.
6.3 The safety issues of targeting CSCs
Although therapies targeting CSCs have made great progress, a number of issues must be investigated and resolved before effective treatments targeting CSCs can enter clinical testing. First of all, we must avoid eliminating normal cells, especially NSCs. The biological characteristics shared by NSCs and CSCs mainly involve their self-renewal and differentiation potential, survival ability, niche-specific microenvironment requirements and specific homing to metastatic sites [96]. Specifically, pathways that regulate NSC self-renewal and cell fate, such as ABC transporters, Wnt and Hedgehog, are also involved in the regulation of CSCs [83]. Thus, the major challenge in targeting CSCs is finding therapies that largely spare NSCs while eradicating quiescent CSCs [97]. Recently, a mathematical model was published that predicts how selective a therapy must be to ensure that enough NSCs survive when CSCs are eradicated [97]. From a clinical point of view, this model suggests criteria by which CSC therapy safety can be assessed [97].
7 Conclusions and perspectives
Although CSCs have been isolated by different groups, there are almost no unanimously recognized CSCs in multiple solid cancer types. This is because there is no standard method for isolating CSCs. Putative CSCs are prospectively isolated using methods based on either physical or immunochemical characteristics. Among these methods, FACS or MACS based on cell surface markers are the most frequently used. If there is no marker for a specific type of CSC, they can also be enriched by efflux of fluorescent dyes, such as Hoechst and Rho. In addition, CSCs can be separated by physical methods, such as Percoll based DGC. In some cases, CSCs may be isolated by different combinations of the above three methods. After isolation, we strongly suggest to establish a standard identification strategy for certifiying CSCs, which may consist of in vitro assessments of self-renewal and multipotentiality and in vivo assessments of serial xenotransplantation into NOD/SCID mice. Next to the stem properties of CSCs, we want to know their origin. At present, there are four theories about the cellular origin of CSCs: the maturation arrest theory, the dedifferentiation theory, the fusion theory, and the multiple origins theory. It is akin to multiple origins and the molecular mechanisms underlying these origins may be more important to clarify. Although the above problems are not completely resolved, many and varied therapeutic approaches to target these cells are being evaluated, and some have already entered clinical trials. The successful CSC therapies mainly concentrate on directly eliminating CSCs by killing them or indirectly eliminating CSCs by modifying them. Because CSCs are heterogeneous and may originate from distinct cells, the treatment of CSCs must be comprehensive by combining several strategies. In conclusion, the field of CSCs is still in its infancy, and the final aim of eliminating CSCs will be far from straightforward.
Abbreviations
- CSCs:
-
Cancer stem cells
- DGC:
-
Density-gradient centrifugation
- EMT:
-
Epithelial to mesenchymal transition
- FACS:
-
Fluorescent activated cells sorting
- HCSCs:
-
Hepatic cancer stem cells
- HNSCs:
-
Hepatic normal stem cells
- NOD/SCID:
-
Non-obese diabetic/severe combined immunodeficiency
- NSCs:
-
Normal stem cells
- NSP:
-
Non side population
- SP:
-
Side population
- SP-HNCs:
-
Side population of hepatic normal cells
- SP-HCCs:
-
Side population of hepatic cancer cells
- TDDS:
-
Targeting drug delivery systems
References
H. Clevers, The cancer stem cell: premises, promises and challenges. Nat. Med. 17(3), 313–319 (2011)
T. Ikegami, Transforming growth factor-beta signaling and liver cancer stem cell. Hepatol Res. 39(9), 847–849 (2009)
M.F. Clarke, J.E. Dick, P.B. Dirks, C.J. Eaves, C.H. Jamieson, D.L. Jones et al., Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 66(19), 9339–9344 (2006)
M. Balic, H. Lin, L. Young, D. Hawes, A. Giuliano, G. McNamara et al., Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin. Cancer Res. 12(19), 5615–5621 (2006)
G. Gu, J. Yuan, M. Wills, S. Kasper, Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 67(10), 4807–4815 (2007)
S. Takaishi, T. Okumura, S. Tu, S.S. Wang, W. Shibata, R. Vigneshwaran et al., Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 27(5), 1006–1020 (2009)
M. Todaro, M.G. Francipane, J.P. Medema, G. Stassi, Colon cancer stem cells: Promise of targeted therapy. Gastroenterology 138(6), 2151–2162 (2010)
T. Chiba, A. Kamiya, O. Yokosuka, A. Iwama, Cancer stem cells in hepatocellular carcinoma: Recent progress and perspective. Cancer Lett. 286(2), 145–153 (2009)
T. Shupe, B.E. Petersen, Evidence regarding a stem cell origin of hepatocellular carcinoma. Stem Cell Rev. 1(3), 261–264 (2005)
M. Baddoo, K. Hill, R. Wilkinson, D. Gaupp, C. Hughes, G.C. Kopen et al., Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J. Cell. Biochem. 89(6), 1235–1249 (2003)
F.I. Ghani, H. Yamazaki, S. Iwata, T. Okamoto, K. Aoe, K. Okabe et al., Identification of cancer stem cell markers in human malignant mesothelioma cells. Biochem. Biophys. Res. Commun. 404(2), 735–742 (2011)
S. Shigdar, J. Lin, Y. Yu, M. Pastuovic, M. Wei, W. Duan, RNA aptamer against a cancer stem cell marker epithelial cell adhesion molecule. Cancer Sci. 102(5), 991–998 (2011)
K. Kohga, T. Tatsumi, T. Takehara, H. Tsunematsu, S. Shimizu, M. Yamamoto et al., Expression of CD133 confers malignant potential by regulating metalloproteinases in human hepatocellular carcinoma. J. Hepatol. 52(6), 872–879 (2010)
L. Vermeulen, M. Todaro, F. de Sousa Mello, M.R. Sprick, K. Kemper, M. Perez Alea et al., Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc. Natl. Acad. Sci. U. S. A. 105(36), 13427–13432 (2008)
L.S. Hart, N.G. Dolloff, D.T. Dicker, C. Koumenis, J.G. Christensen, A. Grimberg et al., Human colon cancer stem cells are enriched by insulin-like growth factor-1 and are sensitive to figitumumab. Cell Cycle 10(14), 2331–2338 (2011)
F.J. Abdul Khalek, G.I. Gallicano, L. Mishra, Colon cancer stem cells. Gastrointest Cancer Res 1, S16–S23 (2010)
W. Song, H. Li, K. Tao, R. Li, Z. Song, Q. Zhao et al., Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma. Int. J. Clin. Pract. 62(8), 1212–1218 (2008)
T. Nagata, C. Sakakura, S. Komiyama, A. Miyashita, M. Nishio, Y. Murayama et al., Expression of cancer stem cell markers CD133 and CD44 in locoregional recurrence of rectal cancer. Anticancer Res. 31(2), 495–500 (2011)
T. Yamashita, J. Ji, A. Budhu, M. Forgues, W. Yang, H.Y. Wang et al., EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 136(3), 1012–1024 (2009)
Z.F. Yang, P. Ngai, D.W. Ho, W.C. Yu, M.N. Ng, C.K. Lau et al., Identification of local and circulating cancer stem cells in human liver cancer. Hepatology 47(3), 919–928 (2008)
H. Yanai, K. Nakamura, S. Hijioka, A. Kamei, T. Ikari, Y. Ishikawa et al., Dlk-1, a cell surface antigen on foetal hepatic stem/progenitor cells, is expressed in hepatocellular, colon, pancreas and breast carcinomas at a high frequency. J. Biochem. 148(1), 85–92 (2010)
B. Christ, P. Stock, M.M. Dollinger, CD13: Waving the flag for a novel cancer stem cell target. Hepatology 53(4), 1388–1390 (2011)
W. Yang, H.X. Yan, L. Chen, Q. Liu, Y.Q. He, L.X. Yu et al., Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 68(11), 4287–4295 (2008)
S. Lingala, Y.Y. Cui, X. Chen, B.H. Ruebner, X.F. Qian, M.A. Zern et al., Immunohistochemical staining of cancer stem cell markers in hepatocellular carcinoma. Exp. Mol. Pathol. 89(1), 27–35 (2010)
Z. Zhu, X. Hao, M. Yan, M. Yao, C. Ge, J. Gu et al., Cancer stem/progenitor cells are highly enriched in CD133 + CD44+ population in hepatocellular carcinoma. Int. J. Cancer 126(9), 2067–2078 (2010)
X.R. Yang, Y. Xu, B. Yu, J. Zhou, S.J. Qiu, G.M. Shi et al., High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut 59(7), 953–962 (2010)
T. Fujii, Y. Zen, K. Harada, H. Niwa, S. Masuda, Y. Kaizaki et al., Participation of liver cancer stem/progenitor cells in tumorigenesis of scirrhous hepatocellular carcinoma–human and cell culture study. Hum. Pathol. 39(8), 1185–1196 (2008)
M.A. Goodell, K. Brose, G. Paradis, A.S. Conner, R.C. Mulligan, Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 183(4), 1797–1806 (1996)
G.J. Spangrude, G.R. Johnson, Resting and activated subsets of mouse multipotent hematopoietic stem cells. Proc Natl Acad Sci USA 87(19), 7433–7437 (1990)
A. Asakura, P. Seale, A. Girgis-Gabardo, M.A. Rudnicki, Myogenic specification of side population cells in skeletal muscle. J. Cell Biol. 159(1), 123–134 (2002)
A.J. Alvi, H. Clayton, C. Joshi, T. Enver, A. Ashworth, M.M. Vivanco et al., Functional and molecular characterisation of mammary side population cells. Breast Cancer Res. 5(1), R1–R8 (2003)
K. Shimano, M. Satake, A. Okaya, J. Kitanaka, N. Kitanaka, M. Takemura et al., Hepatic oval cells have the side population phenotype defined by expression of ATP-binding cassette transporter ABCG2/BCRP1. Am. J. Pathol. 163(1), 3–9 (2003)
C.M. Dekaney, J.M. Rodriguez, M.C. Graul, S.J. Henning, Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology 129(5), 1567–1580 (2005)
M. Ono, T. Maruyama, H. Masuda, T. Kajitani, T. Nagashima, T. Arase et al., Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc. Natl. Acad. Sci. U.S.A. 104(47), 18700–18705 (2007)
T. Chiba, K. Kita, Y.W. Zheng, O. Yokosuka, H. Saisho, A. Iwama et al., Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology 44(1), 240–251 (2006)
G.M. Shi, Y. Xu, J. Fan, J. Zhou, X.R. Yang, S.J. Qiu et al., Identification of side population cells in human hepatocellular carcinoma cell lines with stepwise metastatic potentials. J. Cancer Res. Clin. Oncol. 134(11), 1155–1163 (2008)
Y. Zhang, W.J. Song, F.Q. Zhang, W.H. Liu, K.F. Dou, Differentiation-inducing activity of hydroxycamptothecin on cancer stem-like cells derived from hepatocellular carcinoma. Dig. Dis. Sci. 56(8), 2473–2481 (2011)
W.H. Liu, N.S. Qian, R. Li, K.F. Dou, Replacing Hoechst33342 with rhodamine123 in isolation of cancer stem-like cells from the MHCC97 cell line. Toxicol. In Vitro 24(2), 538–545 (2010)
W.H. Liu, K.S. Tao, N. You, Z.C. Liu, H.T. Zhang, K.F. Dou, Differences in the Properties and Mirna Expression Profiles between Side Populations from Hepatic Cancer Cells and Normal Liver Cells. PLoS One 6(8), e23311 (2011)
Z. Song, R. Li, N. You, K. Tao, K. Dou, Loss of heterozygosity of the tumor suppressor gene Tg737 in the side population cells of hepatocellular carcinomas is associated with poor prognosis. Mol. Biol. Rep. 37(8), 4091–4101 (2010)
A. Golebiewska, N.H. Brons, R. Bjerkvig, S.P. Niclou, Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell 8(2), 136–147 (2011)
F. Montanaro, K. Liadaki, J. Schienda, A. Flint, E. Gussoni, L.M. Kunkel, Demystifying SP cell purification: Viability, yield, and phenotype are defined by isolation parameters. Exp. Cell Res. 298(1), 144–154 (2004)
C. Ferlini, G. Scambia, Assay for apoptosis using the mitochondrial probes, Rhodamine123 and 10-N-nonyl acridine orange. Nat. Protoc. 2(12), 3111–3114 (2007)
A.C. Ribou, J. Vigo, E. Kohen, J.M. Salmon, Microfluorometric study of oxygen dependence of (1″-pyrene butyl)-2-rhodamine ester probe in mitochondria of living cells. J. Photochem. Photobiol. B 70(2), 107–115 (2003)
C. Wu, B.A. Alman, Side population cells in human cancers. Cancer Lett. 268(1), 1–9 (2008)
A.M. Rosca, A. Burlacu, Isolation of a mouse bone marrow population enriched in stem and progenitor cells by centrifugation on a Percoll gradient. Biotechnol. Appl. Biochem. 55(4), 199–208 (2010)
Y. Chang, P.H. Hsieh, C.C. Chao, The efficiency of Percoll and Ficoll density gradient media in the isolation of marrow derived human mesenchymal stem cells with osteogenic potential. Chang Gung Med. J. 32(3), 264–275 (2009)
W.H. Liu, R. Li, K.F. Dou, Convenient and efficient enrichment of the CD133+ liver cells from rat fetal liver cells as a source of liver stem/progenitor cells. Stem Cell Rev. 7(1), 94–102 (2011)
W.H. Liu, X. Wang, N. You, K.S. Tao, T. Wang, L.J. Tang et al., Efficient Enrichment of Hepatic Cancer Stem-Like Cells from a Primary Rat HCC Model via a Density Gradient Centrifugation-Centered Method. PLoS One 7(4), e35720 (2012)
N.A. Lobo, Y. Shimono, D. Qian, M.F. Clarke, The biology of cancer stem cells. Annu. Rev. Cell Dev. Biol. 23, 675–699 (2007)
M.F. Clarke, M. Fuller, Stem cells and cancer: Two faces of eve. Cell 124(6), 1111–1115 (2006)
X. Lu, Y. Kang, Cell fusion hypothesis of the cancer stem cell. Adv. Exp. Med. Biol. 714, 129–140 (2011)
T. Yamashita, M. Honda, K. Nio, Y. Nakamoto, H. Takamura, T. Tani et al., Oncostatin m renders epithelial cell adhesion molecule-positive liver cancer stem cells sensitive to 5-Fluorouracil by inducing hepatocytic differentiation. Cancer Res. 70(11), 4687–4697 (2010)
R. Taghizadeh, M. Noh, Y.H. Huh, E. Ciusani, L. Sigalotti, M. Maio et al., CXCR6, a newly defined biomarker of tissue-specific stem cell asymmetric self-renewal, identifies more aggressive human melanoma cancer stem cells. PLoS One 5(12), e15183 (2010)
A.G. Schepers, H.J. Snippert, D.E. Stange, M. van den Born, J.H. van Es, M. van de Wetering et al., Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337(6095), 730–735 (2012)
A.L. Welm, S. Kim, B.E. Welm, J.M. Bishop, MET and MYC cooperate in mammary tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 102(12), 4324–4329 (2005)
B. Mayer, J.P. Johnson, F. Leitl, K.W. Jauch, M.M. Heiss, F.W. Schildberg et al., E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 53(7), 1690–1695 (1993)
S. Sell, Cellular origin of cancer: Dedifferentiation or stem cell maturation arrest? Environ. Health Perspect. 101(Suppl 5), 15–26 (1993)
R. Fossmark, C.M. Zhao, T.C. Martinsen, S. Kawase, D. Chen, H.L. Waldum, Dedifferentiation of enterochromaffin-like cells in gastric cancer of hypergastrinemic cotton rats. APMIS. 113(6), 436–449 (2005)
A.V. Ougolkov, M.E. Fernandez-Zapico, V.N. Bilim, T.C. Smyrk, S.T. Chari, D.D. Billadeau, Aberrant nuclear accumulation of glycogen synthase kinase-3beta in human pancreatic cancer: association with kinase activity and tumor dedifferentiation. Clin. Cancer Res. 12(17), 5074–5081 (2006)
Y. Oku, T. Shimoji, K. Takifuji, T. Hotta, S. Yokoyama, K. Matsuda et al., Identification of the molecular mechanisms for dedifferentiation at the invasion front of colorectal cancer by a gene expression analysis. Clin. Cancer Res. 14(22), 7215–7222 (2008)
R.J. Gilbertson, T.A. Graham, Cancer: Resolving the stem-cell debate. Nature 488(7412), 462–463 (2012)
J. Chen, Y. Li, T.S. Yu, R.M. McKay, D.K. Burns, S.G. Kernie et al., A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488(7412), 522–526 (2012)
G. Driessens, B. Beck, A. Caauwe, B.D. Simons, C. Blanpain, Defining the mode of tumour growth by clonal analysis. Nature 488(7412), 527–530 (2012)
A.V. Salnikov, G. Kusumawidjaja, V. Rausch, H. Bruns, W. Gross, A. Khamidjanov et al., Cancer stem cell marker expression in hepatocellular carcinoma and liver metastases is not sufficient as single prognostic parameter. Cancer Lett. 275(2), 185–193 (2009)
T. Chiba, S. Miyagi, A. Saraya, R. Aoki, A. Seki, Y. Morita et al., The polycomb gene product BMI1 contributes to the maintenance of tumor-initiating side population cells in hepatocellular carcinoma. Cancer Res. 68(19), 7742–7749 (2008)
J.P. Li, J.Y. Zheng, J.J. Du, R. Zhang, A.G. Yang, What is the relationship among microRNA-181, epithelial cell-adhesion molecule (EpCAM) and beta-catenin in hepatic cancer stem cells. Hepatology 50(6), 2047–2048 (2009). author reply 448
S. Cairo, Y. Wang, A. de Reynies, K. Duroure, J. Dahan, M.J. Redon et al., Stem cell-like micro-RNA signature driven by Myc in aggressive liver cancer. Proc. Natl. Acad. Sci. U.S.A. 107(47), 20471–20476 (2010)
R.J. Isfort, D.B. Cody, C.J. Doersen, W.G. Richards, B.K. Yoder, J.E. Wilkinson et al., The tetratricopeptide repeat containing Tg737 gene is a liver neoplasia tumor suppressor gene. Oncogene 15(15), 1797–1803 (1997)
N. You, W. Liu, X. Zhong, R. Ji, M. Zhang, H. You et al., Tg737 inhibition results in malignant transformation in fetal liver stem/progenitor cells by promoting cell-cycle progression and differentiation arrest. Mol. Carcinog. 51(8), 659–673 (2012)
B. Zhang, X. Pan, G.P. Cobb, T.A. Anderson, microRNAs as oncogenes and tumor suppressors. Dev. Biol. 302(1), 1–12 (2007)
C.E. Rogler, MicroRNAs make inroads into liver development. Gastroenterology 136(3), 770–772 (2009)
E.J. Lee, Y. Gusev, J. Jiang, G.J. Nuovo, M.R. Lerner, W.L. Frankel et al., Expression profiling identifies microRNA signature in pancreatic cancer. Int. J. Cancer 120(5), 1046–1054 (2007)
Q.W. Wong, R.W. Lung, P.T. Law, P.B. Lai, K.Y. Chan, K.F. To et al., MicroRNA-223 is commonly repressed in hepatocellular carcinoma and potentiates expression of Stathmin1. Gastroenterology 135(1), 257–269 (2008)
R. Liu, Z. Zhou, D. Zhao, C. Chen, The Induction of KLF5 Transcription Factor by Progesterone Contributes to Progesterone-Induced Breast Cancer Cell Proliferation and Dedifferentiation. Mol. Endocrinol. 25(7), 1137–1144 (2011)
S. Chumsri, P. Phatak, M.J. Edelman, N. Khakpour, A.W. Hamburger, A.M. Burger, Cancer stem cells and individualized therapy. Cancer Genomics Proteomics 4(3), 165–174 (2007)
G. Federici, V. Espina, L. Liotta, K.H. Edmiston, Breast cancer stem cells: A new target for therapy. Oncology (Williston Park) 25(1), 25–28 (2011)
D. Dingli, F. Michor, Successful therapy must eradicate cancer stem cells. Stem Cells 24(12), 2603–2610 (2006)
Y. Liu, W.L. Lu, J. Guo, J. Du, T. Li, J.W. Wu et al., A potential target associated with both cancer and cancer stem cells: a combination therapy for eradication of breast cancer using vinorelbine stealthy liposomes plus parthenolide stealthy liposomes. J. Control. Release 129(1), 18–25 (2008)
J. Gil, A. Stembalska, K.A. Pesz, M.M. Sasiadek, Cancer stem cells: The theory and perspectives in cancer therapy. J. Appl. Genet. 49(2), 193–199 (2008)
L. Milas, W.N. Hittelman, Cancer stem cells and tumor response to therapy: current problems and future prospects. Semin. Radiat. Oncol. 19(2), 96–105 (2009)
Y.L. Hu, Y.H. Fu, Y. Tabata, J.Q. Gao, Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy. J. Control. Release 147(2), 154–162 (2010)
P. Aimola, V. Desiderio, A. Graziano, P.P. Claudio, Stem cells in cancer therapy: From their role in pathogenesis to their use as therapeutic agents. Drug News Perspect. 23(3), 175–183 (2010)
R. Uchibori, K. Ozawa, [Mesenchymal stem cells (MSCs) as a platform for cancer gene therapy]. Rinsho Ketsueki 51(11), 1641–1646 (2010)
L.J. Dai, M.R. Moniri, Z.R. Zeng, J.X. Zhou, J. Rayat, G.L. Warnock, Potential implications of mesenchymal stem cells in cancer therapy. Cancer Lett. 305(1), 8–20 (2011)
K. Shah, Mesenchymal stem cells engineered for cancer therapy. Adv. Drug Deliv. Rev. 64(8), 739–748 (2012)
I.S. Florian, C. Tomuleasa, O. Soritau, T. Timis, H. Ioani, A. Irimie et al., Cancer stem cells and malignant gliomas. From pathophysiology to targeted molecular therapy. J. BUON 16(1), 16–23 (2011)
H. Beug, Breast cancer stem cells: eradication by differentiation therapy? Cell 138(4), 623–625 (2009)
L. Wijaya, D. Agustina, A.O. Lizandi, M.M. Kartawinata, F. Sandra, Reversing breast cancer stem cell into breast somatic stem cell. Curr. Pharm. Biotechnol. 12(2), 189–195 (2011)
F.A. Kruyt, J.J. Schuringa, Apoptosis and cancer stem cells: Implications for apoptosis targeted therapy. Biochem. Pharmacol. 80(4), 423–430 (2010)
S. Sell, Potential gene therapy strategies for cancer stem cells. Curr. Gene Ther. 6(5), 579–591 (2006)
S. Soltanian, M.M. Matin, Cancer stem cells and cancer therapy. Tumour Biol. 32(3), 425–440 (2011)
O.K. Okamoto, J.F. Perez, Targeting cancer stem cells with monoclonal antibodies: a new perspective in cancer therapy and diagnosis. Expert Rev. Mol. Diagn. 8(4), 387–393 (2008)
M.R. Alison, S.M. Lim, L.J. Nicholson, Cancer stem cells: problems for therapy? J. Pathol. 223(2), 147–161 (2011)
E. Gorelik, A. Lokshin, V. Levina, Lung cancer stem cells as a target for therapy. Anticancer Agents Med Chem. 10(2), 164–171 (2010)
G.J. Leiros, M.E. Balana, Metastatic cancer stem cells: new molecular targets for cancer therapy. Curr. Pharm. Biotechnol. 12(11), 1909–1922 (2011)
M.E. Sehl, J.S. Sinsheimer, H. Zhou, K.L. Lange, Differential destruction of stem cells: implications for targeted cancer stem cell therapy. Cancer Res. 69(24), 9481–9489 (2009)
Acknowledgments
Sincere thanks go to Fu-qin Zhang (the Fourth Military Medical University, China) for data collection. This study was supported by the National Natural Science Foundation of China (No. 81170419, 81172061, 81000309, 81272648, 81001695). The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
Conflicts of interest
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wei-hui Liu, Nan You, Ning Zhang, Hong-tao Yan, Tao Wang, Zhu Huang equally contributed to this work. Hong-bao Liu, Li-jun Tang were co-corresponding authors.
Rights and permissions
About this article
Cite this article
Liu, Wh., You, N., Zhang, N. et al. Interpretation of interlocking key issues of cancer stem cells in malignant solid tumors. Cell Oncol. 35, 397–409 (2012). https://doi.org/10.1007/s13402-012-0110-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-012-0110-8